Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization
Abstract
:1. Introduction
2. Physical Structures of Sperm That Affect Response to the Female Physical Environment
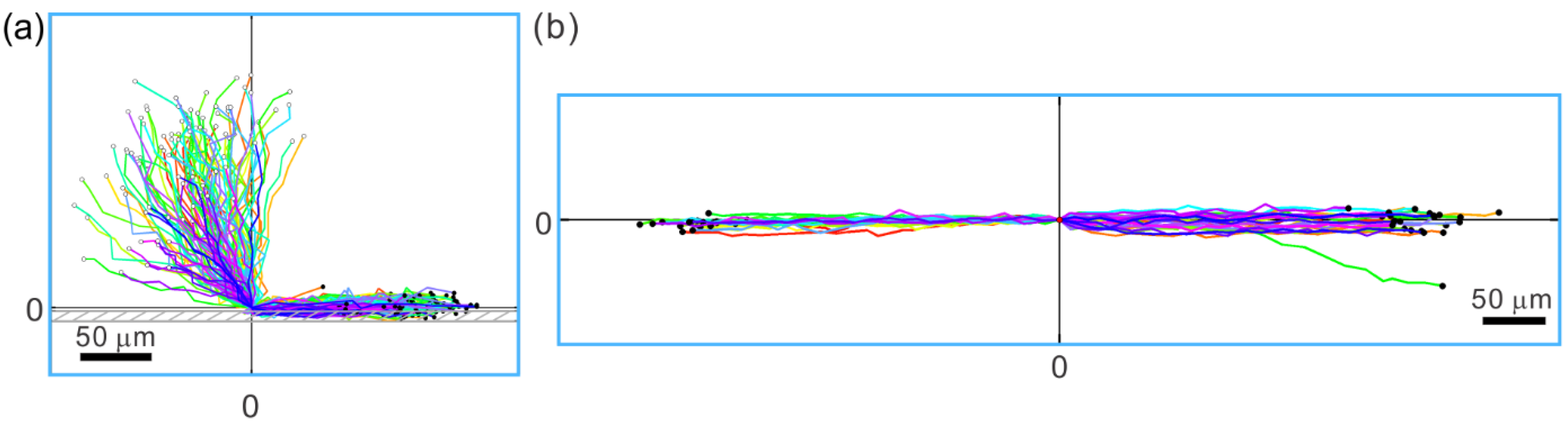
3. Physical Aspects of Female Environment That Affect Direction and Speed of Sperm Movement
3.1. Overview of Mammalian Female Reproductive Tract
3.2. Walls
3.3. Fluid Flow
3.4. Fluid Viscoelasticity
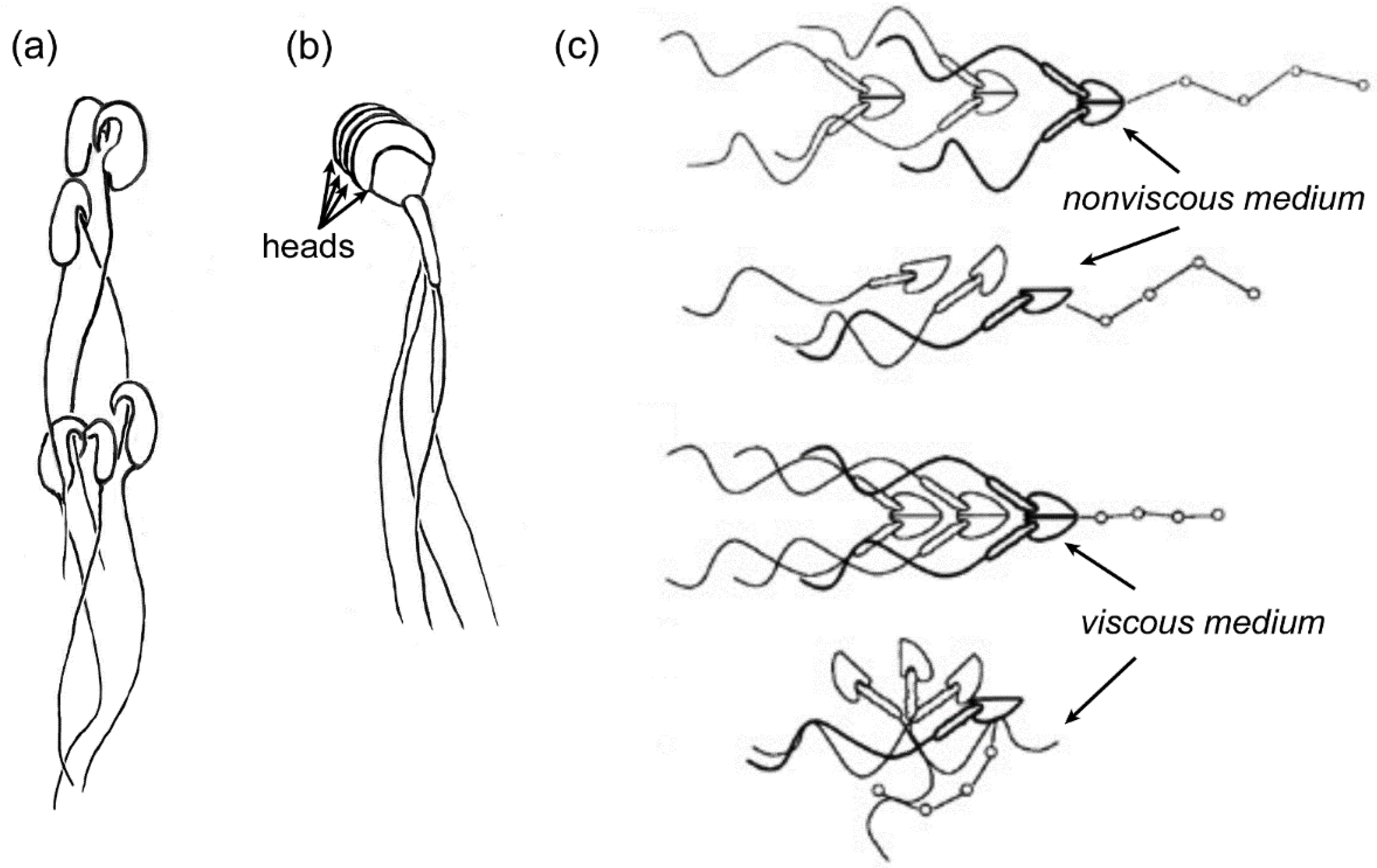
4. Cooperative Movement of Sperm
5. Apparent Co-Evolution of Sperm and Female Reproductive Tract Physical Traits
6. Implications for Clinical Applications
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yániz, J.L.; Lopez-Gatius, F.; Santolaria, P.; Mullins, K.J. Study of the functional anatomy of bovine oviductal mucosa. Anat. Rec. 2000, 260, 268–278. [Google Scholar] [CrossRef]
- Kölle, S. Transport, Distribution and Elimination of Mammalian Sperm Following Natural Mating and Insemination. Reprod. Domest. Anim. 2015, 50, 2–6. [Google Scholar] [CrossRef]
- Suarez, S.S.; Brockman, K.; Lefebvre, R. Distribution of mucus and sperm in bovine oviducts after artificial insemination: The physical environment of the oviductal sperm reservoir. Biol. Reprod. 1997, 56, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Mullins, K.J.; Saacke, R.G. Study of the functional anatomy of bovine cervical mucosa with special reference to mucus secretion and sperm transport. Anat. Rec. 1989, 225, 106–117. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Wigby, S.; Suarez, S.S.; Lazzaro, B.P.; Pizzari, T.; Wolfner, M.F. Chapter Eight-Sperm success and immunity. In Current Topics in Developmental Biology; Lehmann, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 135, pp. 287–313. [Google Scholar]
- Lüpold, S.; Pitnick, S. Sperm form and function: What do we know about the role of sexual selection? Reproduction 2018, 155, R229–R243. [Google Scholar] [CrossRef]
- Fawcett, D.W. The mammalian spermatozoon. Dev. Biol. 1975, 44, 394–436. [Google Scholar] [CrossRef]
- Cummins, J.M.; Woodall, P.F. On mammalian sperm dimensions. Reproduction 1985, 75, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, C.; Dacheux, J.-L.; Hermand, E.; Mazeman, E.; Rousseaux, J.; Rousseaux-Prévost, R. Actin-Binding Properties and Colocalization with Actin During Spermiogenesis of Mammalian Sperm Calicin1. Biol. Reprod. 2000, 63, 1801–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, D.W.; Anderson, W.A.; Phillips, D.M. Morphogenetic factors influencing the shape of the sperm head. Dev. Biol. 1971, 26, 220–251. [Google Scholar] [CrossRef]
- Buffone, M.G.; Hirohashi, N.; Gerton, G.L. Unresolved Questions Concerning Mammalian Sperm Acrosomal Exocytosis. Biol. Reprod. 2014, 90. [Google Scholar] [CrossRef] [Green Version]
- Darszon, A.; Nishigaki, T.; López-González, I.; Visconti, P.E.; Treviño, C.L. Differences and Similarities: The Richness of Comparative Sperm Physiology. Physiology 2020, 35, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.B.; Macauley, L.J.; Lesich, K.A. The Counterbend Phenomenon in Dynein-Disabled Rat Sperm Flagella and What It Reveals about the Interdoublet Elasticity. Biophys. J. 2005, 89, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, N.; Taschner, M.; Engel, B.D.; Lorentzen, E. Structural Studies of Ciliary Components. J. Mol. Biol. 2012, 422, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Elgeti, J.; Winkler, R.G.; Gompper, G. Physics of microswimmers—Single particle motion and collective behavior: A review. Rep. Prog. Phys. 2015, 78, 056601. [Google Scholar] [CrossRef]
