Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy
Abstract
1. Introduction
2. Glutamatergic Mechanisms of Glioma Progression and Tumor-Associated Epilepsy
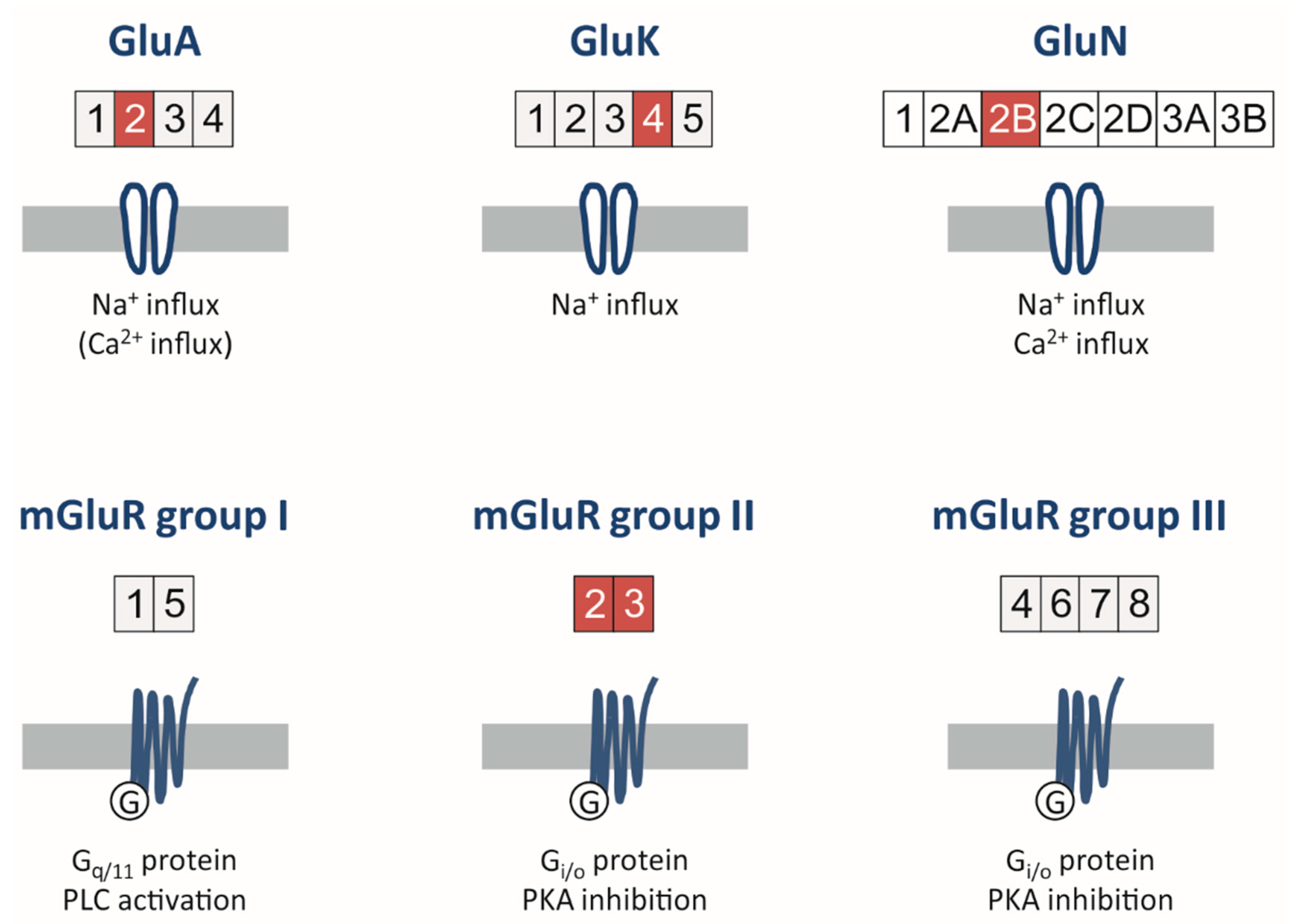
2.1. Ionotropic Glutamate Receptors
2.1.1. AMPA Receptors
2.1.2. Kainate Receptors
2.1.3. NMDA Receptors
2.2. Metabotropic Glutamate Receptors
2.2.1. Group I
2.2.2. Group II
2.2.3. Group III
2.3. Neurogliomal Synapse
2.4. Therapeutic Strategies
2.4.1. Sulfasalazin
2.4.2. Anticonvulsants
2.4.3. Talampanel
2.4.4. Perampanel
2.4.5. Memantine
3. Preclinical Models to Study Glutamate Interaction and Tumor-Associated Epilepsy
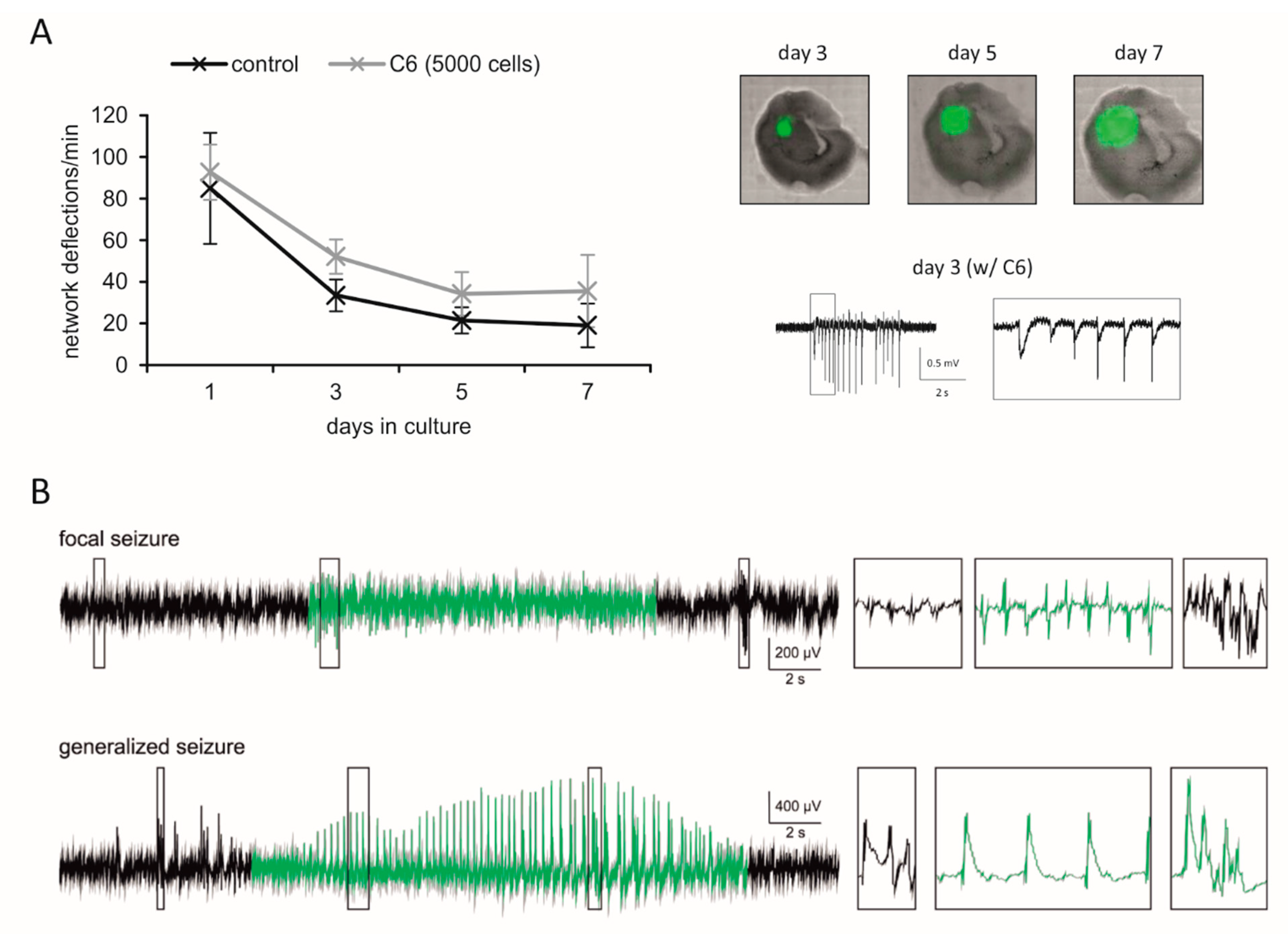
3.1. Cell Culture Models
3.2. Organotypic Brain Slice Cultures
3.3. In Vivo Models
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCSF | artificial cerebrospinal fluid |
| AKT | protein kinase B |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPAR | AMPA receptor |
| ASCT2 | alanine-serine-cysteine amino acid transporter-2 |
| BCAT1 | branched-chain amino acid transaminase 1 |
| cAMP | cyclic adenosine monophosphate |
| CDKN2A/B | cyclin dependent kinase inhibitor 2A/B |
| CDK4 | cyclin-dependent kinase 4 |
| DAAO | D-amino acid oxidase |
| DAG | diacylglycerol |
| D-2HG | D-2-hydroxyglutarate |
| EAAT2 | excitatory amino acid transporter 2 |
| GLT-1 | glutamate transporter 1 |
| IDH1 | isocitrate dehydrogenase 1 |
| iNOS | inducible NO synthase |
| IP3 | inositol-1,4,5-trisphosphate |
| KAR | kainate receptor |
| LEV | levetiracetam |
| MAPK | mitogen-activated protein kinase |
| MGMT | O6-methylguanine-DNA methyltransferase |
| mGluR | metabotropic glutamate receptor |
| Nf1 | neurofibromin 1 |
| NMDA | N-methyl-D-aspartate |
| NMDAR | NMDA receptor |
| PDGFRA | platelet-derived growth factor receptor A |
| PER | perampanel |
| PI3K | phosphatidylinositol-3-kinase |
| PKA | protein kinase A |
| PLC | phospholipase C |
| PTEN | phosphatase and tensin homolog |
| SAS | sulfasalazine |
| SV2A | synaptic vesicle glycoprotein 2A |
| TMZ | temozolomide |
| TPM | topiramate |
| TP53 | tumor protein P53 |
| VPA | valproic acid |
| xCT | solute carrier family 7 member 11 |
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncol. 2013, 15, ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef]
- Nobusawa, S.; Watanabe, T.; Kleihues, P.; Ohgaki, H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin. Cancer Res. 2009, 15, 6002–6007. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Crespo, I.; Vital, A.L.; Gonzalez-Tablas, M.; Patino, M.dC.; Otero, A.; Lopes, M.C.; de Oliveira, C.; Domingues, P.; Orfao, A.; Tabernero, M.D. Molecular and Genomic Alterations in Glioblastoma Multiforme. Am. J. Pathol. 2015, 185, 1820–1833. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Platten, M.; Meisner, C.; Felsberg, J.; Tabatabai, G.; Simon, M.; Nikkhah, G.; Papsdorf, K.; Steinbach, J.P.; Sabel, M.; et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012, 13, 707–715. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Egaña, L.; Auzmendi-Iriarte, J.; Andermatten, J.; Villanua, J.; Ruiz, I.; Elua-Pinin, A.; Aldaz, P.; Querejeta, A.; Sarasqueta, C.; Zubia, F.; et al. Methylation of MGMT promoter does not predict response to temozolomide in patients with glioblastoma in Donostia Hospital. Sci. Rep. 2020, 10, 18445. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs. Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Lassman, A.B.; Joanta-Gomez, A.E.; Pan, P.C.; Wick, W. Current usage of tumor treating fields for glioblastoma. Neurooncol. Adv. 2020, 2, vdaa069. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.L.; Palmisano, S.; Schwartzbaum, J.A.; Svensson, T.; Lönn, S. Comorbid conditions associated with glioblastoma. J. Neurooncol. 2014, 116, 585–591. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, M.S.M.; Wilms, E.B.; Vecht, C.J. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007, 6, 421–430. [Google Scholar] [CrossRef]
- Kerkhof, M.; Vecht, C.J. Seizure characteristics and prognostic factors of gliomas. Epilepsia 2013, 54, 12–17. [Google Scholar] [CrossRef]
- Armstrong, T.S.; Grant, R.; Gilbert, M.R.; Lee, J.W.; Norden, A.D. Epilepsy in glioma patients: Mechanisms, management, and impact of anticonvulsant therapy. Neuro-Oncology 2016, 18, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Huberfeld, G.; Vecht, C.J. Seizures and gliomas—Towards a single therapeutic approach. Nat. Rev. Neurol. 2016, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Roslin, M.; Henriksson, R.; Bergström, P.; Ungerstedt, U.; Bergenheim, A.T. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J. Neurooncol. 2003, 61, 151–160. [Google Scholar] [CrossRef]
- Marcus, H.J.; Carpenter, K.L.H.; Price, S.J.; Hutchinson, P.J. In vivo assessment of high-grade glioma biochemistry using microdialysis: A study of energy-related molecules, growth factors and cytokines. J. Neurooncol. 2010, 97, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Lin, J.H.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- Lyons, S.A.; Chung, W.J.; Weaver, A.K.; Ogunrinu, T.; Sontheimer, H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007, 67, 9463–9471. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, S.; Yoshida, Y.; Sugawara, K.; Aihara, M.; Ohtani, T.; Watanabe, T.; Saito, N.; Tsuzuki, K.; Okado, H.; Miwa, A.; et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J. Neurosci. 2007, 27, 7987–8001. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.C.; Campbell, S.L.; Haas, B.R.; Montana, V.; Robel, S.; Ogunrinu, T.; Sontheimer, H. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 2011, 17, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.I.; Morokoff, A.P.; Bjorksten, A.; D’Abaco, G.; Paradiso, L.; Finch, S.; Wong, D.; Reid, C.A.; Powell, K.L.; Drummond, K.J.; et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology 2012, 79, 883–889. [Google Scholar] [CrossRef]
