Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts
Abstract
1. Introduction
1.1. The Heart and Its Cellular Constituents
1.2. Cardiac Fibroblasts
1.3. Mechanical Activation of Cardiac Fibroblasts
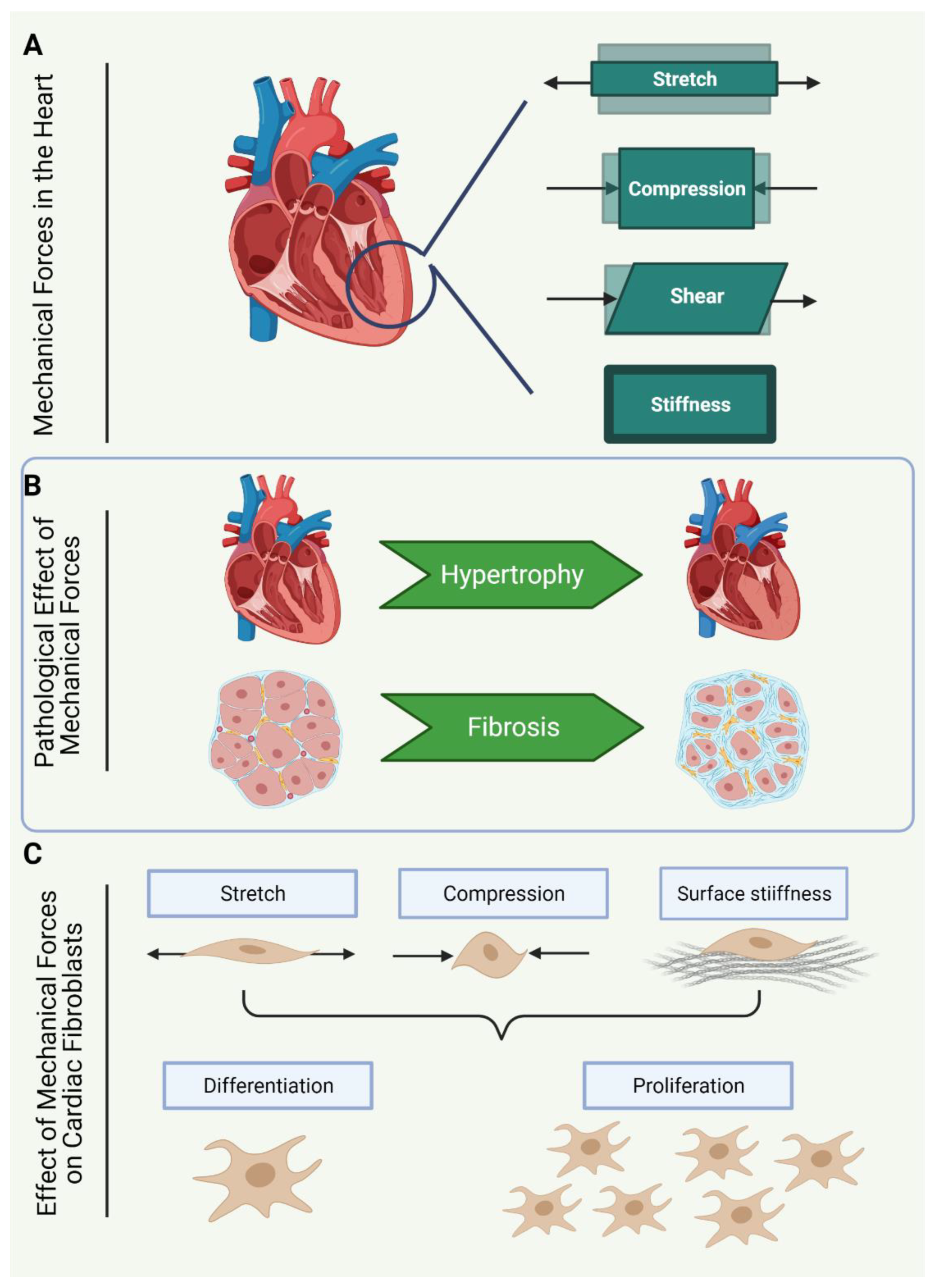
1.4. Mechanical Forces Sensed by Cardiac Fibroblasts
1.5. Cardiac Fibroblasts in Culture
1.6. Mechanosensitive Cation Channels in Cardiac Fibroblasts
2. Transient Receptor Potential (TRP) Channels
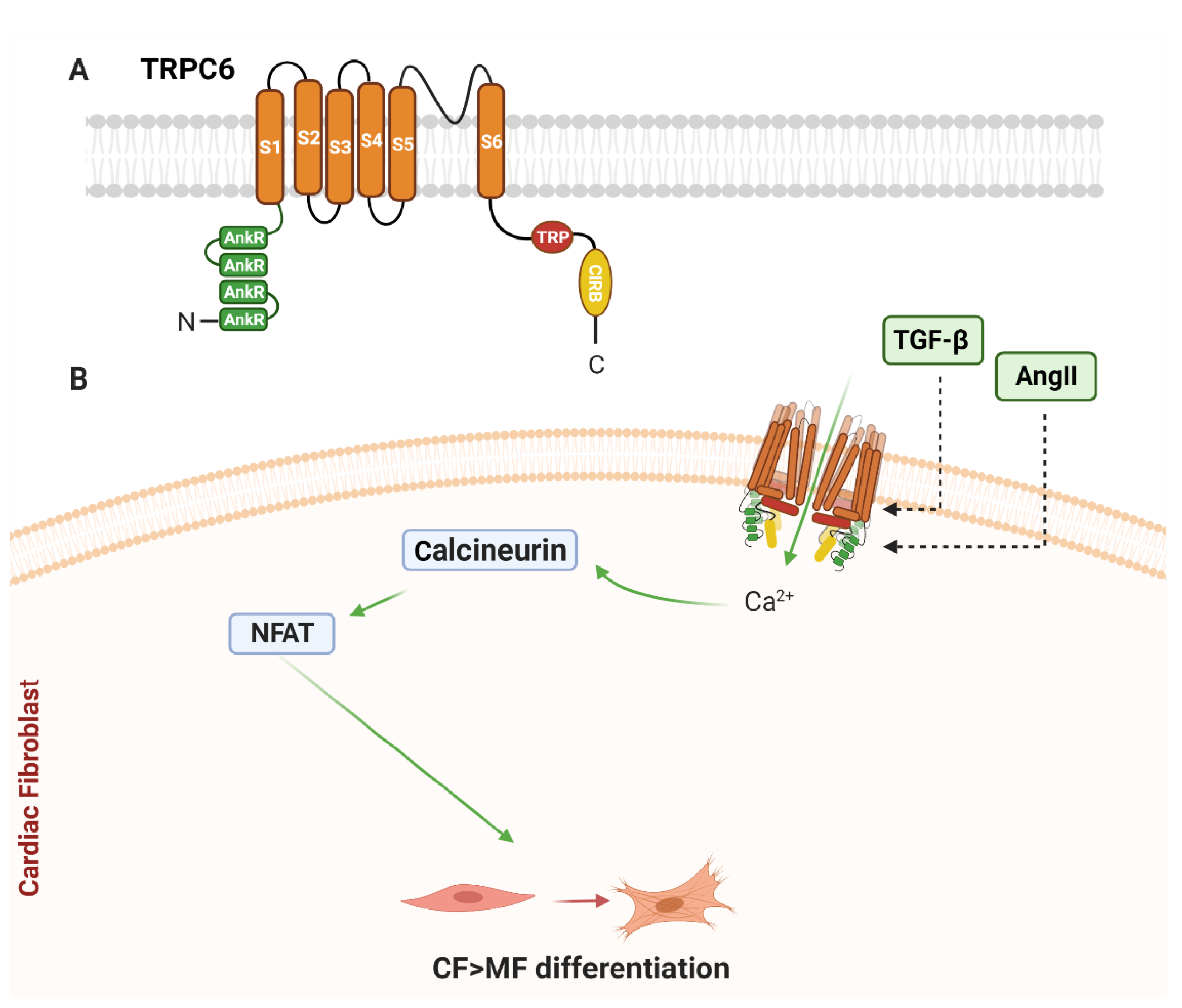
2.1. Canonical Family of Transient Receptor Potential (TRPC) Channels
2.1.1. TRPC6 and Cardiac Remodelling
2.1.2. TRPC6 in Cardiac Fibroblasts
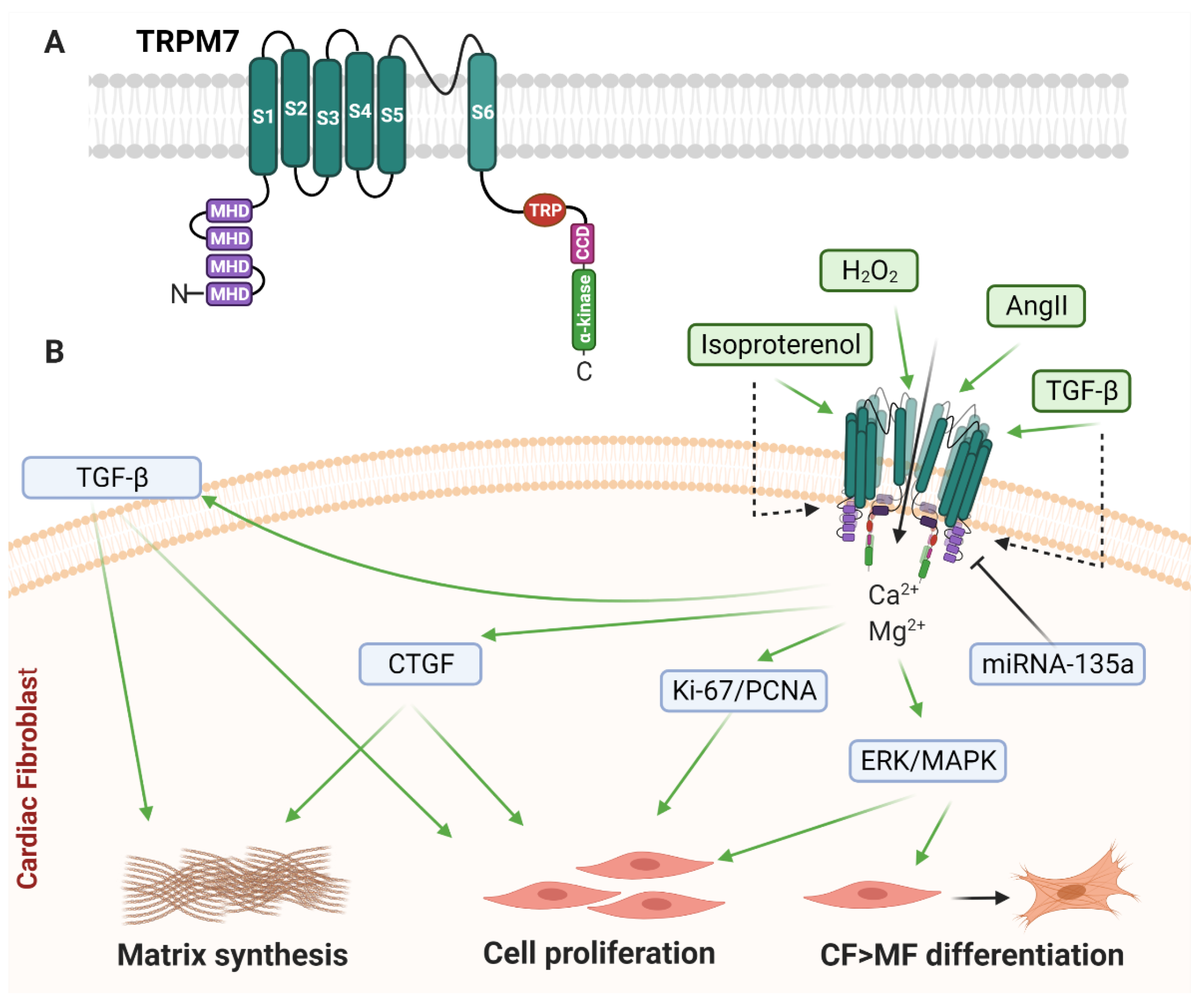
2.2. Melastatin Family of Transient Receptor Potential (TRPM) Channels
2.2.1. TRPM7
2.2.2. TRPM7 and Cardiac Remodelling
2.2.3. TRPM7 in Cardiac Fibroblasts
2.3. Vanilloid Family of Transient Receptor Potential (TRPV) Channels
2.3.1. TRPV1
2.3.2. TRPV1 and Cardiac Remodelling
2.3.3. TRPV1 in Cardiac Fibroblasts
2.4. TRPV4
2.4.1. TRPV4 and Cardiac Remodelling
2.4.2. TRPV4 in Cardiac Fibroblasts
3. Piezo1 Channel
3.1. Piezo1 and Cardiac Remodelling
3.2. Piezo1 in Cardiac Fibroblasts
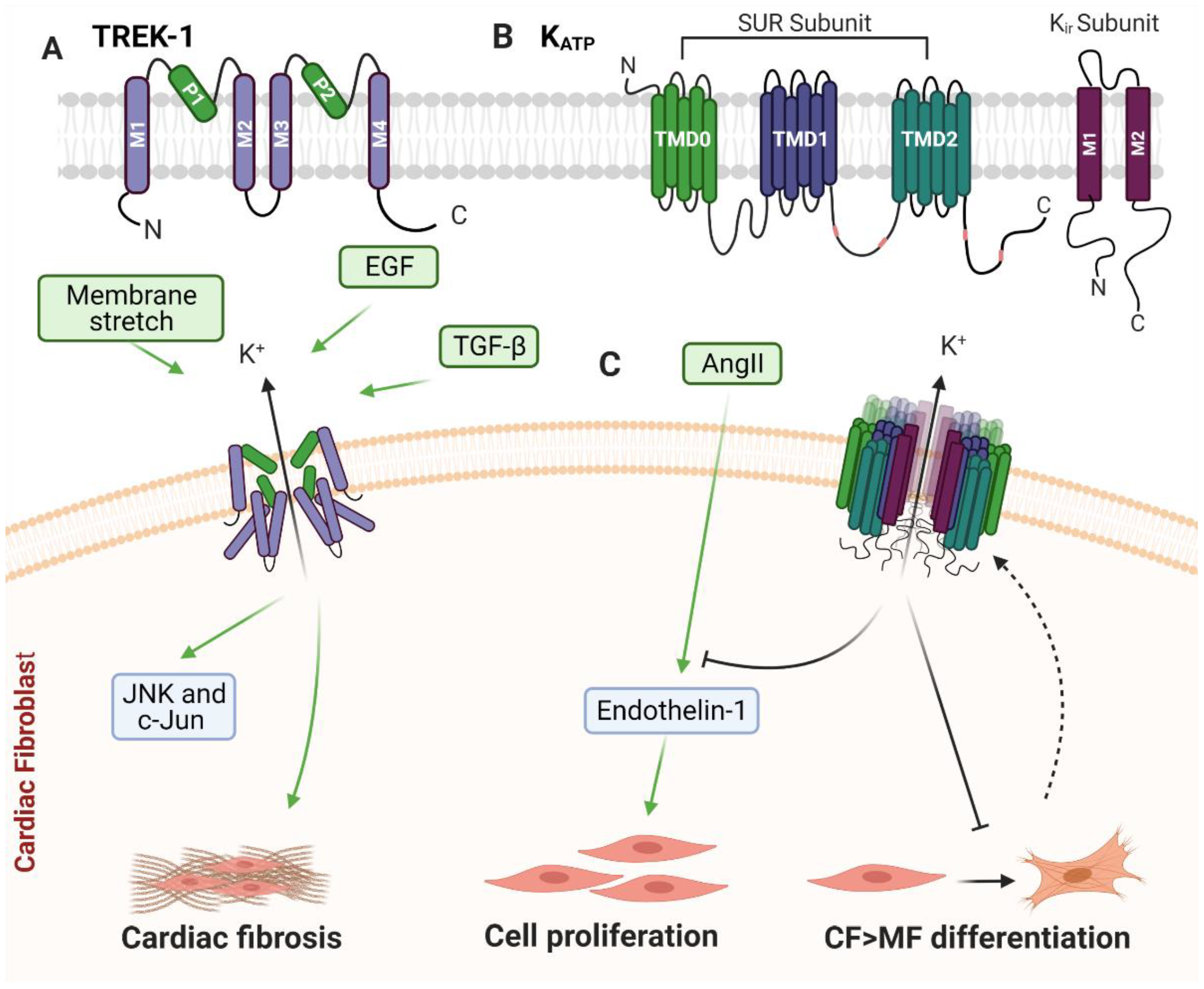
4. TWIK-Related Potassium Channel-1 (TREK-1)
4.1. TREK-1 and Cardiac Remodelling
4.2. TREK-1 in Cardiac Fibroblasts
5. ATP-Sensitive Potassium Channels (KATP)
5.1. KATP and Cardiac Remodelling
5.2. KATP in Cardiac Fibroblasts
6. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Borg, T.K.; Banerjee, I.; Baudino, T.A. Pressure overload induces early morphological changes in the heart. Am. J. Pathol. 2012, 181, 1226–1235. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Herum, K.M.; Lunde, I.G.; McCulloch, A.D.; Christensen, G. The soft- and hard-heartedness of cardiac fibroblasts: Mechanotransduction signaling pathways in fibrosis of the heart. J. Clin. Med. 2017, 6, 53. [Google Scholar] [CrossRef]
- Turner, N.A.; Porter, K.E. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Q.; Li, C.; Li, Y.; Wang, L. Cardiac fibrosis and cardiac fibroblast lineage-tracing: Recent advances. Front. Physiol. 2020, 11, 416. [Google Scholar] [CrossRef]
- Ivey, M.J.; Tallquist, M.D. Defining the cardiac fibroblast. Circ. J. 2016, 80, 2269–2276. [Google Scholar] [CrossRef]
- Turner, N.A.; Porter, K.E. Regulation of myocardial matrix metalloproteinase expression and activity by cardiac fibroblasts. IUBMB Life 2012, 64, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Turner, N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J. Mol. Cell. Cardiol. 2016, 94, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Herum, K.M.; Carlson, C.C.; Christensen, G. Syndecans in heart fibrosis. Cell Tissue Res. 2016, 365, 539–552. [Google Scholar] [CrossRef]
- Engebretsen, K.V.; Lunde, I.G.; Strand, M.E.; Waehre, A.; Sjaastad, I.; Marstein, H.S.; Skrbic, B.; Dahl, C.P.; Askevold, E.T.; Christensen, G.; et al. Lumican is increased in experimental and clinical heart failure, and its production by cardiac fibroblasts is induced by mechanical and proinflammatory stimuli. FEBS J. 2013, 280, 2382–2398. [Google Scholar] [CrossRef] [PubMed]
- Waehre, A.; Vistnes, M.; Sjaastad, I.; Nygård, S.; Husberg, C.; Lunde, I.G.; Aukrust, P.; Yndestad, A.; Vinge, L.E.; Behmen, D.; et al. Chemokines regulate small leucine-rich proteoglycans in the extracellular matrix of the pressure-overloaded right ventricle. J. Appl. Physiol. 2012, 112, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Melleby, A.O.; Strand, M.E.; Romaine, A.; Herum, K.M.; Skrbic, B.; Dahl, C.P.; Sjaastad, I.; Fiane, A.E.; Filmus, J.; Christensen, G.; et al. The heparan sulfate proteoglycan glypican-6 is upregulated in the failing heart, and regulates cardiomyocyte growth through ERK1/2 signaling. PLoS ONE 2016, 11, e0165079. [Google Scholar] [CrossRef]
- Saucerman, J.J.; Tan, P.M.; Buchholz, K.S.; McCulloch, A.D.; Omens, J.H. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat. Rev. Cardiol. 2019, 16, 361–378. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Querejeta, R.; González, A.; Larman, M.; Díez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: Potential role of lysyl oxidase. Hypertension 2012, 60, 677–683. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Lopez, B.; Gaub, R.; Escher, F.; Kühl, U.; Schultheiss, H.P.; Tschöpe, C. Diastolic tissue doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 977–985. [Google Scholar] [CrossRef]
- Herrmann, K.L.; McCulloch, A.D.; Omens, J.H. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1277–H1284. [Google Scholar] [CrossRef]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Gilles, G.K.; McCulloch, A.D.; Herum, K.M. Combining stiffness and stretch to study cardiac fibroblast pro-fibrotic activity. FASEB J. Conf. Exp. Biol. 2018, 32, 896. [Google Scholar]
- Galie, P.A.; Russell, M.W.; Westfall, M.V.; Stegemann, J.P. Interstitial fluid flow and cyclic strain differentially regulate cardiac fibroblast activation via AT1R and TGF-beta1. Exp. Cell Res. 2012, 318, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc. Res. 2003, 57, 793–803. [Google Scholar] [CrossRef]
- Kamkin, A.; Kirischuk, S.; Kiseleva, I. Single mechano-gated channels activated by mechanical deformation of acutely isolated cardiac fibroblasts from rats. Acta Physiol. 2010, 199, 277–292. [Google Scholar] [CrossRef]
- Gaetani, R.; Zizzi, E.A.; Deriu, M.A.; Morbiducci, U.; Pesce, M.; Messina, E. When stiffness matters: Mechanosensing in heart development and disease. Front. Cell Dev. Biol. 2020, 8, 334. [Google Scholar] [CrossRef]
- Thomas, C.H.; Collier, J.H.; Sfeir, C.S.; Healy, K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 2002, 99, 1972–1977. [Google Scholar] [CrossRef]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef]
- Hübner, M.R.; Eckersley-Maslin, M.A.; Spector, D.L. Chromatin organization and transcriptional regulation. Curr. Opin. Genet. Dev. 2013, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, R.; Ross, R.S.; Manso, A.M. Integrins and integrin-related proteins in cardiac fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Tijore, A.; Cox, C.D.; Hariharan, A.; Van Nhieu, G.T.; Martinac, B.; Sheetz, M. Force-dependent Piezo1 recruitment to focal adhesions regulates adhesion maturation and turnover specifically in non-transformed cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Aglialoro, F.; Hofsink, N.; Hofman, M.; Brandhorst, N.; van den Akker, E. Inside out integrin activation mediated by Piezo1 signaling in erythroblasts. Front. Physiol. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P.; Lin, J.-S.; Im, H.-J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391, 1–15. [Google Scholar] [CrossRef]
