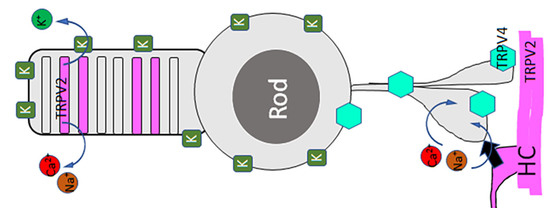
Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Single-Cell and Dual-Cell Patch-Clamp Recording of Photoreceptors
2.3. Multi-Cell Patch-Clamp Recording of Retinal Neurons
2.4. FM 1-43 Probing for MSCs in the Open State
2.5. Antibodies and Immunocytochemistry
2.6. Statistics
3. Results
3.1. The Immunoreactivities of the Mechanosensitive Potassium and Non-Selective Cation Channel in Outer Retinal Neurons
3.2. Pressure-Evoked Currents in Rods
3.3. The Effect of the Pharmacological and Thermal Modulation of Mechanical Sensitive Channels on the Light Response of Rods
3.4. FM 1-43-Identified Open Channels in the Outer Segment (OS) of Photoreceptors
4. Discussion
4.1. Rods and Presynaptic Neurons Express Mechanical Sensitive Channels
4.2. Pressure-Evoked Currents in Rods Are Compartmental and Involve at Least Three Generators
4.3. Pressure-Evoked Mutually Compensating Currents in Rods
4.4. Mechanosensitive Channels Involve the Maintenance of the RP, While TRPV2 Probably Also Involves Phototransduction
4.5. Rigid Shape of the OS of Normal Rods Likely Helps to Open TRPV2 in Rod Disks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
References
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Harasymowycz, P.; Birt, C.; Gooi, P.; Heckler, L.; Hutnik, C.; Jinapriya, D.; Shuba, L.; Yan, D.; Day, R. Medical Management of Glaucoma in the 21st Century from a Canadian Perspective. J. Ophthalmol. 2016, 2016, 6509809. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; De Groot, V.; Audenaert, K.; De Deyn, P.P. Are intracranial pressure fluctuations important in glaucoma? Med. Hypotheses 2011, 77, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Coleman, A.L. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008, 115, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.; Zeimer, R.; Wilensky, J.; Gieser, D.; Vitale, S.; Lindenmuth, K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J. Glaucoma 2000, 9, 134–142. [Google Scholar] [CrossRef]
- Downs, J.C.; Burgoyne, C.F.; Seigfreid, W.P.; Reynaud, J.F.; Strouthidis, N.G.; Sallee, V. 24-hour IOP telemetry in the nonhuman primate: Implant system performance and initial characterization of IOP at multiple timescales. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7365–7375. [Google Scholar] [CrossRef]
- Sugimoto, E.; Aihara, M.; Ota, T.; Araie, M. Effect of light cycle on 24-hour pattern of mouse intraocular pressure. J. Glaucoma 2006, 15, 505–511. [Google Scholar] [CrossRef]
- Sigal, I.A.; Flanagan, J.G.; Tertinegg, I.; Ethier, C.R. Finite element modeling of optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4378–4387. [Google Scholar] [CrossRef]
- Shin, D.H.; Bielik, M.; Hong, Y.J.; Briggs, K.S.; Shi, D.X. Reversal of glaucomatous optic disc cupping in adult patients. Arch. Ophthalmol. 1989, 107, 1599–1603. [Google Scholar] [CrossRef]
- Kang, L.; Gao, J.; Schafer, W.R.; Xie, Z.; Xu, X.Z.C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 2010, 67, 381–391. [Google Scholar] [CrossRef]
- Liu, C.; Montell, C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Xu, X.Z.C. elegans TRP channels. Adv. Exp. Med. Biol. 2011, 704, 323–339. [Google Scholar] [PubMed]
- Krizaj, D. Polymodal Sensory Integration in Retinal Ganglion Cells. Adv. Exp. Med. Biol. 2016, 854, 693–698. [Google Scholar] [PubMed]
- Sappington, R.M.; Sidorova, T.; Ward, N.J.; Chakravarthy, R.; Ho, K.W.; Calkins, D.J. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels 2015, 9, 102–113. [Google Scholar] [CrossRef]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Z.; Jacoby, R.A.; Wu, S.M.; Pang, J.J. The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell Death Dis. 2019, 10, 364–1576. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J. Roles of the ocular pressure, pressure-sensitive ion channel, and elasticity in pressure-induced retinal diseases. Neural Regen Res. 2021, 16, 68–72. [Google Scholar] [CrossRef]