- Inaba, K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011, 17, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.M. Comparative Analysis of Mammalian Sperm Motility. J. Cell Biol. 1972, 53, 561–573. [Google Scholar] [CrossRef]
- Bukatin, A.; Kukhtevich, I.; Stoop, N.; Dunkel, J.; Kantsler, V. Bimodal rheotactic behavior reflects flagellar beat asymmetry in human sperm cells. Proc. Natl. Acad. Sci. USA 2015, 112, 15904–15909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, M.T.; Cupples, G.; Ooi, E.H.; Kirkman-Brown, J.C.; Smith, D.J. Rapid sperm capture: High-throughput flagellar waveform analysis. Hum. Reprod. 2019, 34, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Gadêlha, H.; Hernández-Herrera, P.; Montoya, F.; Darszon, A.; Corkidi, G. Human sperm uses asymmetric and anisotropic flagellar controls to regulate swimming symmetry and cell steering. Sci. Adv. 2020, 6, eaba5168. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Phuyal, S.; Ishimoto, K.; Tung, C.-K.; Gaffney, E.A. Computer-assisted beat-pattern analysis and the flagellar waveforms of bovine spermatozoa. R. Soc. Open Sci. 2020, 7, 200769. [Google Scholar] [CrossRef]
- Baltz, J.M.; Oneeka Williams, P.; Cone, R.A. Dense Fibers Protect Mammalian Sperm Against Damage1. Biol. Reprod. 1990, 43, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemann, C.B.; Lesich, K.A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.G.; Woolley, D.M. Basal Sliding and the Mechanics of Oscillation in a Mammalian Sperm Flagellum. Biophys. J. 2004, 87, 3934–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell. Endocrinol. 2020, 518, 110987. [Google Scholar] [CrossRef]
- Nakamura, N.; Mori, C.; Eddy, E.M. Molecular Complex of Three Testis-Specific Isozymes Associated with the Mouse Sperm Fibrous Sheath: Hexokinase, Phosphofructokinase M, and Glutathione S-Transferase mu class. Biol. Reprod. 2009, 82, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Young, S.A.M.; Miyata, H.; Satouh, Y.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CABYR is essential for fibrous sheath integrity and progressive motility in mouse spermatozoa. J. Cell Sci. 2016, 129, 4379. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S. Chapter 5-Gamete and Zygote Transport. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Academic Press: San Diego, CA, USA, 2015; pp. 197–232. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef] [Green Version]
- Todd Chappell, B.; Mena, L.A.; Maximos, B.; Mollan, S.; Culwell, K.; Howard, B. EVO100 Prevents Chlamydia and Gonorrhea in Women At High-Risk For Infection. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Martyn, F.; McAuliffe, F.M.; Wingfield, M. The role of the cervix in fertility: Is it time for a reappraisal? Hum. Reprod. 2014, 29, 2092–2098. [Google Scholar] [CrossRef]