- Lo, M.; Wang, Y.-Z.; Gout, P.W. The x(c)- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008, 215, 593–602. [Google Scholar] [CrossRef]
- Ye, Z.C.; Rothstein, J.D.; Sontheimer, H. Compromised glutamate transport in human glioma cells: Reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J. Neurosci. 1999, 19, 10767–10777. [Google Scholar] [CrossRef]
- Chung, W.J.; Lyons, S.A.; Nelson, G.M.; Hamza, H.; Gladson, C.L.; Gillespie, G.Y.; Sontheimer, H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 2005, 25, 7101–7110. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, N.E.; Heckel, A.; Hahnen, E.; Engelhorn, T.; Doerfler, A.; Ganslandt, O.; Nimsky, C.; Buchfelder, M.; Eyüpoglu, I.Y. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat. Med. 2008, 14, 629–632. [Google Scholar] [CrossRef]
- Takeuchi, S.; Wada, K.; Toyooka, T.; Shinomiya, N.; Shimazaki, H.; Nakanishi, K.; Nagatani, K.; Otani, N.; Osada, H.; Uozumi, Y.; et al. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery 2013, 72, 33–41. [Google Scholar] [CrossRef]
- Long, Y.; Tao, H.; Karachi, A.; Grippin, A.J.; Jin, L.; Chang, Y.E.; Zhang, W.; Dyson, K.A.; Hou, A.Y.; Na, M.; et al. Dysregulation of Glutamate Transport Enhances Treg Function That Promotes VEGF Blockade Resistance in Glioblastoma. Cancer Res. 2020, 80, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.M.; Buckingham, S.C.; Campbell, S.L.; Robel, S.; Holt, K.T.; Ogunrinu-Babarinde, T.; Warren, P.P.; White, D.M.; Reid, M.A.; Eschbacher, J.M.; et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci. Transl. Med. 2015, 7, 289ra86. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.F.; Heimisdóttir, S.B.; Sørensen, M.D.; Mellegaard, C.S.; Wohlleben, H.; Kristensen, B.W.; Beier, C.P. High expression of cystine-glutamate antiporter xCT (SLC7A11) is an independent biomarker for epileptic seizures at diagnosis in glioma. J. Neurooncol. 2018, 138, 49–53. [Google Scholar] [CrossRef]
- Dührsen, L.; Sauvigny, T.; Ricklefs, F.L.; Mende, K.-C.; Schaper, M.; Matschke, J.; Goebell, E.; Westphal, M.; Martens, T. Seizures as presenting symptom in patients with glioblastoma. Epilepsia 2019, 60, 149–154. [Google Scholar] [CrossRef]
- de Groot, J.F.; Liu, T.J.; Fuller, G.; Yung, W.K.A. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Res. 2005, 65, 1934–1940. [Google Scholar] [CrossRef]
- Buccoliero, A.M.; Caporalini, C.; Scagnet, M.; Mussa, F.; Giordano, F.; Sardi, I.; Migliastro, I.; Moscardi, S.; Conti, V.; Barba, C.; et al. Angiocentric glioma-associated seizures: The possible role of EATT2, pyruvate carboxylase and glutamine synthetase. Seizure 2021, 86, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Tönjes, M.; Barbus, S.; Park, Y.J.; Wang, W.; Schlotter, M.; Lindroth, A.M.; Pleier, S.V.; Bai, A.H.C.; Karra, D.; Piro, R.M.; et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med. 2013, 19, 901–908. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Shi, X.; Zhou, M.; Bao, L.; Hatanpaa, K.J.; Patel, T.; DeBerardinis, R.J.; Wang, Y.; Luo, W. Regulation of branched-chain amino acid metabolism by hypoxia-inducible factor in glioblastoma. Cell. Mol. Life Sci. 2021, 78, 195–206. [Google Scholar] [CrossRef]
- Cho, H.R.; Jeon, H.; Park, C.K.; Park, S.H.; Kang, K.M.; Choi, S.H. BCAT1 is a New MR Imaging-related Biomarker for Prognosis Prediction in IDH1-wildtype Glioblastoma Patients. Sci. Rep. 2017, 7, 17740. [Google Scholar] [CrossRef]
- Yi, L.; Fan, X.; Li, J.; Yuan, F.; Zhao, J.; Nistér, M.; Yang, X. Enrichment of branched chain amino acid transaminase 1 correlates with multiple biological processes and contributes to poor survival of IDH1 wild-type gliomas. Aging 2021, 13, 3645–3660. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- McBrayer, S.K.; Mayers, J.R.; DiNatale, G.J.; Shi, D.D.; Khanal, J.; Chakraborty, A.A.; Sarosiek, K.A.; Briggs, K.J.; Robbins, A.K.; Sewastianik, T.; et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell 2018, 175, 101–116.e25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, Q.; Wang, X.; Liu, Y.; Mao, Y.; Zhou, Q.; Luo, J. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J. Clin. Neurosci. 2016, 31, 56–62. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Worrell, G.A.; Tatum, W.O.; Chaichana, K.L.; Jentoft, M.E.; Cazares, H.G.; Ertekin-Taner, N.; Rosenfeld, S.S.; ReFaey, K.; Quinones-Hinojosa, A. Potential influence of IDH1 mutation and MGMT gene promoter methylation on glioma-related preoperative seizures and postoperative seizure control. Seizure 2019, 69, 283–289. [Google Scholar] [CrossRef]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Pallud, J.; Le Van Quyen, M.; Bielle, F.; Pellegrino, C.; Varlet, P.; Cresto, N.; Baulac, M.; Duyckaerts, C.; Kourdougli, N.; Chazal, G.; et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci. Transl. Med. 2014, 6, 244ra89. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, A.; Yu, K.; Meyer, J.; Aiba, I.; Deneen, B.; Noebels, J.L. Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J. Clin. Investig. 2020, 130, 2286–2300. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Stepulak, A.; Luksch, H.; Gebhardt, C.; Uckermann, O.; Marzahn, J.; Sifringer, M.; Rzeski, W.; Staufner, C.; Brocke, K.S.; Turski, L.; et al. Expression of glutamate receptor subunits in human cancers. Histochem. Cell Biol. 2009, 132, 435–445. [Google Scholar] [CrossRef]