- Patel, N.; Pass, A.; Mason, S.; Gibson, C.R.; Otto, C. Optical Coherence Tomography Analysis of the Optic Nerve Head and Surrounding Structures in Long-Duration International Space Station Astronauts. JAMA Ophthalmol. 2018, 136, 193–200. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, W.; Tian, B.; Wang, C.; Shi, X.; Jiao, X. Observation and management of retinal changes related to diving in professional divers. Chin. Med. J. 2014, 127, 729–733. [Google Scholar]
- Mowatt, L.; Foster, T. Sphenoidal sinus mucocele presenting with acute visual loss in a scuba diver. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]
- Frankfort, B.J.; Khan, A.K.; Tse, D.Y.-Y.; Chung, I.; Pang, J.-J.; Yang, Z.; Gross, R.L.; Wu, S.M. Elevated intraocular pressure causes inner retinal dysfunction before cell loss in a mouse model of experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2013, 54, 762–770. [Google Scholar] [CrossRef]
- Pang, J.J.; Frankfort, B.J.; Gross, R.L.; Wu, S.M. Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice. Proc. Natl. Acad. Sci. USA 2015, 112, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.H.; Qu, J.; John, S.W.; Howell, G.R.; Jakobs, T.C. Synapse Loss and Dendrite Remodeling in a Mouse Model of Glaucoma. PLoS ONE 2015, 10, e0144341. [Google Scholar] [CrossRef] [PubMed]
- El-Danaf, R.N.; Huberman, A.D. Characteristic patterns of dendritic remodeling in early-stage glaucoma: Evidence from genetically identified retinal ganglion cell types. J. Neurosci. 2015, 35, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Velten, I.M.; Horn, F.K.; Korth, M.; Velten, K. The b-wave of the dark adapted flash electroretinogram in patients with advanced asymmetrical glaucoma and normal subjects. Br. J. Ophthalmol. 2001, 85, 403–409. [Google Scholar] [CrossRef] [PubMed]
- North, R.V.; Jones, A.L.; Drasdo, N.; Wild, J.M.; Morgan, J.E. Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1216–1222. [Google Scholar] [CrossRef]
- Drasdo, N.; Aldebasi, Y.H.; Chiti, Z.; Mortlock, K.E.; Morgan, J.E.; North, R.V. The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1266–1272. [Google Scholar]
- Vickers, J.C.; Schumer, R.A.; Podos, S.M.; Wang, R.F.; Riederer, B.M.; Morrison, J.H. Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res. 1995, 680, 23–35. [Google Scholar] [CrossRef]
- Fuchs, M.; Scholz, M.; Sendelbeck, A.; Atorf, J.; Schlegel, C.; Enz, R.; Brandstätter, J.H. Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes. PLoS ONE 2012, 7, e44645. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; de Sevilla Muller, L.P.; Brecha, N.C.; Cuenca, N. Loss of outer retinal neurons and circuitry alterations in the DBA/2J mouse. Invest. Ophthalmol. Vis. Sci. 2014, 55, 6059–6072. [Google Scholar] [CrossRef]
- Persson, A.K.; Kim, I.; Zhao, P.; Estacion, M.; Black, J.A.; Waxman, S.G. Sodium channels contribute to degeneration of dorsal root ganglion neurites induced by mitochondrial dysfunction in an in vitro model of axonal injury. J. Neurosci. 2013, 33, 19250–19261. [Google Scholar] [CrossRef]
- Barsukova, A.G.; Forte, M.; Bourdette, D. Focal increases of axoplasmic Ca2+, aggregation of sodium-calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress. J. Neurosci. 2012, 32, 12028–12037. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Attwell, D. The energetics of CNS white matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Witkovsky, P.; Barabas, P.; Huang, W.; Koehler, C.; Akimov, N.P.; Lee, S.H.; Chauhan, S.; Xing, W.; Rentería, R.C.; et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 2011, 31, 7089–7101. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Arner, K.; Ghosh, F. Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Exp. Eye Res. 2016, 154, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, J.C.; Wensel, T.G. TRP channel gene expression in the mouse retina. Vis. Res. 2011, 51, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Yazulla, S.; Studholme, K.M. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: Colocalization with somatostatin and purinergic P2 × 1 receptors. J. Comp. Neurol. 2004, 474, 407–418. [Google Scholar] [CrossRef]
- Xu, J.W.; Slaughter, M.M. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J. Neurosci. 2005, 25, 7660–7668. [Google Scholar] [CrossRef]