- Spencer, T.E.; Dunlap, K.A.; Filant, J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol. Cell. Endocrinol. 2012, 354, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Kölle, S.; Hughes, B.; Steele, H. Early embryo-maternal communication in the oviduct: A review. Mol. Reprod. Dev. 2020, 87, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, V.K.; Krishna, A. Sperm storage in the female reproductive tract of Scotophilus heathii: Role of androgen. Mol. Reprod. Dev. 2011, 78, 477–487. [Google Scholar] [CrossRef]
- Chang, H.; Suarez, S.S. Rethinking the Relationship Between Hyperactivation and Chemotaxis in Mammalian Sperm. Biol. Reprod. 2010, 83, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedford, J. What marsupial gametes disclose about gamete function in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Rothschild. Non-random distribution of bull spermatozoa in a drop of sperm suspension. Nature 1963, 198, 1221. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Polin, M.; Goldstein, R.E. Ciliary contact interactions dominate surface scattering of swimming eukaryotes. Proc. Natl. Acad. Sci. USA 2013, 110, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Purcell, E.M. Life at low Reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Drescher, K.; Dunkel, J.; Cisneros, L.H.; Ganguly, S.; Goldstein, R.E. Fluid dynamics and noise in bacterial cell–cell and cell–surface scattering. Proc. Natl. Acad. Sci. USA 2011, 108, 10940–10945. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, K.; Gadêlha, H.; Gaffney, E.A.; Smith, D.J.; Kirkman-Brown, J. Coarse-Graining the Fluid Flow around a Human Sperm. Phys. Rev. Lett. 2017, 118, 124501. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Tang, J.X. Accumulation of microswimmers near a surface mediated by collision and rotational Brownian motion. Phys. Rev. Lett. 2009, 103, 078101. [Google Scholar] [CrossRef]
- Berke, A.P.; Turner, L.; Berg, H.C.; Lauga, E. Hydrodynamic attraction of swimming microorganisms by surfaces. Phys. Rev. Lett. 2008, 101, 038102. [Google Scholar] [CrossRef] [Green Version]
- Elgeti, J.; Kaupp, U.B.; Gompper, G. Hydrodynamics of Sperm Cells near Surfaces. Biophys. J. 2010, 99, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosrati, R.; Driouchi, A.; Yip, C.M.; Sinton, D. Two-dimensional slither swimming of sperm within a micrometre of a surface. Nat. Commun. 2015, 6, 8703. [Google Scholar] [CrossRef] [Green Version]
- Denissenko, P.; Kantsler, V.; Smith, D.J.; Kirkman-Brown, J. Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc. Natl. Acad. Sci. USA 2012, 109, 8007–8010. [Google Scholar] [CrossRef] [Green Version]
- Guidobaldi, A.; Jeyaram, Y.; Berdakin, I.; Moshchalkov, V.V.; Condat, C.A.; Marconi, V.I.; Giojalas, L.; Silhanek, A.V. Geometrical guidance and trapping transition of human sperm cells. Phys. Rev. E 2014, 89, 032720. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, R.; Graham, P.J.; Liu, Q.; Sinton, D. Predominance of sperm motion in corners. Sci. Rep. 2016, 6, 26669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, C.-K.; Ardon, F.; Fiore, A.G.; Suarez, S.S.; Wu, M. Cooperative roles of biological flow and surface topography in guiding sperm migration revealed by a microfluidic model. Lab. Chip 2014, 14, 1348. [Google Scholar] [CrossRef] [Green Version]