- Ma, H.; Killaars, A.R.; DelRio, F.W.; Yang, C.; Anseth, K.S. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials 2017, 131, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Mkrtschjan, M.; Gaikwad, S.; Dommaraju, S.; Li, J.; Russell, B. Lipid signaling regulates fibroblast migration and the actin cytoskeleton in response to stiffness and microtopography. FASEB J. Conf. Exp. Biol. 2017, 31, 880. [Google Scholar]
- Mkrtschjan, M.A.; Gaikwad, S.B.; Kappenman, K.J.; Solis, C.; Dommaraju, S.; Le, L.V.; Desai, T.A.; Russell, B. Lipid signaling affects primary fibroblast collective migration and anchorage in response to stiffness and microtopography. J. Cell. Physiol. 2018, 233, 3672–3683. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Corbin, E.A.; Caliari, S.R.; Ouyang, L.; Vega, S.L.; Truitt, R.; Han, L.; Margulies, K.B.; Burdick, J.A. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 2017, 145, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schroer, A.K.; Merryman, W.D. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J. Cell Sci. 2015, 128, 1865–1875. [Google Scholar] [CrossRef]
- Kurotsu, S.; Muraoka, N.; Sadahiro, T.; Isomi, M.; Kojima, H.; Haginiwa, S.; Tani, H.; Tamura, F.; Nara, K.; Suzuki, T.; et al. Stiffness of the scaffold regulates cardiac direct reprogramming. Circ. Conf. 2018, 138, A14577. [Google Scholar]
- Mustonen, E.; Pohjolainen, V.; Aro, J.; Pikkarainen, S.; Leskinen, H.; Ruskoaho, H.; Rysa, J. Upregulation of cardiac matrix gla protein expression in response to hypertrophic stimuli. Blood Press. 2009, 18, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Isu, G.; Lemme, M.; Conficconi, C.; Oertle, P.; Raz, C.; Visone, R.; Cerino, G.; Plodinec, M.; Rasponi, M.; et al. A three-dimensional in vitro dynamic micro-tissue model of cardiac scar formation. Integr. Biol. Quant. Biosci. Nano Macro 2018, 10, 174–183. [Google Scholar] [CrossRef]
- Paik, D.C.; Saito, L.Y.; Sugirtharaj, D.D.; Holmes, J.W. Nitrite-induced cross-linking alters remodeling and mechanical properties of collagenous engineered tissues. Connect. Tissue Res. 2006, 47, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.E.; Black, I.L.D. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J. Biomech. Eng. 2013, 135, 071001. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Fomovsky, G.M.; Chandran, P.L.; Holmes, J.W. Collagen fiber alignment does not explain mechanical anisotropy in fibroblast populated collagen gels. J. Biomech. Eng. 2007, 129, 642–650. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Lewis, K.; Pikes, D.; Marcello, A.; Mujumdar, V.S.; Smiley, L.M.; Moore, C.K. Stretch-induced membrane type matrix metalloproteinase and tissue plasminogen activator in cardiac fibroblast cells. J. Cell. Physiol. 1998, 176, 374–382. [Google Scholar] [CrossRef]
- Yost, M.J.; Simpson, D.; Wrona, K.; Ridley, S.; Ploehn, H.J.; Borg, T.K.; Terracio, L. Design and construction of a uniaxial cell stretcher. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H3124–H3130. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef]
- Van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 2016, 93, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.F.; Gillis, T.E. Short-term cyclical stretch phosphorylates p38 and ERK1/2 MAPKs in cultured fibroblasts from the hearts of rainbow trout, Oncorhynchus mykiss. Biol. Open 2020, 9, bio049296. [Google Scholar] [CrossRef] [PubMed]
- Ruwhof, C.; van Wamel, A.E.; Egas, J.M.; van der Laarse, A. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol. Cell. Biochem. 2000, 208, 89–98. [Google Scholar] [CrossRef]
- Ugolini, G.S.; Rasponi, M.; Pavesi, A.; Santoro, R.; Kamm, R.; Fiore, G.B.; Pesce, M.; Soncini, M. On-chip assessment of human primary cardiac fibroblasts proliferative responses to uniaxial cyclic mechanical strain. Biotechnol. Bioeng. 2016, 113, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Lee, J.; Yazdi, I.K.; Miri, A.K.; Lin, Y.D.; Seo, J.; Zhang, Y.S.; Khademhosseini, A.; Shin, S.R. Cardiac fibrotic remodeling on a chip with dynamic mechanical stimulation. Adv. Healthc. Mater. 2019, 8, e1801146. [Google Scholar] [CrossRef]
- Rook, M.; Van Ginneken, A.; de Jonge, B.; El Aoumari, A.; Gros, D.; Jongsma, H. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am. J. Physiol. Cell Physiol. 1992, 263, C959–C977. [Google Scholar] [CrossRef]
- Abramochkin, D.V.; Lozinsky, I.T.; Kamkin, A. Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart. J. Mol. Cell. Cardiol. 2014, 70, 27–36. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Isenberg, G.; Wagner, K.D.; Gunther, J.; Theres, H.; Scholz, H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog. Biophys. Mol. Biol. 2003, 82, 111–120. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Lozinsky, I.; Scholz, H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res. Cardiol. 2005, 100, 337–345. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Lozinsky, I.; Gunther, J.; Scholz, H. Mechanically induced potentials in atrial fibroblasts from rat hearts are sensitive to hypoxia/reoxygenation. Pflug. Arch. Eur. J. Physiol. 2003, 446, 169–174. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Pylaev, A.; Leiterer, K.P.; Theres, H.; Scholz, H.; Gunther, J.; Isenberg, G. A possible role for atrial fibroblasts in postinfarction bradycardia. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H842–H849. [Google Scholar] [CrossRef]
- Peyronnet, R.; Nerbonne, J.M.; Kohl, P. Cardiac mechano-gated ion channels and arrhythmias. Circ. Res. 2016, 118, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Chiodo, L.; Loppini, A. Biophysics and modeling of mechanotransduction in neurons: A review. Mathematics 2021, 9, 323. [Google Scholar] [CrossRef]
- Liu, C.; Montell, C. Forcing open Trp channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Cox, C.D.; Ridone, P.; Rohde, P.R.; Cordero-Morales, J.F.; Vásquez, V.; Laver, D.R.; Martinac, B. Mammalian Trp ion channels are insensitive to membrane stretch. J. Cell Sci. 2019, 132, jcs238360. [Google Scholar] [CrossRef]
- Gottlieb, P.; Folgering, J.; Maroto, R.; Raso, A.; Wood, T.G.; Kurosky, A.; Bowman, C.; Bichet, D.; Patel, A.; Sachs, F. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch. Eur. J. Physiol. 2008, 455, 1097–1103. [Google Scholar] [CrossRef]
- Rocio Servin-Vences, M.; Moroni, M.; Lewin, G.R.; Poole, K. Direct measurement of TRPV4 and Piezo1 activity reveals multiple mechanotransduction pathways in chondrocytes. eLife 2017, 6, e21074. [Google Scholar] [CrossRef]
- Sheng, J.; Shim, W.; Wei, H.; Lim, S.Y.; Liew, R.; Lim, T.S.; Ong, B.H.; Chua, Y.L.; Wong, P. Hydrogen sulphide suppresses human atrial fibroblast proliferation and transformation to myofibroblasts. J. Cell. Mol. Med. 2013, 17, 1345–1354. [Google Scholar] [CrossRef]