- Brocke, K.S.; Staufner, C.; Luksch, H.; Geiger, K.D.; Stepulak, A.; Marzahn, J.; Schackert, G.; Temme, A.; Ikonomidou, C. Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol. Ther. 2010, 9, 455–468. [Google Scholar] [CrossRef]
- Pickard, L.; Noël, J.; Henley, J.M.; Collingridge, G.L.; Molnar, E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J. Neurosci. 2000, 20, 7922–7931. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Bacci, A.; Kharazia, V.; Huguenard, J.R. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J. Neurosci. 2002, 22, 3005–3015. [Google Scholar] [CrossRef]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.; Patt, S.; Schrey, M.; Rich, A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc. Natl. Acad. Sci. USA 2001, 98, 14687–14692. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, S.; Tsuzuki, K.; Yoshida, Y.; Yamada, N.; Hagimura, N.; Okado, H.; Miwa, A.; Kurihara, H.; Nakazato, Y.; Tamura, M.; et al. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 2002, 8, 971–978. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Krauss, G.L.; Wechsler, R.T.; Wang, X.F.; DiVentura, B.; Brandt, C.; Trinka, E.; O’Brien, T.J.; Laurenza, A.; Patten, A.; et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology 2015, 85, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Piña-Garza, J.E.; Rosenfeld, W.; Saeki, K.; Villanueva, V.; Yoshinaga, H.; Patten, A.; Williams, B.; Malhotra, M. Efficacy and safety of adjunctive perampanel in adolescent patients with epilepsy: Post hoc analysis of six randomized studies. Epilepsy Behav. 2020, 104, 106876. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; Heinemann, S. Cloned glutamate receptors. Ann. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef]
- Fogarty, D.J.; Pérez-Cerdá, F.; Matute, C. KA1-like kainate receptor subunit immunoreactivity in neurons and glia using a novel anti-peptide antibody. Brain Res. Mol. Brain Res. 2000, 81, 164–176. [Google Scholar] [CrossRef]
- Cauley, K.; Kukekov, V.; Young, D. Kainate/AMPA receptors expressed on human fetal astrocytes in long-term culture. J. Neurosci. Res. 1997, 47, 311–321. [Google Scholar] [CrossRef]
- Liu, Q.S.; Xu, Q.; Arcuino, G.; Kang, J.; Nedergaard, M. Astrocyte-mediated activation of neuronal kainate receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Gryder, D.S.; Rogawski, M.A. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J. Neurosci. 2003, 23, 7069–7074. [Google Scholar] [CrossRef] [PubMed]
- Irvine, M.W.; Costa, B.M.; Dlaboga, D.; Culley, G.R.; Hulse, R.; Scholefield, C.L.; Atlason, P.; Fang, G.; Eaves, R.; Morley, R.; et al. Piperazine-2,3-dicarboxylic acid derivatives as dual antagonists of NMDA and GluK1-containing kainate receptors. J. Med. Chem. 2012, 55, 327–341. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Matta, J.A.; Ashby, M.C.; Sanz-Clemente, A.; Roche, K.W.; Isaac, J.T.R. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 2011, 70, 339–351. [Google Scholar] [CrossRef]
- Evans, R.C.; Morera-Herreras, T.; Cui, Y.; Du, K.; Sheehan, T.; Kotaleski, J.H.; Venance, L.; Blackwell, K.T. The effects of NMDA subunit composition on calcium influx and spike timing-dependent plasticity in striatal medium spiny neurons. PLoS Comput. Biol. 2012, 8, e1002493. [Google Scholar] [CrossRef]
- Philpot, B.D.; Sekhar, A.K.; Shouval, H.Z.; Bear, M.F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 2001, 29, 157–169. [Google Scholar] [CrossRef]
- Liu, L.; Wong, T.P.; Pozza, M.F.; Lingenhoehl, K.; Wang, Y.; Sheng, M.; Auberson, Y.P.; Wang, Y.T. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004, 304, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- McQuate, A.; Barria, A. Rapid exchange of synaptic and extrasynaptic NMDA receptors in hippocampal CA1 neurons. J. Neurophysiol. 2020, 123, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Hosseinmardi, N.; Mirnajafi-Zadeh, J.; Javan, M.; Semnanian, S.; Naghdi, N.; Fathollahi, Y. Long-term potentiation enhancing effect of epileptic insult in the CA1 area is dependent on prior-application of primed-burst stimulation. Exp. Brain Res. 2020, 238, 897–903. [Google Scholar] [CrossRef]
- Müller, L.; Tokay, T.; Porath, K.; Köhling, R.; Kirschstein, T. Enhanced NMDA receptor-dependent LTP in the epileptic CA1 area via upregulation of NR2B. Neurobiol. Dis. 2013, 54, 183–193. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Park, H.; Kang, T.C. Src/CK2/PTEN-Mediated GluN2B and CREB Dephosphorylations Regulate the Responsiveness to AMPA Receptor Antagonists in Chronic Epilepsy Rats. Int. J. Mol. Sci. 2020, 21, 9633. [Google Scholar] [CrossRef]
- Conti, F.; Barbaresi, P.; Melone, M.; Ducati, A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb. Cortex 1999, 9, 110–120. [Google Scholar] [CrossRef]
- Lee, M.C.; Ting, K.K.; Adams, S.; Brew, B.J.; Chung, R.; Guillemin, G.J. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS ONE 2010, 5, e14123. [Google Scholar] [CrossRef]
- Xing, W.J.; Zou, Y.; Han, Q.L.; Dong, Y.C.; Deng, Z.L.; Lv, X.H.; Jiang, T.; Ren, H. Effects of epidermal growth factor receptor and phosphatase and tensin homologue gene expression on the inhibition of U87MG glioblastoma cell proliferation induced by protein kinase inhibitors. Clin. Exp. Pharmacol. Physiol. 2013, 40, 13–21. [Google Scholar] [CrossRef]
- Markert, J.M.; Fuller, C.M.; Gillespie, G.Y.; Bubien, J.K.; McLean, L.A.; Hong, R.L.; Lee, K.; Gullans, S.R.; Mapstone, T.B.; Benos, D.J. Differential gene expression profiling in human brain tumors. Physiol. Genom. 2001, 5, 21–33. [Google Scholar] [CrossRef]