- Tung, C.-K.; Hu, L.; Fiore, A.G.; Hickman, D.G.; Ardon, F.; Gilbert, R.O.; Suarez, S.S.; Wu, M. Microgrooves and fluid flows provide preferential pathway to sperm over pathogen Tritrichomonas foetus. Proc. Natl. Acad. Sci. USA 2015, 112, 5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretherton, F.P.; Rothschild. Rheotaxis of Spermatozoa. Proc. R. Soc. B 1961, 153, 490–502. [Google Scholar] [CrossRef]
- Schiffer, C.; Rieger, S.; Brenker, C.; Young, S.; Hamzeh, H.; Wachten, D.; Tüttelmann, F.; Röpke, A.; Kaupp, U.B.; Wang, T.; et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling. EMBO J. 2020, 39, e102363. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife 2014, 3, e02403. [Google Scholar] [CrossRef] [Green Version]
- Tung, C.-K.; Ardon, F.; Roy, A.; Koch, D.L.; Suarez, S.S.; Wu, M. Emergence of Upstream Swimming via a Hydrodynamic Transition. Phys. Rev. Lett. 2015, 114, 108102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, J.; Meriano, J.; Ru, C.; Xie, S.; Luo, J.; Sun, Y. Human sperm rheotaxis: A passive physical process. Sci. Rep. 2016, 6, 23553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolley, D. Motility of spermatozoa at surfaces. Reproduction 2003, 126, 259–270. [Google Scholar] [CrossRef]
- Lauga, E.; DiLuzio, W.R.; Whitesides, G.M.; Stone, H.A. Swimming in circles: Motion of bacteria near solid boundaries. Biophys. J. 2006, 90, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, S.; Hamaguchi, M.S.; Naruse, M.; Ishijima, S.A.; Hamaguchi, Y. Rotational movement of a spermatozoon around its long axis. J. Exp. Biol. 1992, 163, 15–31. [Google Scholar] [CrossRef]
- Friedrich, B.M.; Riedel-Kruse, I.H.; Howard, J.; Jülicher, F. High-precision tracking of sperm swimming fine structure provides strong test of resistive force theory. J. Exp. Biol. 2010, 213, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Moreno, C.; Torres Luque, A.; Oliszewski, R.; Rosa, R.J.; Otero, M.C. Characterization of native Escherichia coli populations from bovine vagina of healthy heifers and cows with postpartum uterine disease. PLoS ONE 2020, 15, e0228294. [Google Scholar] [CrossRef] [PubMed]
- Patteson, A.E.; Gopinath, A.; Goulian, M.; Arratia, P.E. Running and tumbling with E. coli in polymeric solutions. Sci. Rep. 2015, 5, 15761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathijssen, A.J.T.M.; Figueroa-Morales, N.; Junot, G.; Clément, É.; Lindner, A.; Zöttl, A. Oscillatory surface rheotaxis of swimming E. coli bacteria. Nat. Commun. 2019, 10, 3434. [Google Scholar] [CrossRef] [Green Version]
- Gaddum-Rosse, P.; Blandau, R.J. Comparative Observations on Ciliary Currents in Mammalian Oviducts. Biol. Reprod. 1976, 14, 605–609. [Google Scholar] [CrossRef]
- Gaddum-Rosse, P.; Blandau, R.J.; Thiersch, J.B. Ciliary activity in the human and Macaca nemestrina oviduct. Am. J. Anat. 1973, 138, 269–275. [Google Scholar] [CrossRef]
- Hino, T.; Yanagimachi, R. Active peristaltic movements and fluid production of the mouse oviduct: Their roles in fluid and sperm transport and fertilization. Biol. Reprod. 2019, 101, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Usui, T.; Yamashita, M.; Kanemori, Y.; Baba, T. Surfing and Swimming of Ejaculated Sperm in the Mouse Oviduct. Biol. Reprod. 2016, 94. [Google Scholar] [CrossRef] [Green Version]