- Jakob, D.; Klesen, A.; Allegrini, B.; Darkow, E.; Aria, D.; Emig, R.; Chica, A.S.; Rog-Zielinska, E.A.; Guth, T.; Beyersdorf, F.; et al. Piezo1 and BKca channels in human atrial fibroblasts: Interplay and remodelling in atrial fibrillation. BioRxiv 2021. [Google Scholar] [CrossRef]
- Iribe, G.; Jin, H.; Kaihara, K.; Naruse, K. Effects of axial stretch on sarcolemmal BKca channels in post-hatch chick ventricular myocytes. Exp. Physiol. 2010, 95, 699–711. [Google Scholar] [CrossRef]
- Zagorodnyuk, V.P.; Chen, B.N.; Costa, M.; Brookes, S.J. 4-aminopyridine- and dendrotoxin-sensitive potassium channels influence excitability of vagal mechano-sensitive endings in guinea-pig oesophagus. Br. J. Pharmacol. 2002, 137, 1195–1206. [Google Scholar] [CrossRef]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Rohde, P.R.; Laver, D.R.; Martinac, B. Mechanosensitivity of TRPC6 ion channel reconstituted in the liposomes. Biophys. J. 2016, 110, 610a–611a. [Google Scholar] [CrossRef]
- Wilson, C.; Dryer, S.E. A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades. Am. J. Physiol. Ren. Physiol. 2014, 306, F1018–F1025. [Google Scholar] [CrossRef]
- Numata, T.; Shimizu, T.; Okada, Y. TRPM7 is a stretch-and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007, 292, C460–C467. [Google Scholar] [CrossRef]
- Numata, T.; Shimizu, T.; Okada, Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell. Physiol. Biochem. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Swain, S.M.; Romac, J.M.J.; Shahid, R.A.; Pandol, S.J.; Liedtke, W.; Vigna, S.R.; Liddle, R.A. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J. Clin. Investig. 2020, 130, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Lewis, A.H.; Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife 2015, 4, e12088. [Google Scholar] [CrossRef]
- Brohawn, S.G.; Su, Z.; MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef]
- Berrier, C.; Pozza, A.; de Lavalette, A.d.L.; Chardonnet, S.; Mesneau, A.; Jaxel, C.; Le Maire, M.; Ghazi, A. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J. Biol. Chem. 2013, 288, 27307–27314. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Lamorgese, M. Ischemia potentiates the mechanosensitive modulation of atrial ATP-sensitive potassium channels. Ann. N. Y. Acad. Sci. 1994, 723, 392–395. [Google Scholar] [CrossRef]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.; Johnson, C.; Burdyga, T.; Melanaphy, D. TRPM channels in the vasculature. Adv. Exp. Med. Biol. 2011, 704, 707–729. [Google Scholar]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically activated ion channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [PubMed]
- Löf, C.; Viitanen, T.; Sukumaran, P.; Törnquist, K. TRPC2: Of mice but not men. Adv. Exp. Med. Biol. 2011, 704, 125–134. [Google Scholar]
- Morine, K.J.; Paruchuri, V.; Qiao, X.; Aronovitz, M.; Huggins, G.S.; DeNofrio, D.; Kiernan, M.S.; Karas, R.H.; Kapur, N.K. Endoglin selectively modulates transient receptor potential channel expression in left and right heart failure. Cardiovasc. Pathol. 2016, 25, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Bush, E.W.; Hood, D.B.; Papst, P.J.; Chapo, J.A.; Minobe, W.; Bristow, M.R.; Olson, E.N.; McKinsey, T.A. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 2006, 281, 33487–33496. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Numaga-Tomita, T.; Kitajima, N.; Toyama, T.; Harada, E.; Shimauchi, T.; Nishimura, A.; Ishikawa, T.; Kumagai, Y.; Birnbaumer, L.; et al. TRPC6 counteracts TRPC3-NOX2 protein complex leading to attenuation of hyperglycemia-induced heart failure in mice. Sci. Rep. 2017, 7, 7511. [Google Scholar] [CrossRef]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Asanov, A.; Sampieri, A.; Moreno, C.; Pacheco, J.; Salgado, A.; Sherry, R.; Vaca, L. Combined single channel and single molecule detection identifies subunit composition of STIM1-activated transient receptor potential canonical (TRPC) channels. Cell Calcium 2015, 57, 1–13. [Google Scholar] [CrossRef]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; Del Toro, R.; Martín-Bórnez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP channels: Current perspectives in the adverse cardiac remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.L.; de Souza, L.B.; Ambudkar, I.S. Role of TRPC channels in store-operated calcium entry. In Calcium Entry Pathways in Non-Excitable Cells; Springer: Berlin/Heidelberg, Germany, 2016; pp. 87–109. [Google Scholar]
- Sabourin, J.; Bartoli, F.; Antigny, F.; Gomez, A.M.; Benitah, J.-P. Transient receptor potential canonical (TRPC)/Orai1-dependent store-operated Ca2+ channels: New targets of aldosterone in cardiomyocytes. J. Biol. Chem. 2016, 291, 13394–13409. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kurahara, L.-H.; Hiraishi, K. Trp channels in cardiac and intestinal fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Iribe, G.; Nishida, M.; Naruse, K. Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology. Prog. Biophys. Mol. Biol. 2017, 130, 264–272. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Liu, P.; Jiang, Y.; Zhao, Y.; Wei, H.; Niu, W. TRPC1 expression and distribution in rat hearts. Eur. J. Histochem. 2009, 53, e26. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient receptor potential canonical (TRPC) channels: Then and now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Inoue, R.; Jensen, L.J.; Jian, Z.; Shi, J.; Hai, L.; Lurie, A.I.; Henriksen, F.H.; Salomonsson, M.; Morita, H.; Kawarabayashi, Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase c/diacylglycerol and phospholipase a2/ω-hydroxylase/20-hete pathways. Circ. Res. 2009, 104, 1399–1409. [Google Scholar] [CrossRef]
- Davis, J.; Burr, A.R.; Davis, G.F.; Birnbaumer, L.; Molkentin, J.D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 2012, 23, 705–715. [Google Scholar] [CrossRef]
- Liu, C.; Shang, Q.; Shi, G.; Wang, X. TRPC6-dependent inflammation mechanism of cardiac fibrosis induced by high salt in wistar rats. Int. J. Cardiol. 2011, 152, S92–S93. [Google Scholar] [CrossRef]
- Kapur, N.K.; Qiao, X.; Paruchuri, V.; Mackey, E.E.; Daly, G.H.; Ughreja, K.; Morine, K.J.; Levine, J.; Aronovitz, M.J.; Hill, N.S.; et al. Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J. Am. Heart Assoc. 2014, 3, e000965. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Rainer, P.P.; Hahn, V.S.; Lee, D.-i.; Jo, S.-H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef]
- Camacho Londoño, J.E.; Marx, A.; Kraft, A.E.; Schürger, A.; Richter, C.; Dietrich, A.; Lipp, P.; Birnbaumer, L.; Freichel, M. Angiotensin-II-evoked Ca2+ entry in murine cardiac fibroblasts does not depend on TRPC channels. Cells 2020, 9, 322. [Google Scholar] [CrossRef]