- Hu, G.; Wei, B.; Wang, L.; Wang, L.; Kong, D.; Jin, Y.; Sun, Z. Analysis of gene expression profiles associated with glioma progression. Mol. Med. Rep. 2015, 12, 1884–1890. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Cai, S.; Saadatzadeh, M.R.; Hanenberg, H.; Pollok, K.E.; Cohen-Gadol, A.A.; Chen, J. Phosphorylation of NMDA 2B at S1303 in human glioma peritumoral tissue: Implications for glioma epileptogenesis. Neurosurg. Focus 2014, 37, E17. [Google Scholar] [CrossRef]
- Vecht, C.; Duran-Peña, A.; Houillier, C.; Durand, T.; Capelle, L.; Huberfeld, G. Seizure response to perampanel in drug-resistant epilepsy with gliomas: Early observations. J. Neurooncol. 2017, 133, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Dunn-Pirio, A.M.; Woodring, S.; Lipp, E.; Herndon, J.E.; Healy, P.; Weant, M.; Randazzo, D.; Desjardins, A.; Friedman, H.S.; Peters, K.B. Adjunctive perampanel for glioma-associated epilepsy. Epilepsy Behav. Case Rep. 2018, 10, 114–117. [Google Scholar] [CrossRef]
- Izumoto, S.; Miyauchi, M.; Tasaki, T.; Okuda, T.; Nakagawa, N.; Nakano, N.; Kato, A.; Fujita, M. Seizures and Tumor Progression in Glioma Patients with Uncontrollable Epilepsy Treated with Perampanel. Anticancer Res. 2018, 38, 4361–4366. [Google Scholar] [CrossRef]
- Maschio, M.; Pauletto, G.; Zarabla, A.; Maialetti, A.; Ius, T.; Villani, V.; Fabi, A.; Koudriavtseva, T.; Giannarelli, D. Perampanel in patients with brain tumor-related epilepsy in real-life clinical practice: A retrospective analysis. Int. J. Neurosci. 2019, 129, 593–597. [Google Scholar] [CrossRef]
- Chonan, M.; Saito, R.; Kanamori, M.; Osawa, S.I.; Watanabe, M.; Suzuki, H.; Nakasato, N.; Tominaga, T. Experience of Low Dose Perampanel to Add-on in Glioma Patients with Levetiracetam-uncontrollable Epilepsy. Neurol. Med. Chir. 2020, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; Zarabla, A.; Maialetti, A.; Villani, V.; Koudriavtseva, T.; Russo, E.; Nozzolillo, A.; Sueri, C.; Belcastro, V.; Balestrini, S.; et al. Perampanel Confirms to Be Effective and Well-Tolerated as an Add-On Treatment in Patients with Brain Tumor-Related Epilepsy (PERADET Study). Front. Neurol. 2020, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988, 241, 835–837. [Google Scholar] [CrossRef]
- Hashimoto, A.; Nishikawa, T.; Hayashi, T.; Fujii, N.; Harada, K.; Oka, T.; Takahashi, K. The presence of free D-serine in rat brain. FEBS Lett. 1992, 296, 33–36. [Google Scholar] [CrossRef]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef]
- Neims, A.H.; Zieverink, W.D.; Smilack, J.D. Distribution of D-amino acid oxidase in bovine and human nervous tissues. J. Neurochem. 1966, 13, 163–168. [Google Scholar] [CrossRef]
- Hayashi, F.; Takahashi, K.; Nishikawa, T. Uptake of D- and L-serine in C6 glioma cells. Neurosci. Lett. 1997, 239, 85–88. [Google Scholar] [CrossRef]
- Shao, Z.; Kamboj, A.; Anderson, C.M. Functional and immunocytochemical characterization of D-serine transporters in cortical neuron and astrocyte cultures. J. Neurosci. Res. 2009, 87, 2520–2530. [Google Scholar] [CrossRef]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O.J.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Balu, D.; Wolosker, H. D-Serine, the Shape-Shifting NMDA Receptor Co-agonist. Neurochem. Res. 2020, 45, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Sikka, P.; Walker, R.; Cockayne, R.; Wood, M.J.A.; Harrison, P.J.; Burnet, P.W.J. D-Serine metabolism in C6 glioma cells: Involvement of alanine-serine-cysteine transporter (ASCT2) and serine racemase (SRR) but not D-amino acid oxidase (DAO). J. Neurosci. Res. 2010, 88, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, P.; Campomenosi, P.; Pollegioni, L.; Sacchi, S. The degradation (by distinct pathways) of human D-amino acid oxidase and its interacting partner pLG72--two key proteins in D-serine catabolism in the brain. FEBS J. 2014, 281, 708–723. [Google Scholar] [CrossRef]
- Shoji, K.; Mariotto, S.; Ciampa, A.R.; Suzuki, H. Regulation of serine racemase activity by D-serine and nitric oxide in human glioblastoma cells. Neurosci. Lett. 2006, 392, 75–78. [Google Scholar] [CrossRef]
- Shoji, K.; Mariotto, S.; Ciampa, A.R.; Suzuki, H. Mutual regulation between serine and nitric oxide metabolism in human glioblastoma cells. Neurosci. Lett. 2006, 394, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Cuny, E.; Loiseau, H.; Penchet, G.; Ellie, E.; Arsaut, J.; Vital, A.; Vincendeau, P.; Demotes-Mainard, J. Association of elevated glial expression of interleukin-1beta with improved survival in patients with glioblastomas multiforme. J. Neurosurg. 2002, 96, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Shishido, Y.; Ichise-Shishido, S.; Kawazoe, T.; Ono, K.; Iwana, S.; Tomita, Y.; Yorita, K.; Sakai, T.; Fukui, K. Potential role for astroglial D-amino acid oxidase in extracellular D-serine metabolism and cytotoxicity. J. Biochem. 2006, 139, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Yin, S.; Noetzel, M.J.; Johnson, K.A.; Zamorano, R.; Jalan-Sakrikar, N.; Gregory, K.J.; Conn, P.J.; Niswender, C.M. Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J. Neurosci. 2014, 34, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Munguba, H.; Gutzeit, V.A.; Singh, D.R.; Kristt, M.; Dittman, J.S.; Levitz, J. Defining the Homo- and Heterodimerization Propensities of Metabotropic Glutamate Receptors. Cell Rep. 2020, 31, 107605. [Google Scholar] [CrossRef]
- Nakada, M.; Kita, D.; Watanabe, T.; Hayashi, Y.; Teng, L.; Pyko, I.V.; Hamada, J.-I. Aberrant signaling pathways in glioma. Cancers 2011, 3, 3242–3278. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Silginer, M.; Ventura, E.; Macnair, W.; Burghardt, I.; Claassen, M.; Gatti, S.; Wichmann, J.; Riemer, C.; Schneider, H.; et al. Negative allosteric modulators of metabotropic glutamate receptor 3 target the stem-like phenotype of glioblastoma. Mol. Ther. Oncolytics 2020, 20, 166–174. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Li, H.; Zhao, Z.; Liao, Y.; Wang, X.; Su, J.; Sang, S.; Liu, Q. Anti-cancer effect of metabotropic glutamate receptor 1 inhibition in human glioma U87 cells: Involvement of PI3K/Akt/mTOR pathway. Cell. Physiol. Biochem. 2015, 35, 419–432. [Google Scholar] [CrossRef]