- Overstreet, J.W.; Cooper, G.W. Sperm Transport in the Reproductive Tract of the Female Rabbit: I. The Rapid Transit Phase of Transport. Biol. Reprod. 1978, 19, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.H.F.; Coy, P.; Gadea, J.; Rath, D. Considerations of viscosity in the preliminaries to mammalian fertilisation. J. Assist. Reprod. Genet. 2011, 28, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.G. The Structure and Rheology of Complex. Fluids; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Denn, M.M. Issues in Viscoelastic Fluid Mechanics. Annu. Rev. Fluid Mech. 1990, 22, 13–32. [Google Scholar] [CrossRef]
- Hyakutake, T.; Suzuki, H.; Yamamoto, S. Effect of non-Newtonian fluid properties on bovine sperm motility. J. Biomech. 2015, 48, 2941–2947. [Google Scholar] [CrossRef]
- Tuson, H.H.; Copeland, M.F.; Carey, S.; Sacotte, R.; Weibel, D.B. Flagellum Density Regulates Proteus mirabilis Swarmer Cell Motility in Viscous Environments. J. Bacteriol. 2013, 195, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Swei, J.; Talbot, J.B. Viscosity correlation for aqueous polyvinylpyrrolidone (PVP) solutions. J. Appl. Polym. Sci. 2003, 90, 1153–1155. [Google Scholar] [CrossRef]
- Van Steirteghem, A.C.; Nagy, Z.; Joris, H.; Liu, J.; Staessen, C.; Smitz, J.; Wisanto, A.; Devroey, P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum. Reprod. 1993, 8, 1061–1066. [Google Scholar] [CrossRef]
- Hyakutake, T.; Suzuki, H.; Yamamoto, S. Effect of viscosity on motion characteristics of bovine sperm. J. Aero Aqua Bio-Mech. 2015, 4, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Tung, C.-K.; Lin, C.; Harvey, B.; Fiore, A.G.; Ardon, F.; Wu, M.; Suarez, S.S. Fluid viscoelasticity promotes collective swimming of sperm. Sci. Rep. 2017, 7, 3152. [Google Scholar] [CrossRef]
- Ishimoto, K.; Gaffney, E.A. Boundary element methods for particles and microswimmers in a linear viscoelastic fluid. J. Fluid Mech. 2017, 831, 228–251. [Google Scholar] [CrossRef]
- Ishimoto, K.; Gaffney, E.A. Hydrodynamic Clustering of Human Sperm in Viscoelastic Fluids. Sci. Rep. 2018, 8, 15600. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.J.; Gaffney, E.A.; Gadêlha, H.; Kapur, N.; Kirkman-Brown, J.C. Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity. Cell Motil. 2009, 66, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S.; Dai, X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol. Reprod. 1992, 46, 686–691. [Google Scholar] [CrossRef]
- Teran, J.; Fauci, L.; Shelley, M. Viscoelastic Fluid Response Can Increase the Speed and Efficiency of a Free Swimmer. Phys. Rev. Lett. 2010, 104, 038101. [Google Scholar] [CrossRef]
- Spagnolie, S.E.; Liu, B.; Powers, T.R. Locomotion of Helical Bodies in Viscoelastic Fluids: Enhanced Swimming at Large Helical Amplitudes. Phys. Rev. Lett. 2013, 111, 068101. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, J.K.; Lynch, S.; Barrett, A.; Fauci, L.; Cortez, R. Enhanced flagellar swimming through a compliant viscoelastic network in Stokes flow. J. Fluid Mech. 2016, 792, 775–797. [Google Scholar] [CrossRef]