- Pelucchi, B.; Grimaldi, A.; Moriondo, A. Vertebrate rod photoreceptors express both BK and IK calcium-activated potassium channels, but only BK channels are involved in receptor potential regulation. J. Neurosci. Res. 2008, 86, 194–201. [Google Scholar] [CrossRef]
- Sakaba, T.; Ishikane, H.; Tachibana, M. Ca2+ -activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neurosci. Res. 1997, 27, 219–228. [Google Scholar] [CrossRef]
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bang, H.; Gnatenco, C.; Kim, D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 2001, 442, 64–72. [Google Scholar] [CrossRef]
- Maingret, F.; Fosset, M.; Lesage, F.; Lazdunski, M.; Honore, E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 1999, 274, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Maingret, F.; Patel, A.J.; Lesage, F.; Lazdunski, M.; Honore, E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J. Biol. Chem. 2000, 275, 10128–10133. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Lazdunski, M.; Honore, E. Lipid and mechano-gated 2P domain K(+) channels. Curr. Opin. Cell Biol. 2001, 13, 422–428. [Google Scholar] [CrossRef]
- Patel, A.J.; Honoré, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998, 17, 4283–4290. [Google Scholar] [CrossRef]
- Lesage, F.; Terrenoire, C.; Romey, G.; Lazdunski, M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J. Biol. Chem. 2000, 275, 28398–28405. [Google Scholar] [CrossRef]
- Cox, C.D.; Bavi, N.; Martinac, B. Origin of the Force: The Force-From-Lipids Principle Applied to Piezo Channels. Curr. Top. Membr. 2017, 79, 59–96. [Google Scholar]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Yeh, J.J.; E Boyd, A.; A Parada, C.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Li, W.; Feng, Z.; Sternberg, P.W.; Xu, X.Z. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 2006, 440, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Loukin, S.; Zhou, X.; Su, Z.; Saimi, Y.; Kung, C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J. Biol. Chem. 2010, 285, 27176–27181. [Google Scholar] [CrossRef] [PubMed]
- Ciura, S.; Liedtke, W.; Bourque, C.W. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J. Neurosci. 2011, 31, 14669–14676. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Jo, A.O.; Frye, A.M.; Vazquez-Chona, F.; Macaulay, N.; Thoreson, W.B.; Križaj, D. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J. Neurosci. 2014, 34, 15689–15700. [Google Scholar] [CrossRef]
- McGahon, M.K.; Fernández, J.A.; Dash, D.P.; McKee, J.; Simpson, D.A.; Zholos, A.V.; McGeown, J.G.; Curtis, T.M. TRPV2 Channels Contribute to Stretch-Activated Cation Currents and Myogenic Constriction in Retinal Arterioles. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5637–5647. [Google Scholar] [CrossRef]
- Meyers, J.R.; MacDonald, R.B.; Duggan, A.; Lenzi, D.; Standaert, D.G.; Corwin, J.T.; Corey, D.P. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003, 23, 4054–4065. [Google Scholar] [CrossRef]
- Drew, L.J.; Wood, J.N. FM1-43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol. Pain 2007, 3, 1. [Google Scholar] [CrossRef]
- Gale, J.E.; Marcotti, W.; Kennedy, H.J.; Kros, C.J.; Richardson, G.P. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001, 21, 7013–7025. [Google Scholar] [CrossRef]
- Zanini, D.; Gopfert, M.C. TRPs in hearing. Handb. Exp. Pharmacol. 2014, 223, 899–916. [Google Scholar]
- Asai, Y.; Holt, J.R.; Geleoc, G.S. A quantitative analysis of the spatiotemporal pattern of transient receptor potential gene expression in the developing mouse cochlea. J. Assoc. Res. Otolaryngol. 2010, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, C.; Kim, S.-J.; Kim, H.-J.; Oh, G.-S.; Shen, A.; So, H.-S.; Park, R. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp. Mol. Med. 2013, 45, e12. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Paul, D.L.; Wu, S.M. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J. Physiol. 2012, 590, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Pang, J.J.; Wu, S.M. Sign-preserving and sign-inverting synaptic interactions between rod and cone photoreceptors in the dark-adapted retina. J. Physiol. 2013, 591, 5711–5726. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J. Neurosci. Methods 1987, 20, 139–149. [Google Scholar] [CrossRef]