- Dalley, C.B.; Wroblewska, B.; Wolfe, B.B.; Wroblewski, J.T. The Role of Metabotropic Glutamate Receptor 1 Dependent Signaling in Glioma Viability. J. Pharmacol. Exp. Ther. 2018, 367, 59–70. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Qi, C.; Zhao, X.; Liu, B.; Hao, F.; Zhao, Z. Inhibition of metabotropic glutamate receptor 5 facilitates hypoxia-induced glioma cell death. Brain Res. 2019, 1704, 241–248. [Google Scholar] [CrossRef]
- Ciceroni, C.; Bonelli, M.; Mastrantoni, E.; Niccolini, C.; Laurenza, M.; Larocca, L.M.; Pallini, R.; Traficante, A.; Spinsanti, P.; Ricci-Vitiani, L.; et al. Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas. Cell Death Differ. 2013, 20, 396–407. [Google Scholar] [CrossRef]
- Arcella, A.; Carpinelli, G.; Battaglia, G.; D’Onofrio, M.; Santoro, F.; Ngomba, R.T.; Bruno, V.; Casolini, P.; Giangaspero, F.; Nicoletti, F. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro-Oncology 2005, 7, 236–245. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Arcella, A.; Bruno, V.; Ngomba, R.T.; Battaglia, G.; Lombari, V.; Ragona, G.; Calogero, A.; Nicoletti, F. Pharmacological blockade of mGlu2/3 metabotropic glutamate receptors reduces cell proliferation in cultured human glioma cells. J. Neurochem. 2003, 84, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Yelskaya, Z.; Carrillo, V.; Dubisz, E.; Gulzar, H.; Morgan, D.; Mahajan, S.S. Synergistic inhibition of survival, proliferation, and migration of U87 cells with a combination of LY341495 and Iressa. PLoS ONE 2013, 8, e64588. [Google Scholar] [CrossRef]
- Ciceroni, C.; Arcella, A.; Mosillo, P.; Battaglia, G.; Mastrantoni, E.; Oliva, M.A.; Carpinelli, G.; Santoro, F.; Sale, P.; Ricci-Vitiani, L.; et al. Type-3 metabotropic glutamate receptors negatively modulate bone morphogenetic protein receptor signaling and support the tumourigenic potential of glioma-initiating cells. Neuropharmacology 2008, 55, 568–576. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, X.; Luan, Y.; Liu, Y.; Li, X.; Liu, C.; Lu, H.; Chen, X.; Liu, Y. Activity of Metabotropic Glutamate Receptor 4 Suppresses Proliferation and Promotes Apoptosis with Inhibition of Gli-1 in Human Glioblastoma Cells. Front. Neurosci. 2018, 12, 320. [Google Scholar] [CrossRef]
- Iacovelli, L.; Arcella, A.; Battaglia, G.; Pazzaglia, S.; Aronica, E.; Spinsanti, P.; Caruso, A.; De Smaele, E.; Saran, A.; Gulino, A.; et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas. J. Neurosci. 2006, 26, 8388–8397. [Google Scholar] [CrossRef]
- Jantas, D.; Grygier, B.; Gołda, S.; Chwastek, J.; Zatorska, J.; Tertil, M. An endogenous and ectopic expression of metabotropic glutamate receptor 8 (mGluR8) inhibits proliferation and increases chemosensitivity of human neuroblastoma and glioma cells. Cancer Lett. 2018, 432, 1–16. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef]
- Climans, S.A.; Brandes, A.A.; Cairncross, J.G.; Ding, K.; Fay, M.; Laperriere, N.; Menten, J.; Nishikawa, R.; O’Callaghan, C.J.; Perry, J.R.; et al. Temozolomide and seizure outcomes in a randomized clinical trial of elderly glioblastoma patients. J. Neurooncol. 2020, 149, 65–71. [Google Scholar] [CrossRef]
- Garcia, C.G.; Kahn, S.A.; Geraldo, L.H.M.; Romano, I.; Domith, I.; E Silva, D.C.L.; Assunção, F.D.S.; Ferreira, M.J.; Portugal, C.C.; De Souza, J.M.; et al. Combination Therapy with Sulfasalazine and Valproic Acid Promotes Human Glioblastoma Cell Death Through Imbalance of the Intracellular Oxidative Response. Mol. Neurobiol. 2018, 55, 6816–6833. [Google Scholar] [CrossRef]
- Takeuchi, S.; Wada, K.; Nagatani, K.; Otani, N.; Osada, H.; Nawashiro, H. Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol. India 2014, 62, 42–47. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Lafky, J.M.; Uhm, J.H.; Giannini, C.; Kumar, S.K.; Kimlinger, T.K.; Northfelt, D.W.; Flynn, P.J.; Jaeckle, K.A.; et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A north central cancer treatment group trial. Clin. Cancer Res. 2013, 19, 4816–4823. [Google Scholar] [CrossRef]
- Peereboom, D.M.; Ahluwalia, M.S.; Ye, X.; Supko, J.G.; Hilderbrand, S.L.; Phuphanich, S.; Nabors, L.B.; Rosenfeld, M.R.; Mikkelsen, T.; Grossman, S.A. New Approaches to Brain Tumor Therapy Consortium. NABTT 0502: A phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro-Oncology 2013, 15, 490–496. [Google Scholar] [CrossRef]
- Zustovich, F.; Landi, L.; Lombardi, G.; Porta, C.; Galli, L.; Fontana, A.; Amoroso, D.; Galli, C.; Andreuccetti, M.; Falcone, A.; et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: A phase II study. Anticancer Res. 2013, 33, 3487–3494. [Google Scholar] [CrossRef]
- Hottinger, A.F.; Aissa, A.B.; Espeli, V.; Squiban, D.; Dunkel, N.; Vargas, M.I.; Hundsberger, T.; Mach, N.; Schaller, K.; Weber, D.C.; et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br. J. Cancer 2014, 110, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Sehm, T.; Rauh, M.; Wiendieck, K.; Buchfelder, M.; Eyüpoglu, I.Y.; Savaskan, N.E. Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget 2016, 7, 74630–74647. [Google Scholar] [CrossRef]