- Kraft, R.; Harteneck, C. The mammalian melastatin-related transient receptor potential cation channels: An overview. Pflügers Arch. 2005, 451, 204–211. [Google Scholar] [CrossRef]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, M.; Yi, X.; Guo, F.; Zhou, Y.; Chen, S.; Wu, X. TRPM7 channels mediate the functional changes in cardiac fibroblasts induced by angiotensin II. Int. J. Mol. Med. 2017, 39, 1291–1298. [Google Scholar] [CrossRef]
- Guo, J.-L.; Yu, Y.; Jia, Y.-Y.; Ma, Y.-Z.; Zhang, B.-Y.; Liu, P.-Q.; Chen, S.-R.; Jiang, J.-M. Transient receptor potential melastatin 7 (TRPM7) contributes to H2O2-induced cardiac fibrosis via mediating Ca2+ influx and extracellular signal–regulated kinase 1/2 (ERK1/2) activation in cardiac fibroblasts. J. Pharmacol. Sci. 2014, 125, 184–192. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Q.-y.; Zhou, Y.; Lu, X.-c.; Liu, Y.-h.; Wu, Y.; Guo, Q.; Ma, Y.-t.; Tang, Y.-q. AstragalosideIV against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine 2017, 30, 10–17. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Pan, Y.; Lu, C.; Xu, H.; Wang, X.; Liu, T.; Feng, K.; Tang, Y. MicroRNA-135a inhibits cardiac fibrosis induced by isoproterenol via TRPM7 channel. Biomed. Pharmacother. 2018, 104, 252–260. [Google Scholar] [CrossRef]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakamoto, K.; Kimura, J. Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J. Pharmacol. Sci. 2012, 118, 186–197. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, Y.; Xie, J.; Jiang, J.; Yue, L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr. Top. Med. Chem. 2013, 13, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Winkler, P.A.; Sun, W.; Lü, W.; Du, J. Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 2018, 562, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Shin, S.K.; Song, M.-Y.; Lee, J.E.; Park, K.-S. Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem. Biophys. Res. Commun. 2012, 417, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS ONE 2008, 3, e1876. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP 2 hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef]
- Valinsky, W.C.; Jolly, A.; Miquel, P.; Touyz, R.M.; Shrier, A. Aldosterone upregulates transient receptor potential melastatin 7 (TRPM7). J. Biol. Chem. 2016, 291, 20163–20172. [Google Scholar] [CrossRef] [PubMed]
- Callera, G.E.; He, Y.; Yogi, A.; Montezano, A.C.; Paravicini, T.; Yao, G.; Touyz, R.M. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J. Hypertens. 2009, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Kozak, J.A.; Shimizu, Y.; McLachlin, D.T.; Yamaguchi, H.; Wei, F.-Y.; Tomizawa, K.; Matsui, H.; Chait, B.T.; Cahalan, M.D. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/CHAK1. J. Biol. Chem. 2005, 280, 20793–20803. [Google Scholar] [CrossRef] [PubMed]
- Numata, T. Mechanosensor TRPM7 channel and its physiological role in cell volume regulation. In Proceedings of the Annual Meeting of the Physiological Society of Japan, Tokyo, Japan, 25–27 March 2008; Physiological Society of Japan: Tokyo, Japan, 2008; p. 009. [Google Scholar]
- Pedersen, S.F.; Nilius, B. Transient receptor potential channels in mechanosensing and cell volume regulation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 428, pp. 183–207. [Google Scholar]
- Zhao, R.; Afthinos, A.; Zhu, T.; Mistriotis, P.; Li, Y.; Serra, S.A.; Zhang, Y.; Yankaskas, C.L.; He, S.; Valverde, M.A. Cell sensing and decision-making in confinement: The role of TRPM7 in a tug of war between hydraulic pressure and cross-sectional area. Sci. Adv. 2019, 5, eaaw7243. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Huang, C.-C.; Yen, M.-H.; Lee, O.K. Shear flow regulates osteogenic differentiation of mesenchymal stem cells through TRPM7-mediated osterix pathway. Biophys. J. 2014, 106, 521a. [Google Scholar] [CrossRef]
- Rios, F.J.; Zou, Z.-G.; Harvey, A.P.; Harvey, K.Y.; Nosalski, R.; Anyfanti, P.; Camargo, L.L.; Lacchini, S.; Ryazanov, A.G.; Ryazanova, L. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc. Res. 2020, 116, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.; Zou, Z.-g.; Camargo, L.L.; Harvey, A.P.; Lacchini, S.; Anyfanti, P.; Montezano, A.C.; Touyz, R.M. A9907 cardiovascular inflammation and fibrosis in TRPM7-kinase deficient mice. J. Hypertens. 2018, 36, e58. [Google Scholar] [CrossRef]
- Sah, R.; Mesirca, P.; Van den Boogert, M.; Rosen, J.; Mably, J.; Mangoni, M.E.; Clapham, D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA 2013, 110, E3037–E3046. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Sun, H.-Y.; Chen, K.-H.; Du, X.-L.; Liu, B.; Cheng, L.-C.; Li, X.; Jin, M.-W.; Li, G.-R. Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res. Cardiol. 2012, 107, 282. [Google Scholar] [CrossRef]
- Yang, Z.; Quan, L.; Qiao, G.; Yonghui, L.; Xinwei, W.; Yiqun, T. Effects of TRPM7 on cardiac fibrosis and drug intervention. Asia Pac. Tradit. Med. 2015, 23, 2. [Google Scholar]
- Blythe, N.M.; Muraki, K.; Ludlow, M.J.; Stylianidis, V.; Gilbert, H.T.J.; Evans, E.L.; Cuthbertson, K.; Foster, R.; Swift, J.; Li, J.; et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 2019, 294, 17395–17408. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.; Xiao, C.; Jia, Y.; Guo, J.; Jiang, J.; Liu, P. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef]
- Beltran, L.; Beltran, M.; Aguado, A.; Gisselmann, G.; Hatt, H. 2-aminoethoxydiphenyl borate activates the mechanically gated human KCNK channels KCNK 2 (TREK-1), KCNK 4 (TRAAK), and KCNK 10 (TREK-2). Front. Pharmacol. 2013, 4, 63. [Google Scholar] [CrossRef]
- Colton, C.K.; Zhu, M.X. 2-aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb. Exp. Pharmacol. 2007, 179, 173–187. [Google Scholar]
- Wei, Y.; Wu, Y.; Feng, K.; Zhao, Y.; Tao, R.; Xu, H.; Tang, Y. Astragaloside iv inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/SMADs pathway. J. Ethnopharmacol. 2020, 249, 112404. [Google Scholar] [CrossRef]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qi, H.; Cao, Y.; Shi, P.; Song, C.; Ba, L.; Chen, Y.; Gao, J.; Li, S.; Li, B.; et al. Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. J. Cell. Mol. Med. 2018, 22, 6055–6067. [Google Scholar] [CrossRef] [PubMed]