- Werblin, F.S. Transmission along and between rods in the tiger salamander retina. J. Physiol. 1978, 280, 449–470. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Barrow, A.; Jacoby, R.A.; Wu, S.M. How do tonic glutamatergic synapses evade receptor desensitization? J. Physiol. 2008, 586, 2889–2902. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Wu, S.M. Ionotropic glutamate receptors mediate OFF responses in light-adapted ON bipolar cells. Vis. Res. 2012, 68, 48–58. [Google Scholar] [CrossRef]
- Maple, B.R.; Wu, S.M. Glycinergic synaptic inputs to bipolar cells in the salamander retina. J. Physiol. 1998, 506 Pt 3, 731–744. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Wu, S.M. Segregation and integration of visual channels: Layer-by-layer computation of ON-OFF signals by amacrine cell dendrites. J. Neurosci. 2002, 22, 4693–4701. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Lem, J.; Bramblett, D.E.; Paul, D.L.; Wu, S.M. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc. Natl. Acad. Sci. USA 2010, 107, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Field, G.D.; Rieke, F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron 2002, 35, 733–747. [Google Scholar] [CrossRef]
- Guha, A.; Barrow, R.M.; Balachandar, R. An experimental and numerical study of water jet cleaning process. J. Mater. Process. Technol. 2011, 211, 610–618. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.W.; Cohen, M.R.; Jiang, J.; Samanta, A.; Lodowski, D.T.; Zhou, Z.H.; Moiseenkova-Bell, V.Y. Structure of the full-length TRPV2 channel by cryo-EM. Nat. Commun. 2016, 7, 11130. [Google Scholar] [CrossRef]
- Juvin, V.; Penna, A.; Chemin, J.; Lin, Y.L.; Rassendren, F.A. Pharmacological characterization and molecular determinants of the activation of transient receptor potential V2 channel orthologs by 2-aminoethoxydiphenyl borate. Mol. Pharmacol. 2007, 72, 1258–1268. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Kolodziejczyk, K.; Kougioumtzidou, E.; Attwell, D. Proton-gated Ca(2+)-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 2016, 529, 523–527. [Google Scholar] [CrossRef]
- Clapham, D.E. SnapShot: Mammalian TRP channels. Cell 2007, 129, 220. [Google Scholar] [CrossRef]
- Leffler, A.; Linte, R.M.; Nau, C.; Reeh, P.; Babes, A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur. J. Neurosci. 2007, 26, 12–22. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, G.; Lee, A.J.; Stornetta, R.L.; Zhu, J.J. The organization of two new cortical interneuronal circuits. Nat. Neurosci. 2013, 16, 210–218. [Google Scholar] [CrossRef]
- Wang, G.; Wyskiel, D.R.; Yang, W.; Wang, Y.; Milbern, L.C.; Lalanne, T.; Jiang, X.; Shen, Y.; Sun, Q.Q.; Zhu, J.J. An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits. Nat. Protoc. 2015, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.; Cocucci, E.; Podini, P.; Kirchhausen, T.; Clementi, E.; Meldolesi, J. Macropinocytosis: Regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 2006, 119, 4758–4769. [Google Scholar] [CrossRef]
- Pang, J.J.; Wu, S.M. Morphology and immunoreactivity of retrogradely double-labeled ganglion cells in the mouse retina. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4886–4896. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J.; Gao, F.; Wu, S.M. Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. J. Comp. Neurol. 2010, 518, 2456–2474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Z.; Wu, S.M. Development of cholinergic amacrine cells is visual activity-dependent in the postnatal mouse retina. J. Comp. Neurol. 2005, 484, 331–343. [Google Scholar] [CrossRef]
- Fink, M.; Duprat, F.; Lesage, F.; Reyes, R.; Romey, G.; Heurteaux, C.; Lazdunski, M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996, 15, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Foster, S.; Scott, M.A.; Sherwood, P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. J. Comp. Neurol. 2008, 506, 584–603. [Google Scholar] [CrossRef]