- Dahlmanns, M.; Yakubov, E.; Chen, D.; Sehm, T.; Rauh, M.; Savaskan, N.; Wrosch, J.K. Chemotherapeutic xCT inhibitors sorafenib and erastin unraveled with the synaptic optogenetic function analysis tool. Cell Death Discov. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Weller, M.; Gorlia, T.; Cairncross, J.G.; van den Bent, M.J.; Mason, W.; Belanger, K.; Brandes, A.A.; Bogdahn, U.; Macdonald, D.R.; Forsyth, P.; et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 2011, 77, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.D.; Eljamel, S. Impact of particular antiepileptic drugs on the survival of patients with glioblastoma multiforme. J. Neurosurg. 2013, 118, 859–865. [Google Scholar] [CrossRef]
- Barker, C.A.; Bishop, A.J.; Chang, M.; Beal, K.; Chan, T.A. Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 504–509. [Google Scholar] [CrossRef]
- Kerkhof, M.; Dielemans, J.C.; van Breemen, M.S.; Zwinkels, H.; Walchenbach, R.; Taphoorn, M.J.; Vecht, C.J. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-Oncology 2013, 15, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Krauze, A.V.; Myrehaug, S.D.; Chang, M.G.; Holdford, D.J.; Smith, S.; Shih, J.; Tofilon, P.J.; Fine, H.A.; Camphausen, K. A Phase 2 Study of Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients With Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Redjal, N.; Reinshagen, C.; Le, A.; Walcott, B.P.; McDonnell, E.; Dietrich, J.; Nahed, B.V. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J. Neurooncol. 2016, 127, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Texakalidis, P.; McDonald, K.L.; Mekary, R.A.; Smith, T.R. The survival effect of valproic acid in glioblastoma and its current trend: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 174, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, S.; Frijlink, E.; Kroonen, J.; Spliet, W.G.M.; van Hecke, W.; Seute, T.; Snijders, T.J.; Robe, P.A. Effects of valproic acid on histone deacetylase inhibition in vitro and in glioblastoma patient samples. Neurooncol. Adv. 2019, 1, vdz025. [Google Scholar] [CrossRef]
- Lange, F.; Weßlau, K.; Porath, K.; Hörnschemeyer, J.; Bergner, C.; Krause, B.J.; Mullins, C.S.; Linnebacher, M.; Köhling, R.; Kirschstein, T. AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PLoS ONE 2019, 14, e0211644. [Google Scholar] [CrossRef] [PubMed]
- Engh, J.A. Anti-convulsants and gene expression in malignant gliomas. Neurosurgery 2010, 67, N24–N26. [Google Scholar] [CrossRef][Green Version]
- Cardona, A.F.; Rojas, L.; Wills, B.; Bernal, L.; Ruiz-Patiño, A.; Arrieta, O.; Hakim, E.J.; Hakim, F.; Mejía, J.A.; Useche, N.; et al. Efficacy and safety of Levetiracetam vs. other antiepileptic drugs in Hispanic patients with glioblastoma. J. Neurooncol. 2018, 136, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Min, K.L.; Chang, M.J. Effect of anti-epileptic drugs on the survival of patients with glioblastoma multiforme: A retrospective, single-center study. PLoS ONE 2019, 14, e0225599. [Google Scholar] [CrossRef] [PubMed]
- Roh, T.H.; Moon, J.H.; Park, H.H.; Kim, E.H.; Hong, C.K.; Kim, S.H.; Kang, S.G.; Chang, J.H. Association between survival and levetiracetam use in glioblastoma patients treated with temozolomide chemoradiotherapy. Sci. Rep. 2020, 10, 10783. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Nagatomo, K.; Tokumaru, J.; Takaki, M.; Willmore, L.J. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007, 1151, 55–61. [Google Scholar] [CrossRef]
- Pichardo-Macías, L.A.; Ramírez-Mendiola, B.A.; Contreras García, I.J.; Zamudio Hernández, S.R.; Chávez Pacheco, J.L.; Sánchez Huerta, K.B.; Mendoza Torreblanca, J.G. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res. 2018, 140, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vecht, C.J.; Wilms, E.B. Seizures in low- and high-grade gliomas: Current management and future outlook. Expert Rev. Anticancer Ther. 2010, 10, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Deneka-Hannemann, S.; Jarosz, B.; Zgrajka, W.; Stoma, F.; Trojanowski, T.; Turski, W.A.; Rzeski, W. Kynurenic acid inhibits proliferation and migration of human glioblastoma T98G cells. Pharmacol. Rep. 2014, 66, 130–136. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ye, X.; Chamberlain, M.; Mikkelsen, T.; Batchelor, T.; Desideri, S.; Piantadosi, S.; Fisher, J.; Fine, H.A. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: A multicenter phase II trial. J. Clin. Oncol. 2009, 27, 4155–4161. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Kreisl, T.N.; Kim, L.; Duic, J.P.; Butman, J.A.; Albert, P.S.; Fine, H.A. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer 2010, 116, 1776–1782. [Google Scholar] [CrossRef]
- Rösche, J.; Piek, J.; Hildebrandt, G.; Grossmann, A.; Kirschstein, T.; Benecke, R. Perampanel in the treatment of a patient with glioblastoma multiforme without IDH1 mutation and without MGMT promotor methylation. Fortschr. Neurol. Psychiatr. 2015, 83, 286–289. [Google Scholar] [CrossRef]
- Müller-Längle, A.; Lutz, H.; Hehlgans, S.; Rödel, F.; Rau, K.; Laube, B. NMDA Receptor-Mediated Signaling Pathways Enhance Radiation Resistance, Survival and Migration in Glioblastoma Cells-A Potential Target for Adjuvant Radiotherapy. Cancers 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Lenting, K.; Verhaak, R.; Laan, M.T.; Wesseling, P.; Leenders, W. Glioma: Experimental models and reality. Acta Neuropathol. 2017, 133, 263–282. [Google Scholar] [CrossRef]
- Torsvik, A.; Stieber, D.; Enger, P.Ø.; Golebiewska, A.; Molven, A.; Svendsen, A.; Westermark, B.; Niclou, S.P.; Olsen, T.K.; Enger, M.C.; et al. U-251 revisited: Genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med. 2014, 3, 812–824. [Google Scholar] [CrossRef]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Mayer, J.; Kirschstein, T.; Resch, T.; Porath, K.; Krause, B.J.; Köhling, R.; Lange, F. Perampanel attenuates epileptiform phenotype in C6 glioma. Neurosci. Lett. 2020, 715, 134629. [Google Scholar] [CrossRef]