- Entin-Meer, M.; Keren, G. Potential roles in cardiac physiology and pathology of the cation channel TRPV2 expressed in cardiac cells and cardiac macrophages: A mini-review. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H181–H188. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The role of transient receptor potential vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Wang, H.-J.; Wang, W.; Cornish, K.G.; Rozanski, G.J.; Zucker, I.H. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 2014, 64, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liang, Y.; Wang, X.; Lu, Z.; Li, L.; Zhu, S.; Liu, D.; Yan, Z.; Zhu, Z. TRPV1 activation attenuates high-salt diet-induced cardiac hypertrophy and fibrosis through PPAR-δ upregulation. PPAR Res. 2014, 2014, 491963. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, L.; Zhang, Q.; Wu, F.; Wang, L. The role of TRPV1 channels in atherosclerosis. Channels 2020, 14, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary capsaicin ameliorates pressure overload–induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am. J. Hypertens. 2014, 27, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Simon, S.A. TRPV1 Receptors and Signal Transduction; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 69–84. [Google Scholar]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Hudspeth, A.; Friedman, J.M.; Heller, S. Vanilloid receptor–related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vannucchi, M.G.; Zardo, C.; Spinelli, M.; Beneforti, P.; Turini, D.; Faussone-Pellegrini, M.S. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur. Urol. 2004, 46, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, A.; Brady, C.M.; Yiangou, Y.; Davis, J.; Fowler, C.J.; Anand, P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 2005, 65, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W. Chapter 22: TRPV Channels’ Function in Osmo-and Mechanotransduction; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2007. [Google Scholar]
- Scotland, R.S.; Chauhan, S.; Davis, C.; De Felipe, C.; Hunt, S.; Kabir, J.; Kotsonis, P.; Oh, U.; Ahluwalia, A. Vanilloid receptor TRPV1, sensory c-fibers, and vascular autoregulation: A novel mechanism involved in myogenic constriction. Circ. Res. 2004, 95, 1027–1034. [Google Scholar] [CrossRef]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.H. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 2005, 112, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Wang, D.H. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1791–H1798. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-J.; Chen, Y.-S.; Huang, H.-S.; Ma, M.-C. Hypoxic preconditioning protects rat hearts against ischemia–reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res. Cardiol. 2014, 109, 414. [Google Scholar] [CrossRef]
- Ren, J.-Y.; Song, J.-X.; Lu, M.-Y.; Chen, H. Cardioprotection by ischemic postconditioning is lost in isolated perfused heart from diabetic rats: Involvement of transient receptor potential vanilloid 1, calcitonin gene-related peptide and substance P. Regul. Pept. 2011, 169, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rubinstein, J.; Prieto, A.R.; Thang, L.V.; Wang, D.H. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension 2009, 53, 243–250. [Google Scholar] [CrossRef]
- Huang, W.; Rubinstein, J.; Prieto, A.R.; Wang, D.H. Enhanced postmyocardial infarction fibrosis via stimulation of the transforming growth factor-β-Smad2 signaling pathway: Role of transient receptor potential vanilloid type 1 channels. J. Hypertens. 2010, 28, 367–376. [Google Scholar] [CrossRef]
- Zhong, B.; Rubinstein, J.; Ma, S.; Wang, D.H. Genetic ablation of Trpv1 exacerbates pressure overload-induced cardiac hypertrophy. Biomed. Pharmacother. 2018, 99, 261–270. [Google Scholar] [CrossRef]
- Buckley, C.L.; Stokes, A.J. Mice lacking functional TRPV1 are protected from pressure overload cardiac hypertrophy. Channels 2011, 5, 367–374. [Google Scholar] [CrossRef][Green Version]
- Lang, H.; Li, Q.; Yu, H.; Li, P.; Lu, Z.; Xiong, S.; Yang, T.; Zhao, Y.; Huang, X.; Gao, P.; et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function. Br. J. Pharmacol. 2015, 172, 5548–5558. [Google Scholar] [CrossRef]
- Yoshie, K.; Rajendran, P.S.; Massoud, L.; Mistry, J.; Swid, M.A.; Wu, X.; Sallam, T.; Zhang, R.; Goldhaber, J.I.; Salavatian, S.; et al. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 2020, 5, e124477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Li, D.; Zhang, Y.; Tang, B.; Li, G.; Yang, Y.; Yang, D. Transgenic overexpression of transient receptor potential vanilloid subtype 1 attenuates isoproterenol-induced myocardial fibrosis in mice. Int. J. Mol. Med. 2016, 38, 601–609. [Google Scholar] [CrossRef]
- Smith, R.S.; Agata, J.; Xia, C.-F.; Chao, L.; Chao, J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005, 76, 2457–2471. [Google Scholar] [CrossRef]
- Kazakov, A.; Hall, R.; Jagoda, P.; Bachelier, K.; Müller-Best, P.; Semenov, A.; Lammert, F.; Böhm, M.; Laufs, U. Inhibition of endothelial nitric oxide synthase induces and enhances myocardial fibrosis. Cardiovasc. Res. 2013, 100, 211–221. [Google Scholar] [CrossRef]
- Liu, C.-P.; Yeh, J.-L.; Wu, B.-N.; Chai, C.-Y.; Chen, I.-J.; Lai, W.-T. KMUP-3 attenuates ventricular remodelling after myocardial infarction through eNOS enhancement and restoration of MMP-9/TIMP-1 balance. Br. J. Pharmacol. 2011, 162, 126–135. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Liddle, R.A. Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 2021, 296, 100171. [Google Scholar] [CrossRef]
- Michalick, L.; Kuebler, W.M. TRPV4—A missing link between mechanosensation and immunity. Front. Immunol. 2020, 11, 413. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef]
- Jones, J.L.; Peana, D.; Veteto, A.B.; Lambert, M.D.; Nourian, Z.; Karasseva, N.G.; Hill, M.A.; Lindman, B.R.; Baines, C.P.; Krenz, M.; et al. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc. Res. 2018, 115, 46–56. [Google Scholar] [CrossRef]
- Jones, J.L.; Lambert, M.D.; Whitfield, J.T.; Domeier, T.L. The TRPV4 ion channel alters intracellular calcium transients in cardiomyocytes of aged mice. Biophys. J. 2016, 110, 99a. [Google Scholar] [CrossRef][Green Version]
- Jones, J.L.; Peana, D.; Lambert, M.D.; Domeier, T.L. TRPV4 enhances cardiomyocyte calcium transients and cardiac contractility following hypoosmotic stress and ischemia-reperfusion. Biophys. J. 2017, 112, 95a–96a. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Huang, H.; Jiang, Y.; Wei, H.; Liu, P.; Wang, W.; Niu, W. Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat. Eur. J. Histochem. 2012, 56, e32. [Google Scholar] [CrossRef]
- Cappelli, H.; Adapala, R.; Thoppil, R.; Ohanyan, V.; Luli, J.; Luther, D.; Paruchiri, S.; Meszaros, J.G.; Chilian, W.; Thodeti, C. TRPV4 deficiency protects myocardium following myocardial infarction and transverse aortic constriction. FASEB J. 2014, 28, 893.11. [Google Scholar]
- Adapala, R.K. Role of Mechanosensitive Ion Channel TRPV4 in Cardiac Remodeling. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2018. [Google Scholar]
- Adapala, R.K.; Minasyan, A.; Kanugula, A.K.; Cappelli, H.C.; Paruchuri, S.; Meszaros, G.J.; Thodeti, C.K. Targeting TRPV4 channels protects heart from pathological remodeling following myocardial infarction. Circulation 2017, 136, A24061. [Google Scholar]