- Woolley, D.M.; Crockett, R.F.; Groom, W.D.I.; Revell, S.G. A study of synchronisation between the flagella of bull spermatozoa, with related observations. J. Exp. Biol. 2009, 212, 2215–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, E.; Turgut, A.E.; Dorigo, M.; Huepe, C. Elasticity-Based Mechanism for the Collective Motion of Self-Propelled Particles with Springlike Interactions: A Model System for Natural and Artificial Swarms. Phys. Rev. Lett. 2013, 111, 268302. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, S.F.; Holt, W.V.; Keaveny, E.E. Collective dynamics of sperm cells. Philos. Trans. R. Soc. B: Biol. Sci. 2020, 375, 20190384. [Google Scholar] [CrossRef]
- Rothschild, L. The Activity of Ram Spermatozoa. J. Exp. Biol. 1948, 25, 219. [Google Scholar] [CrossRef]
- David, I.; Kohnke, P.; Lagriffoul, G.; Praud, O.; Plouarboué, F.; Degond, P.; Druart, X. Mass sperm motility is associated with fertility in sheep. Anim. Reprod. Sci. 2015, 161, 75–81. [Google Scholar] [CrossRef]
- ELzanaty, S.; Malm, J.; Giwercman, A. Visco-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. Int. J. Androl. 2004, 27, 94–100. [Google Scholar] [CrossRef]
- Creppy, A.; Plouraboué, F.; Praud, O.; Druart, X.; Cazin, S.; Yu, H.; Degond, P. Symmetry-breaking phase transitions in highly concentrated semen. J. R. Soc. Interface 2016, 13, 20160575. [Google Scholar] [CrossRef] [PubMed]
- Vicsek, T.; Czirók, A.; Ben-Jacob, E.; Cohen, I.; Shochet, O. Novel Type of Phase Transition in a System of Self-Driven Particles. Phys. Rev. Lett. 1995, 75, 1226–1229. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.; Tu, Y. Long-Range Order in a Two-Dimensional Dynamical XY Model: How Birds Fly Together. Phys. Rev. Lett. 1995, 75, 4326–4329. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Dvorakova, K.; Jenkins, N.; Breed, W. Exceptional sperm cooperation in the wood mouse. Nature 2002, 418, 174–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, H.S.; Hoekstra, H.E. Competition drives cooperation among closely related sperm of deer mice. Nature 2010, 463, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Immler, S.; Moore, H.D.M.; Breed, W.G.; Birkhead, T.R. By Hook or by Crook? Morphometry, Competition and Cooperation in Rodent Sperm. PLoS ONE 2007, 2, e170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourmente, M.; Zarka-Trigo, D.; Roldan, E.R.S. Is the hook of muroid rodent’s sperm related to sperm train formation? J. Evol. Biol. 2016, 29, 1168–1177. [Google Scholar] [CrossRef] [Green Version]
- Martan, J.; Shepherd, B.A. Spermatozoa in rouleaux in the female guinea pig genital tract. Anat. Rec. 1973, 175, 625–629. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Mahi, C.A. The sperm acrosome reaction and fertilization in the guinea-pig: A study in vivo. Reproduction 1976, 46, 49. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, S.P.; Swann, N.J.; Primakoff, P.; Myles, D.G. A Role for the WH-30 Protein in Sperm-Sperm Adhesion during Rouleaux Formation in the Guinea Pig. Dev. Biol. 1993, 156, 243–252. [Google Scholar] [CrossRef]