- Caragher, S.; Chalmers, A.J.; Gomez-Roman, N. Glioblastoma’s Next Top Model: Novel Culture Systems for Brain Cancer Radiotherapy Research. Cancers 2019, 11, 44. [Google Scholar] [CrossRef]
- Klein, E.; Hau, A.C.; Oudin, A.; Golebiewska, A.; Niclou, S.P. Glioblastoma Organoids: Pre-Clinical Applications and Challenges in the Context of Immunotherapy. Front. Oncol. 2020, 10, 604121. [Google Scholar] [CrossRef]
- Soubéran, A.; Tchoghandjian, A. Practical Review on Preclinical Human 3D Glioblastoma Models: Advances and Challenges for Clinical Translation. Cancers 2020, 12, 2347. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Seufert, S.; Hauke, J.; Tränkle, C.; Eyüpoglu, I.Y.; Hahnen, E. Dissection of mitogenic and neurodegenerative actions of cystine and glutamate in malignant gliomas. Oncogene 2011, 30, 43–53. [Google Scholar] [CrossRef]
- Lange, F.; Hartung, J.; Liebelt, C.; Boisserée, J.; Resch, T.; Porath, K.; Hörnschemeyer, J.; Reichart, G.; Sellmann, T.; Neubert, V.; et al. Perampanel Add-on to Standard Radiochemotherapy in vivo Promotes Neuroprotection in a Rodent F98 Glioma Model. Front. Neurosci. 2020, 14, 598266. [Google Scholar] [CrossRef]
- Bouckaert, C.; Germonpré, C.; Verhoeven, J.; Chong, S.A.; Jacquin, L.; Mairet-Coello, G.; André, V.M.; Leclercq, K.; Vanhove, C.; De Vos, F.; et al. Development of a Rat Model for Glioma-Related Epilepsy. Int. J. Mol. Sci. 2020, 21, 6999. [Google Scholar] [CrossRef] [PubMed]
- Köhling, R.; Senner, V.; Paulus, W.; Speckmann, E.J. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotransplants. Neurobiol. Dis. 2006, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Senner, V.; Köhling, R.; Püttmann-Cyrus, S.; Straub, H.; Paulus, W.; Speckmann, E.J. A new neurophysiological/neuropathological ex vivo model localizes the origin of glioma-associated epileptogenesis in the invasion area. Acta Neuropathol. 2004, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Kim, S.H.; Dovas, A.; Zhao, H.T.; Goldberg, A.R.; Xu, W.; Yagielski, A.J.; Cambareri, M.K.; Patel, K.B.; Mela, A.; et al. Glioma-Induced Alterations in Neuronal Activity and Neurovascular Coupling during Disease Progression. Cell Rep. 2020, 31, 107500. [Google Scholar] [CrossRef]
- Mullins, C.S.; Schneider, B.; Lehmann, A.; Stockhammer, F.; Mann, S.; Classen, C.F.; Linnebacher, M. A comprehensive approach to patient-individual glioblastoma multiforme model establishment. J. Cancer Sci. Ther. 2014, 6, 6. [Google Scholar] [CrossRef]
- Stringer, B.W.; Day, B.W.; D’Souza, R.C.J.; Jamieson, P.R.; Ensbey, K.S.; Bruce, Z.C.; Lim, Y.C.; Goasdoué, K.; Offenhäuser, C.; Akgül, S.; et al. A reference collection of patient-derived cell line and xenograft models of proneural, classical and mesenchymal glioblastoma. Sci. Rep. 2019, 9, 4902. [Google Scholar] [CrossRef]
- Johansson, P.; Krona, C.; Kundu, S.; Doroszko, M.; Baskaran, S.; Schmidt, L.; Vinel, C.; Almstedt, E.; Elgendy, R.; Elfineh, L.; et al. A Patient-Derived Cell Atlas Informs Precision Targeting of Glioblastoma. Cell Rep. 2020, 32, 107897. [Google Scholar] [CrossRef]
- Ye, L.F.; Reznik, E.; Korn, J.M.; Lin, F.; Yang, G.; Malesky, K.; Gao, H.; Loo, A.; Pagliarini, R.; Mikkelsen, T.; et al. Patient-derived glioblastoma cultures as a tool for small-molecule drug discovery. Oncotarget 2020, 11, 443–451. [Google Scholar] [CrossRef]
- Gomez-Roman, N.; Stevenson, K.; Gilmour, L.; Hamilton, G.; Chalmers, A.J. A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro-Oncology 2017, 19, 229–241. [Google Scholar] [CrossRef]
- Ghoochani, A.; Yakubov, E.; Sehm, T.; Fan, Z.; Hock, S.; Buchfelder, M.; Eyüpoglu, I.Y.; Savaskan, N.E. A versatile ex vivo technique for assaying tumor angiogenesis and microglia in the brain. Oncotarget 2016, 7, 1838–1853. [Google Scholar] [CrossRef]
- Chadwick, E.J.; Yang, D.P.; Filbin, M.G.; Mazzola, E.; Sun, Y.; Behar, O.; Pazyra-Murphy, M.F.; Goumnerova, L.; Ligon, K.L.; Stiles, C.D.; et al. A Brain Tumor/Organotypic Slice Co-culture System for Studying Tumor Microenvironment and Targeted Drug Therapies. J. Vis. Exp. 2015, 7, e53304. [Google Scholar] [CrossRef]
- Eisemann, T.; Costa, B.; Strelau, J.; Mittelbronn, M.; Angel, P.; Peterziel, H. An advanced glioma cell invasion assay based on organotypic brain slice cultures. BMC Cancer 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, A.; Hess, S.; Bhuwania, R.; Linder, S.; Kloppenburg, P.; Noegel, A.A.; Clemen, C.S. CRN2 enhances the invasiveness of glioblastoma cells. Neuro-Oncology 2013, 15, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Romero-Leguizamón, C.R.; Elnagar, M.R.; Kristiansen, U.; Kohlmeier, K.A. Increasing cellular lifespan with a flow system in organotypic culture of the Laterodorsal Tegmentum (LDT). Sci. Rep. 2019, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Kirschstein, T.; Köhling, R. Animal models of tumour-associated epilepsy. J. Neurosci. Methods 2016, 260, 109–117. [Google Scholar] [CrossRef]
- Mathieu, D.; Lecomte, R.; Tsanaclis, A.M.; Larouche, A.; Fortin, D. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can. J. Neurol. Sci. 2007, 34, 296–306. [Google Scholar] [CrossRef]
- von Eckardstein, K.L.; Patt, S.; Kratzel, C.; Kiwit, J.C.W.; Reszka, R. Local chemotherapy of F98 rat glioblastoma with paclitaxel and carboplatin embedded in liquid crystalline cubic phases. J. Neurooncol. 2005, 72, 209–215. [Google Scholar] [CrossRef]
- Schültke, E.; Bräuer-Krisch, E.; Blattmann, H.; Requardt, H.; Laissue, J.A.; Hildebrandt, G. Survival of rats bearing advanced intracerebral F 98 tumors after glutathione depletion and microbeam radiation therapy: Conclusions from a pilot project. Radiat. Oncol. 2018, 13, 89. [Google Scholar] [CrossRef]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2019, 125, 424–433. [Google Scholar] [CrossRef]