- Adapala, R.; Kanugula, A.K.; Ohanyan, V.A.; Paruchuri, S.M.; Chilian, W.M.; Thodeti, C.K. Endothelial TRPV4 deletion protects myocardium against pressure overload induced hypertrophy via preserved angiogenesis and reduced cardiac fibrosis. Circulation 2019, 140, A15843. [Google Scholar]
- Jia, X.; Xiao, C.; Sheng, D.; Yang, M.; Cheng, Q.; Wu, J.; Zhang, S. TRPV4 mediates cardiac fibrosis via the TGF-β1/Smad3 signaling pathway in diabetic rats. Cardiovasc. Toxicol. 2020, 20, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Itoh, Y.; Muraki, K. Cardiac fibroblasts have functional TRPV4 activated by 4α-phorbol 12, 13-didecanoate. Life Sci. 2009, 85, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.K.; Thoppil, R.J.; Luther, D.J.; Paruchuri, S.; Meszaros, J.G.; Chilian, W.M.; Thodeti, C.K. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell. Cardiol. 2013, 54, 45–52. [Google Scholar] [CrossRef]
- Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Chilian, W.M.; Thodeti, C.K. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res. Cardiol. 2020, 115, 1–14. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Wu, J.; Young, M.; Lewis, A.H.; Martfeld, A.N.; Kalmeta, B.; Grandl, J. Inactivation of mechanically activated Piezo1 ion channels is determined by the C-terminal extracellular domain and the inner pore helix. Cell Rep. 2017, 21, 2357–2366. [Google Scholar] [CrossRef]
- Wu, J.; Goyal, R.; Grandl, J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, K.; Geng, J.; Chi, S.; Wang, Y.; Zhi, P.; Zhang, M.; Xiao, B. Ion permeation and mechanotransduction mechanisms of mechanosensitive Piezo channels. Neuron 2016, 89, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Kalli, A.C. Force sensing by Piezo channels in cardiovascular health and disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Qiu, Z.; Woo, S.-H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.; Shi, J.; Gaunt, H.; Hyman, A.; Webster, P.; Williams, C.; Forbes, K.; Walker, J.; Simpson, N.; Beech, D. Piezo1 channels are mechanosensors in human fetoplacental endothelial cells. MHR Basic Sci. Reprod. Med. 2018, 24, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Zeng, W.-Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. Piezos mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Faucherre, A.; Ou Maati, H.M.; Nasr, N.; Pinard, A.; Theron, A.; Odelin, G.; Desvignes, J.-P.; Salgado, D.; Collod-Béroud, G.; Avierinos, J.-F.; et al. Piezo1 is required for outflow tract and aortic valve development. J. Mol. Cell. Cardiol. 2020, 143, 51–62. [Google Scholar] [CrossRef]
- Duchemin, A.-L.; Vignes, H.; Vermot, J. Mechanically activated Piezo channels modulate outflow tract valve development through the YAP1 and KLF2-Notch signaling axis. Elife 2019, 8, e44706. [Google Scholar] [CrossRef]
- Wong, T.Y.; Juang, W.C.; Tsai, C.T.; Tseng, C.J.; Lee, W.H.; Chang, S.N.; Cheng, P.W. Mechanical stretching simulates cardiac physiology and pathology through mechanosensor Piezo1. J. Clin. Med. 2018, 7, 410. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, K.; Wu, K.; Zhang, M.; Wang, S.; Cheng, H.; Zhou, Z.; Xiao, B. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat. Commun. 2021, 12, 869. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef]
- Guo, Y.R.; MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife 2017, 6, e33660. [Google Scholar] [CrossRef]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.-Q. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef]
- Coste, B.; Murthy, S.E.; Mathur, J.; Schmidt, M.; Mechioukhi, Y.; Delmas, P.; Patapoutian, A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; De Vecchis, D.; Hyman, A.J.; Povstyan, O.V.; Ludlow, M.J.; Shi, J.; Beech, D.J.; Kalli, A.C. Modeling of full-length Piezo1 suggests importance of the proximal N-terminus for dome structure. Biophys. J. 2021, 8, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.-A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals Piezo1 is gated by bilayer tension. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein Piezo1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef] [PubMed]
- Deivasikamani, V.; Dhayalan, S.; Abudushalamu, Y.; Mughal, R.; Visnagri, A.; Cuthbertson, K.; Scragg, J.L.; Munsey, T.S.; Viswambharan, H.; Muraki, K.; et al. Piezo1 channel activation mimics high glucose as a stimulator of insulin release. Sci. Rep. 2019, 9, 16876. [Google Scholar] [CrossRef]
- Lewis, A.H.; Cui, A.F.; McDonald, M.F.; Grandl, J. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Rep. 2017, 19, 2572–2585. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Wang, J.; Fu, W.; Li, X. Study on the mechanism of excessive apoptosis of nucleus pulposus cells induced by shRNA-Piezo1 under abnormal mechanical stretch stress. J. Cell. Biochem. 2019, 120, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Bavi, N.; Richardson, J.; Heu, C.; Martinac, B.; Poole, K. Piezo1-mediated currents are modulated by substrate mechanics. ACS Nano 2019, 13, 13545–13559. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, P.A.; Bae, C.; Sachs, F. Gating the mechanical channel Piezo1. Channels 2012, 6, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.O.; Massey, A.E.; Mata-Daboin, A.D.; Sierra-Valdez, F.J.; Chauhan, S.C.; Cordero-Morales, J.F.; Vásquez, V. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Sachs, F.; Gottlieb, P.A. Protonation of the human Piezo1 ion channel stabilizes inactivation. J. Biol. Chem. 2015, 290, 5167–5173. [Google Scholar] [CrossRef]
- Guo, Y.; Merten, A.-L.; Schöler, U.; Yu, Z.-Y.; Cvetkovska, J.; Fatkin, D.; Feneley, M.P.; Martinac, B.; Friedrich, O. In vitro cell stretching technology (isostretcher) as an approach to unravel Piezo1-mediated cardiac mechanotransduction. Prog. Biophys. Mol. Biol. 2021, 159, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, B.; Yuan, G.; Chen, Y.; Liang, F.; Zeng, H.; Zheng, S.; Cao, L.; Geng, D.; Zhou, S. Stretch-activated channel Piezo1 is up-regulated in failure heart and cardiomyocyte stimulated by AngII. Am. J. Transl. Res. 2017, 9, 2945. [Google Scholar] [PubMed]
- Meléndez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef]
- Hirota, H.; Yoshida, K.; Kishimoto, T.; Taga, T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc. Natl. Acad. Sci. USA 1995, 92, 4862–4866. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Blythe, N.M. Cardiac fibroblast p38 MAPK: A critical regulator of myocardial remodeling. J. Cardiovasc. Dev. Dis. 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Fuseler, J.W.; Intwala, A.R.; Baudino, T.A. IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1694–H1704. [Google Scholar] [CrossRef]