- Rodger, J.C.; Bedford, J.M. Separation of sperm pairs and sperm—Egg interaction in the opossum, Didelphis virginiana. Reproduction 1982, 64, 171. [Google Scholar] [CrossRef] [Green Version]
- Moore, H. Gamete biology of the new world marsupial, the grey short-tailed opossum, Monodelphis domestica. Reprod. Fertil. Dev. 1996, 8, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.D.M.; Taggart, D.A. Sperm Pairing in the Opossum Increases the Efficiency of Sperm Movement in a Viscous Environment1. Biol. Reprod. 1995, 52, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.A.; O’Brien, H.P.; Moore, H.D.M. Ultrastructural characteristics of in vivo and in vitro fertilization in the grey short-tailed opossum, Monodelphis domestica. Anat. Rec. 1993, 237, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kessel, R.; Kardon, R. Tissues and Organs: A Text.-Atlas of Scanning Electron. Microscopy; W. H. Freeman and Co.: San Francisco, CA, USA, 1979. [Google Scholar]
- Roberts, S.J. Veterinary Obstetrics and Genital Diseases (Theriogenology), 3rd ed.; Roberts, S.J., Ed.; David and Charles: Woodstock, VT, USA; North Pomfret, VT, USA; Pomfret, VT, USA, 1986. [Google Scholar]
- Katz, D.F.; Morales, P.; Samuels, S.J.; Overstreet, J.W. Mechanisms of filtration of morphologically abnormal human sperm by cervical mucus. Fertil. Steril. 1990, 54, 513–516. [Google Scholar] [CrossRef]
- Mortimer, D.; Leslie, E.E.; Kelly, R.W.; Templeton, A.A. Morphological selection of human spermatozoa in vivo and in vitro. J. Reprod. Fertil. 1982, 64, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, E.; Franken, D.R.; Wet, J.D.; Grobler, S. Sperm Selection Capacity of Cervical Mucus. Arch. Androl. 1984, 12, 5–7. [Google Scholar] [CrossRef]
- Bergman, A.; Amit, A.; Yedwab, G.; David, M.P.; Homonnai, Z.T.; Paz, G.F. Filtering capacity of bovine cervical mucus towards abnormal forms of human-ejaculated spermatozoa. Int. J. Androl. 1981, 4, 675–684. [Google Scholar] [CrossRef]
- Bianchi, P.G.; De Agostini, A.; Fournier, J.; Guidetti, C.; Tarozzi, N.; Bizzaro, D.; Manicardi, G.C. Human Cervical Mucus Can Act in Vitro as a Selective Barrier Against Spermatozoa Carrying Fragmented DNA and Chromatin Structural Abnormalities. J. Assist. Reprod. Genet. 2004, 21, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, K.; McGettigan, P.A.; Reid, C.J.; Browne, J.A.; Irwin, J.A.; Tharmalingam, T.; Corfield, A.; Baird, A.; Loftus, B.J.; Evans, A.C.O.; et al. Molecular aspects of mucin biosynthesis and mucus formation in the bovine cervix during the periestrous period. Physiol. Genom. 2012, 44, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Fléchon, J.E.; Hunter, R.H.F. Distribution of spermatozoa in the utero-tubal junction and isthmus of pigs, and their relationship with the luminal epithelium after mating: A scanning electron microscope study. Tissue Cell 1981, 13, 127–139. [Google Scholar] [CrossRef]
- Gaddum-Rosse, P. Some observations on sperm transport through the uterotubal junction of the rat. Am. J. Anat. 1981, 160, 333–341. [Google Scholar] [CrossRef]