- Rosati, A.; Buttolo, L.; Stefini, R.; Todeschini, A.; Cenzato, M.; Padovani, A. Efficacy and safety of levetiracetam in patients with glioma: A clinical prospective study. Arch. Neurol. 2010, 67, 343–346. [Google Scholar] [CrossRef]
- Laghari, A.A.; Ahmed, S.I.; Qadeer, N.; Shamim, M.S. Choice of therapeutic anti-seizure medication in patients with brain tumour. J. Pak. Med. Assoc. 2019, 69, 442–444. [Google Scholar] [PubMed]
- Fukuyama, K.; Ueda, Y.; Okada, M. Effects of Carbamazepine, Lacosamide and Zonisamide on Gliotransmitter Release Associated with Activated Astroglial Hemichannels. Pharmaceuticals 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Ueda, Y. Brivaracetam prevents astroglial l-glutamate release associated with hemichannel through modulation of synaptic vesicle protein. Biomed. Pharmacother. 2021, 138, 111462. [Google Scholar] [CrossRef] [PubMed]

| Reference | Patients Enrolled | Perampanel Therapy | Seizure Reponse |
|---|---|---|---|
| Vecht et al., 2017 [93] | 12 patients | 2–12 mg/d | seizure-free = 6/12 |
| 9 male, 3 female | follow-up = 6 months | ≥50% reduction = 3/12 | |
| median = 41 years | responder rate = 75% | ||
| Dunn-Pirio et al., 2018 [94] | 8 patients | 2–8 mg/d | seizure-free = 5/8 |
| 6 male, 2 female | follow-up = 16 weeks | ≥50% reduction = 1/8 | |
| median = 45 years | responder rate = 75% | ||
| Izumoto et al., 2018 [95] | 10 patients | 4–8 mg/d | seizure-free = 6/10 |
| 6 male, 4 female | follow-up = 6 months | ≥50% reduction = 4/10 | |
| median = 59 years | responder rate = 100% | ||
| Maschio et al., 2019 [96] | 11 patients | 7.3 mg/d | seizure-free = 5/12 |
| 9 male, 2 female | follow-up = 12 months | ≥50% reduction = 4/12 | |
| median = 54 years | responder rate = 82% | ||
| Chonan et al., 2020 [97] | 18 patients | 2–4 mg/d | seizure-free = 17/18 |
| 9 male, 9 female | follow-up = 10.6 months | ≥50% reduction = 0/18 | |
| median = 49 years | responder rate = 94% | ||
| Coppola et al., 2020 1 [98] | 36 patients | 2–12 mg/d | seizure-free = 7/21 |
| 23 male, 13 female | follow-up = 12 months | ≥50% reduction = 12/21 | |
| median = 46 years | responder rate = 90% |
| Title (Trial) | Status | Interventions | Location |
|---|---|---|---|
| Perampanel for the reduction of seizure frequency in patients with high-grade glioma and focal epilepsy (NCT04650204) | Not yet recruiting | Perampanel | Jacksonville, FL, USA |
| Effect of perampanel on peritumoral hyperexcitability in HGG (NCT04497142) | Recruiting | Perampanel | Boston, MA, USA |
| Sulfasalazine and stereotactic radiosurgery for recurrent glioblastoma (NCT04205357) | Recruiting | Sulfasalazine | Bergen, Norway |
| Efficacy and Safety of perampanel in combination in glioma-refractory epilepsy (NCT03636958) | Recruiting | Perampanel | Marseille, France |
| Memantine for prevention of cognitive late effects in pediatric patients receiving cranial radiation therapy for localized brain tumors (NCT03194906) | Recruiting | Memantine | Memphis, TN, USA |
| Temozolomide, memantine hydrochloride, mefloquine, and metformin hydrochloride in treating patients with glioblastoma multiforme after radiation therapy (NCT01430351) | Active, not recruiting | Memantine, mefloquine, metformin | Houston, TX, USA |
| Level | Model | Glioma | Advantages/Disadvantages |
|---|---|---|---|
| in vitro | permanent cell lines | rodent and human cells [45,128,164] | (+) high throughput |
| (−) genetic drift | |||
| (−) no microenvironment | |||
| patient-derived cell lines | human (primary) glioblastoma cells [117,148] | (+) high throughput (+) genetic status of primary tumor and clinical data accessible (−) no microenvironment | |
| spheroids/organoids | human and rodent glioblastoma [165,166,167] | (+) median throughput (−) no microenvironment | |
| ex vivo | organotypic slice cultures with glio- blastoma cells | human or rodent glioblastoma [168] | (+) median throughput (+) genetic manipulation feasible (+) interaction with healthy brain tissue |
| (−) only short-time monitoring (1-3 weeks) | |||
| (−) microenvironment lacking immune system | |||
| (−) animal consuming research | |||
| in vivo 1 | orthotopic rat glioma | F98 and C6 rat [169,170,171,172] | (+) glioma-associated seizures (+) immunocompetent (−) ethical issues related to animal studies (−) low throughput (−) no genetic variances |
| orthotopic mice glioma | murine glioma [58,173] | (+) glioma-associated seizures (+) immunocompetent (+) genetic alterations based on human glioma (−) ethical issues related to animal studies (−) low throughput (−) low genetic variances | |
| orthotopic human glioblastoma | GBM12/GBM22 [34] | (+) glioma-associated seizures (−) ethical issues related to animal studies (−)low throughput (−) immunodeficient host (−) no genetic variances |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, F.; Hörnschemeyer, M.F.; Kirschstein, T. Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy. Cells 2021, 10, 1226. https://doi.org/10.3390/cells10051226
Lange F, Hörnschemeyer MF, Kirschstein T. Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy. Cells. 2021; 10(5):1226. https://doi.org/10.3390/cells10051226
Chicago/Turabian StyleLange, Falko, Max Frederik Hörnschemeyer, and Timo Kirschstein. 2021. "Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy" Cells 10, no. 5: 1226. https://doi.org/10.3390/cells10051226
APA StyleLange, F., Hörnschemeyer, M. F., & Kirschstein, T. (2021). Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy. Cells, 10(5), 1226. https://doi.org/10.3390/cells10051226