- Bageghni, S.A.; Hemmings, K.E.; Zava, N.; Denton, C.P.; Porter, K.E.; Ainscough, J.F.; Drinkhill, M.J.; Turner, N.A. Cardiac fibroblast-specific p38α MAP kinase promotes cardiac hypertrophy via a putative paracrine interleukin-6 signaling mechanism. FASEB J. 2018, 32, 4941–4954. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Bugg, D.; Ghearing, N.; Dorn, L.E.; Kim, P.; Sargent, M.A.; Gunaje, J.; Otsu, K.; Davis, J. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation 2017, 136, 549–561. [Google Scholar] [CrossRef]
- Emig, R.; Knodt, W.; Krussig, M.J.; Zgierski-Johnston, C.M.; Gorka, O.; Groß, O.; Kohl, P.; Ravens, U.; Peyronnet, R. Piezo1 channels contribute to the regulation of human atrial fibroblast mechanical properties and matrix stiffness sensing. Cells 2021, 10, 663. [Google Scholar] [CrossRef]
- Schewe, M.; Nematian-Ardestani, E.; Sun, H.; Musinszki, M.; Cordeiro, S.; Bucci, G.; de Groot, B.L.; Tucker, S.J.; Rapedius, M.; Baukrowitz, T. A non-canonical voltage-sensing mechanism controls gating in K2P K+ channels. Cell 2016, 164, 937–949. [Google Scholar] [CrossRef]
- Patel, A.J.; Honore, E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001, 24, 339–346. [Google Scholar] [CrossRef]
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Role of TREK-1 in health and disease, focus on the central nervous system. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Rinne, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021, 159, 126–135. [Google Scholar] [CrossRef]
- Brohawn, S.G. How ion channels sense mechanical force: Insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 2015, 1352, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.M.; Lee, T.E.; Watson, L.J.; Mao, L.; Chandok, G.; Wang, H.G.; Frangakis, S.; Pitt, G.S.; Shah, S.H.; Wolf, M.J.; et al. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J. Clin. Investig. 2018, 128, 4843–4855. [Google Scholar] [CrossRef]
- Honoré, E. The neuronal background K2P channels: Focus on TREK1. Nat. Rev. Neurosci. 2007, 8, 251–261. [Google Scholar] [CrossRef]
- Murbartián, J.; Lei, Q.; Sando, J.J.; Bayliss, D.A. Sequential phosphorylation mediates receptor-and kinase-induced inhibition of TREK-1 background potassium channels. J. Biol. Chem. 2005, 280, 30175–30184. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; Thümmler, S.; Duprat, F.; Feliciangeli, S.; Vinh, J.; Escoubas, P.; Guy, N.; Lazdunski, M.; Lesage, F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006, 25, 5864–5872. [Google Scholar] [CrossRef] [PubMed]
- Maingret, F.; Lauritzen, I.; Patel, A.J.; Heurteaux, C.; Reyes, R.; Lesage, F.; Lazdunski, M.; Honoré, E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000, 19, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Honoré, E.; Maingret, F.; Lazdunski, M.; Patel, A.J. An intracellular proton sensor commands lipid-and mechano-gating of the K+ channel TREK-1. EMBO J. 2002, 21, 2968–2976. [Google Scholar] [CrossRef]
- Kang, D.; Choe, C.; Kim, D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 2005, 564, 103–116. [Google Scholar] [CrossRef]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
- Alloui, A.; Zimmermann, K.; Mamet, J.; Duprat, F.; Noel, J.; Chemin, J.; Guy, N.; Blondeau, N.; Voilley, N.; Rubat-Coudert, C. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006, 25, 2368–2376. [Google Scholar] [CrossRef]
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318. [Google Scholar] [CrossRef]
- Patel, A.J.; Honoré, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honoré, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998, 17, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Sachs, F. Dynamic properties of stretch-activated K+ channels in adult rat atrial myocytes. Prog. Biophys. Mol. Biol. 2003, 82, 121–135. [Google Scholar] [CrossRef]
- Terrenoire, C.; Lauritzen, I.; Lesage, F.; Romey, G.; Lazdunski, M. A TREK-1–like potassium channel in atrial cells inhibited by β-adrenergic stimulation and activated by volatile anesthetics. Circ. Res. 2001, 89, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Unudurthi, S.D.; Wu, X.; Qian, L.; Amari, F.; Onal, B.; Li, N.; Makara, M.A.; Smith, S.A.; Snyder, J.; Fedorov, V.V. Two-pore K+ channel TREK-1 regulates sinoatrial node membrane excitability. J. Am. Heart Assoc. 2016, 5, e002865. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Wiedmann, F.; Kallenberger, S.M.; Ratte, A.; Schulte, J.S.; Scholz, B.; Muller, F.U.; Voigt, N.; Zafeiriou, M.P.; Ehrlich, J.R.; et al. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog. Biophys. Mol. Biol. 2017, 130, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Wiedmann, F.; Tristram, F.; Anand, P.; Wenzel, W.; Lugenbiel, P.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Cardiac expression and atrial fibrillation-associated remodeling of K₂P2.1 (TREK-1) K⁺ channels in a porcine model. Life Sci. 2014, 97, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lugenbiel, P.; Wenz, F.; Syren, P.; Geschwill, P.; Govorov, K.; Seyler, C.; Frank, D.; Schweizer, P.A.; Franke, J.; Weis, T. TREK-1 (K2P 2.1) K+ channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res. Cardiol. 2017, 112, 8. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, S.; Waters, C.M.; Schwingshackl, A.; Mancarella, S. TREK-1 protects the heart against ischemia-reperfusion-induced injury and from adverse remodeling after myocardial infarction. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Wiedmann, F.; Voigt, N.; Zhou, X.-B.; Heijman, J.; Lang, S.; Albert, V.; Kallenberger, S.; Ruhparwar, A.; Szabó, G. Upregulation of K2P3. 1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 2015, 132, 82–92. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Li, P.; Yuan, H.; Feng, N.; Peng, Y.; Wang, L.; Wang, X. An increased TREK-1–like potassium current in ventricular myocytes during rat cardiac hypertrophy. J. Cardiovasc. Pharmacol. 2013, 61, 302–310. [Google Scholar] [CrossRef]
- Nichols, C.G. Adenosine triphosphate-sensitive potassium currents in heart disease and cardioprotection. Card. Electrophysiol. Clin. 2016, 8, 323–335. [Google Scholar] [CrossRef]
- Sperelakis, N. Cell Physiology Source Book: Essentials of Membrane Biophysics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Huang, H.; Liang, L.; Liu, P.; Wei, H.; Sachs, F.; Niu, W.; Wang, W. Mechanical effects on KATP channel gating in rat ventricular myocytes. PLoS ONE 2013, 8, e63337. [Google Scholar] [CrossRef][Green Version]
- Terzic, A.; Kurachi, Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J. Physiol. 1996, 492, 395–404. [Google Scholar] [CrossRef]