- Zhang, H.; Cuenca, N.; Ivanova, T.; Church-Kopish, J.; Frederick, J.M.; MacLeish, P.R.; Baehr, W. Identification and light-dependent translocation of a cone-specific antigen, cone arrestin, recognized by monoclonal antibody 7G6. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2858–2867. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Cheron, G.; Lohof, A.; D’Alcantara, P.; Meyer, M.; Parmentier, M.; Schurmans, S. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc. Natl. Acad. Sci. USA 1999, 96, 5257–5262. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, A.J.; Wu, S.M. Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. J. Comp. Neurol. 2006, 499, 432–441. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Wu, S.M. Immuocytochemical analysis of spatial organization of photoreceptors and amacrine and ganglion cells in the tiger salamander retina. Vis. Neurosci. 2004, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J.; Yang, Z.; Jacoby, R.A.; Wu, S.M. Cone synapses in mammalian retinal rod bipolar cells. J. Comp. Neurol. 2018, 526, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.M. Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res. 2009, 49, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B.; Babai, N.; Bartoletti, T.M. Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J. Neurosci. 2008, 28, 5691–5695. [Google Scholar] [CrossRef] [PubMed]
- Deniz, S.; Wersinger, E.; Schwab, Y.; Mura, C.; Erdélyi, F.; Szabo, G.; Rendon, A.; Sahel, J.-A.; Picaud, S.; Roux, M.J. Mammalian retinal horizontal cells are unconventional GABAergic neurons. J. Neurochem. 2011, 116, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Boije, H.; Shirazi, F.S.; Edqvist, P.H.; Hallbook, F. Horizontal Cells, the Odd Ones Out in the Retina, Give Insights into Development and Disease. Front. Neuroanat. 2016, 10, 77. [Google Scholar] [CrossRef]
- Wu, S.M. Synaptic transmission in the outer retina. Annu. Rev. Physiol. 1994, 56, 141–168. [Google Scholar] [CrossRef]
- Hughes, S.; Foster, R.G.; Peirson, S.N.; Hankins, M.W. Expression and localisation of two-pore domain (K2P) background leak potassium ion channels in the mouse retina. Sci. Rep. 2017, 7, 46085. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, X.; Wolff, D.; Alvarez, O.; Latorre, R. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle. Biophys. J. 1987, 52, 707–716. [Google Scholar] [CrossRef]
- Kanda, H.; Ling, J.; Tonomura, S.; Noguchi, K.; Matalon, S.; Gu, J.G. TREK-1 and TRAAK Are Principal K(+) Channels at the Nodes of Ranvier for Rapid Action Potential Conduction on Mammalian Myelinated Afferent Nerves. Neuron 2019, 104, 960–971. [Google Scholar] [CrossRef]
- Piechotta, P.L.; Rapedius, M.; Stansfeld, P.J.; Bollepalli, M.K.; Erhlich, G.; Andres-Enguix, I.; Fritzenschaft, H.; Decher, N.; Sansom, M.S.P.; Tucker, S.J.; et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011, 30, 3607–3619. [Google Scholar] [CrossRef]
- Liedtke, W.; Tobin, D.M.; Bargmann, C.I.; Friedman, J.M. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2003, 100, 14531–14536. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 278, 32037–32046. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Cook, B. TRP channel proteins and signal transduction. Physiol. Rev. 2002, 82, 429–472. [Google Scholar] [CrossRef]
- Audo, I.; Kohl, S.; Leroy, B.P.; Munier, F.L.; Guillonneau, X.; Mohand-Saïd, S.; Bujakowska, K.; Nandrot, E.F.; Lorenz, B.; Preising, M.; et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 2009, 85, 720–729. [Google Scholar] [CrossRef]
- Koike, C.; Obara, T.; Uriu, Y.; Numata, T.; Sanuki, R.; Miyata, K.; Koyasu, T.; Ueno, S.; Funabiki, K.; Tani, A.; et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA 2010, 107, 332–337. [Google Scholar] [CrossRef]
- Barro-Soria, R.; Stindl, J.; Müller, C.; Foeckler, R.; Todorov, V.; Castrop, H.; Strauß, O. Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: Role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS ONE 2012, 7, e49624. [Google Scholar] [CrossRef]
- Link, T.M.; Park, U.; Vonakis, B.M.; Raben, D.M.; Soloski, M.J.; Caterina, M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010, 11, 232–239. [Google Scholar] [CrossRef]