- Ogbaghebriel, A.; Shrier, A. Differential responsiveness of atrial and ventricular myocytes to potassium channel openers. J. Cardiovasc. Pharmacol. 1995, 25, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, M.; Carter, C.C.; Youssef, N.; Light, P.E. The mechano-sensitivity of cardiac ATP-sensitive potassium channels is mediated by intrinsic MgATPase activity. J. Mol. Cell. Cardiol. 2017, 108, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Paucek, P.; Yarov-Yarovoy, V.; Sun, X.; Schindler, P.A. The mitochondrial KATP channel as a receptor for potassium channel openers. J. Biol. Chem. 1996, 271, 8796–8799. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; Hillman, R.M.; Scott, C.A.; Chilton, E.L. Ischemia reperfusion injury produces, and ischemic preconditioning prevents, rat cardiac fibroblast differentiation: Role of KATP channels. J. Cardiovasc. Dev. Dis. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, A.; Rosner, E.; Lanning, J.; Parachuru, L.; Chowdhury, P.D.; Han, S.; Lopez, G.; Tong, X.; Yoshida, H.; Nakamura, T.Y. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Benamer, N.; Ou Maati, H.M.; Demolombe, S.; Cantereau, A.; Delwail, A.; Bois, P.; Bescond, J.; Faivre, J.F. Molecular and functional characterization of a new potassium conductance in mouse ventricular fibroblasts. J. Mol. Cell. Cardiol. 2009, 46, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Benamer, N.; Vasquez, C.; Mahoney, V.M.; Steinhardt, M.J.; Coetzee, W.A.; Morley, G.E. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1231–H1239. [Google Scholar] [CrossRef]
- Chi, L.; Uprichard, A.C.; Lucchesi, B.R. Profibrillatory actions of pinacidil in a conscious canine model of sudden coronary death. J. Cardiovasc. Pharmacol. 1990, 15, 452–464. [Google Scholar] [CrossRef]
- Di Diego, J.M.; Antzelevitch, C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of ATP-regulated potassium current promote phase 2 reentry? Circulation 1993, 88, 1177–1189. [Google Scholar] [CrossRef]
- D’Alonzo, A.J.; Zhu, J.L.; Darbenzio, R.B.; Dorso, C.R.; Grover, G.J. Proarrhythmic effects of pinacidil are partially mediated through enhancement of catecholamine release in isolated perfused guinea-pig hearts. J. Mol. Cell. Cardiol. 1998, 30, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C.; McPherson, C.D.; Sontag, D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ. Res. 1991, 69, 571–581. [Google Scholar] [CrossRef]
- Yamada, S.; Kane, G.C.; Behfar, A.; Liu, X.K.; Dyer, R.B.; Faustino, R.S.; Miki, T.; Seino, S.; Terzic, A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6. 2-null mutant. J. Physiol. 2006, 577, 1053–1065. [Google Scholar] [CrossRef]
- Liou, J.-Y.; Hong, H.-J.; Sung, L.-C.; Chao, H.-H.; Chen, P.-Y.; Cheng, T.-H.; Chan, P.; Liu, J.-C. Nicorandil inhibits angiotensin-II-induced proliferation of cultured rat cardiac fibroblasts. Pharmacology 2011, 87, 144–151. [Google Scholar] [CrossRef]
- Schultz, F.; Hasan, A.; Alvarez-Laviada, A.; Miragoli, M.; Bhogal, N.; Wells, S.; Poulet, C.; Chambers, J.; Williamson, C.; Gorelik, J. The protective effect of ursodeoxycholic acid in an in vitro model of the human fetal heart occurs via targeting cardiac fibroblasts. Prog. Biophys. Mol. Biol. 2016, 120, 149–163. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, Z.; Black, C.M.; de Crombrugghe, B.; Denton, C.P. Ligand-dependent genetic recombination in fibroblasts: A potentially powerful technique for investigating gene function in fibrosis. Am. J. Pathol. 2002, 160, 1609–1617. [Google Scholar] [CrossRef]
- Acharya, A.; Baek, S.T.; Huang, G.; Eskiocak, B.; Goetsch, S.; Sung, C.Y.; Banfi, S.; Sauer, M.F.; Olsen, G.S.; Duffield, J.S.; et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012, 139, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]


| Measurements of Mechanosensitivity in Cation Channels | ||||
|---|---|---|---|---|
| Channel | Model Used for Electrophysiological Reading | Nature of the Intervention | Channel Specific Signal Confirmed | Type of Mechanical Stimuli |
| TRPC6 | Cell-attached patch [65,72] | Pipette applied negative pressure | No [65] Yes [72] | 25% tonic mechanical stretch; membrane stretch Osmotic swelling; membrane stretch |
| Excised patch readings of protein reconstituted in liposome [65,73] | Pipette applied negative pressure | Yes (spontaneously active but not stretch responsive) | ||
| Whole-cell patch [74] | Hypo-osmotic treatment | Yes, confirmed with inhibitor: SKF-96365 | ||
| TRPM7 | Excised inside-out patch [75] | Pipette applied negative pressure; suction; osmotic swelling; perfusion-induced mechanical stress | Yes, confirmed in TRPM7 null cells; inhibitor: 2-APB; functional KO | Osmotic swelling; membrane stretch |
| Whole-cell patch [76] | ||||
| TRPV1 | Cell-attached patch [65] | Pipette applied negative pressure (not responsive) | No response detected | Osmotic swelling; membrane stretch |
| TRPV4 | Cell-attached patch [65] | Pipette applied negative pressure (not responsive) | No response detected | Osmotic swelling; membrane stretch |
| Whole cell [77] | Cell indentation with glass rod (no loss of reading with Trpv4 KO) | Yes, confirmed in cells isolated from Trpv4 KO mice +/− | ||
| Outside-out patch [77] | High-speed pressure clamp (no loss of reading with Trpv4 KO) | Yes, confirmed in cells isolated from Trpv4 KO mice +/− TRPV4 agonist | ||
| Piezo1 | Whole cell [78] | Cell indentation with glass rod | Yes, confirmed with siRNA knockdown | Membrane stretch |
| Outside-out patch [79] | High-speed pressure clamp applied positive pressure | Yes, confirmed with siRNA knockdown | ||
| TREK-1 | Inside-out patch; outside out; cell attached [80] | Pipette applied negative pressure (No effect) | Yes (TREK-1 over expressed in COS cells) | Membrane stretch; cell swelling |
| Inside-out patch of protein reconstituted in liposomes (channel spontaneously active) [81] | Pipette applied positive pressure (inactivates) | Yes, confirmed with TREK-1 inserted in liposome | ||
| KATP | Cell-attached; inside-out; excised-patch; perforated patch whole cell [82] | Negative pressure applied; hypotonic osmotic swelling | Yes, mechanosensitivity dependent on SUR subunit | Membrane stretch; cell swelling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, L.; Turner, N.A. Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells 2021, 10, 990. https://doi.org/10.3390/cells10050990
Stewart L, Turner NA. Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells. 2021; 10(5):990. https://doi.org/10.3390/cells10050990
Chicago/Turabian StyleStewart, Leander, and Neil A. Turner. 2021. "Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts" Cells 10, no. 5: 990. https://doi.org/10.3390/cells10050990
APA StyleStewart, L., & Turner, N. A. (2021). Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells, 10(5), 990. https://doi.org/10.3390/cells10050990