- Volland, S.; Hughes, L.; Kong, C.; Burgess, B.L.; Linberg, K.A.; Luna, G.; Zhou, Z.H.; Fisher, S.K.; Williams, D.S. Three-dimensional organization of nascent rod outer segment disk membranes. Proc. Natl. Acad. Sci. USA 2015, 112, 14870–14875. [Google Scholar] [CrossRef]
- Ding, J.D.; Salinas, R.Y.; Arshavsky, V.Y. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J. Cell Biol. 2015, 211, 495–502. [Google Scholar] [CrossRef]
- Dosey, T.L.; Wang, Z.; Fan, G.; Zhang, Z.; Serysheva, I.I.; Chiu, W.; Wensel, T.G. Structures of TRPV2 in distinct conformations provide insight into role of the pore turret. Nat. Struct. Mol. Biol. 2019, 26, 40–49. [Google Scholar] [CrossRef]
- Zubcevic, L.; Herzik, M.A.; Jr Chung, B.C.; Liu, Z.; Lander, G.C.; Lee, S.Y. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 2016, 23, 180–186. [Google Scholar] [CrossRef]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef]
- Sawamura, S.; Shirakawa, H.; Nakagawa, T.; Mori, Y.; Kaneko, Y. TRP Channels in the Brain: What Are They There For? In Neurobiology of TRP Channels, 2nd ed.; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Arnadottir, J.; Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef]
- Molnar, T.; Barabas, P.; Birnbaumer, L.; Punzo, C.; Kefalov, V.; Krizaj, D. Store-operated channels regulate intracellular calcium in mammalian rods. J. Physiol. 2012, 590, 3465–3481. [Google Scholar] [CrossRef]
- Bocchero, U.; Falleroni, F.; Mortal, S.; Li, Y.; Cojoc, D.; Lamb, T.; Torre, V. Mechanosensitivity is an essential component of phototransduction in vertebrate rods. PLoS Biol. 2020, 18, e3000750. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Xu, X.S. Mechanosensitive Channels: In Touch with Piezo. Curr. Biol. 2010, 20, R936–R938. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Matsumoto, N.; Imai, M.; Suzuki, M. Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol. 2003, 285, C96–C101. [Google Scholar] [CrossRef]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Choi, H.J.; Sun, D.; Jakobs, T.C. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 2015, 21, 749–766. [Google Scholar]
- Baxter, S.L.; Keenan, W.T.; Athanas, A.J.; Proudfoot, J.A.; Zangwill, L.M.; Ayyagari, R.; Liebmann, J.M.; Girkin, C.A.; Patapoutian, A.; Weinreb, R.N. Investigation of associations between Piezo1 mechanoreceptor gain-of-function variants and glaucoma-related phenotypes in humans and mice. Sci. Rep. 2020, 10, 19013–76026. [Google Scholar] [CrossRef]
- Morozumi, W.; Inagaki, S.; Iwata, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Piezo channel plays a part in retinal ganglion cell damage. Exp. Eye Res. 2020, 191, 107900. [Google Scholar] [CrossRef]
- Gao, L.; Yang, P.; Qin, P.; Lu, Y.; Li, X.; Tian, Q.; Li, Y.; Xie, C.; Tian, J.-B.; Zhang, C.; et al. Selective potentiation of 2-APB-induced activation of TRPV1-3 channels by acid. Sci. Rep. 2016, 6, 20791. [Google Scholar] [CrossRef]
- Cervetto, L.; Lagnado, L.; Perry, R.J.; Robinson, D.W.; McNaughton, P.A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature 1989, 337, 740–743. [Google Scholar] [CrossRef]





| Channel | Location |
|---|---|
| TRAAK | Photoreceptor inner segments, INL (ACs, BCs), mouse [41] |
| TRAAK | INL, RGL, Müller, mouse [98] |
| BK | Rod terminals, salamander [38] |
| BK | Rods, salamander [39] |
| BK | Photoreceptors, goldfish [40] |
| TRPV2 (VRL1) | Photoreceptor axon terminals, IPL, and OPL in rat, cat, primate [37] |
| TRPV2 | Photoreceptor axons, OPL, mouse [36] |
| TRPV4 | OPL, IPL, Müller, mouse [34] |
| TRPV4 | OPL, IPL, pig [35] |
| TRPV4 | OPL, IPL, BCs, RGCs, Müller, primate [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.-J.; Gao, F.; Wu, S.M. Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons. Cells 2021, 10, 1288. https://doi.org/10.3390/cells10061288
Pang J-J, Gao F, Wu SM. Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons. Cells. 2021; 10(6):1288. https://doi.org/10.3390/cells10061288
Chicago/Turabian StylePang, Ji-Jie, Fan Gao, and Samuel M. Wu. 2021. "Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons" Cells 10, no. 6: 1288. https://doi.org/10.3390/cells10061288
APA StylePang, J.-J., Gao, F., & Wu, S. M. (2021). Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons. Cells, 10(6), 1288. https://doi.org/10.3390/cells10061288