- Nakanishi, T.; Isotani, A.; Yamaguchi, R.; Ikawa, M.; Baba, T.; Suarez, S.S.; Okabe, M. Selective Passage Through the Uterotubal Junction of Sperm from a Mixed Population Produced by Chimeras of Calmegin-Knockout and Wild-Type Male Mice. Biol. Reprod. 2004, 71, 957, 959–965. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, Q.; Guo, S.; Ma, C.; Lu, Y.; Shi, J.; Liu, S.; Zhou, T.; Noda, T.; Qian, J.; et al. Cooperation-based sperm clusters mediate sperm oviduct entry and fertilization. Protein Cell 2021. [Google Scholar] [CrossRef]
- Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arús, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020, 12, 1123–1130. [Google Scholar] [CrossRef]
- Kamphuis, E.I.; Bhattacharya, S.; van der Veen, F.; Mol, B.W.J.; Templeton, A. Are we overusing IVF? BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ombelet, W.; Martens, G.; Bruckers, L. Pregnant after assisted reproduction: A risk pregnancy is born! 18-years perinatal outcome results from a population-based registry in Flanders, Belgium. Facts Views Vis. Obgyn 2016, 8, 193–204. [Google Scholar] [PubMed]
- Aitken, R.J.; Clarkson, J.S. Significance of Reactive Oxygen Species and Antioxidants in Defining the Efficacy of Sperm Preparation Techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Cambi, M.; Boni, L.; Iorio, A.L.; Passaro, C.; Luppino, B.; Nadalini, M.; Marchiani, S.; Tamburrino, L.; et al. Variation of DNA Fragmentation Levels During Density Gradient Sperm Selection for Assisted Reproduction Techniques: A Possible New Male Predictive Parameter of Pregnancy? Medicine 2016, 95, e3624. [Google Scholar] [CrossRef]
- Jafek, A.; Feng, H.; Brady, H.; Petersen, K.; Chaharlang, M.; Aston, K.; Gale, B.; Jenkins, T.; Samuel, R. An automated instrument for intrauterine insemination sperm preparation. Sci. Rep. 2020, 10, 21385. [Google Scholar] [CrossRef]
- Schuster, T.G.; Cho, B.; Keller, L.M.; Takayama, S.; Smith, G.D. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod. Biomed. Online 2003, 7, 75–81. [Google Scholar] [CrossRef]
- Seo, D.B.; Agca, Y.; Feng, Z.C.; Critser, J.K. Development of sorting, aligning, and orienting motile sperm using microfluidic device operated by hydrostatic pressure. Microfluid. Nanofluidics 2007, 3, 561–570. [Google Scholar] [CrossRef]
- Zaferani, M.; Palermo, G.D.; Abbaspourrad, A. Strictures of a microchannel impose fierce competition to select for highly motile sperm. Sci. Adv. 2019, 5, eaav2111. [Google Scholar] [CrossRef] [Green Version]
- Oseguera-López, I.; Ruiz-Díaz, S.; Ramos-Ibeas, P.; Pérez-Cerezales, S. Novel Techniques of Sperm Selection for Improving IVF and ICSI Outcomes. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef]
- Nosrati, R.; Vollmer, M.; Eamer, L.; San Gabriel, M.C.; Zeidan, K.; Zini, A.; Sinton, D. Rapid selection of sperm with high DNA integrity. Lab. Chip 2014, 14, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Riordon, J.; Tarlan, F.; You, J.B.; Zhang, B.; Graham, P.J.; Kong, T.; Wang, Y.; Lagunov, A.; Hannam, T.; Jarvi, K.; et al. Two-dimensional planar swimming selects for high DNA integrity sperm. Lab. Chip 2019, 19, 2161–2167. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, C.-K.; Suarez, S.S. Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells 2021, 10, 1297. https://doi.org/10.3390/cells10061297
Tung C-K, Suarez SS. Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells. 2021; 10(6):1297. https://doi.org/10.3390/cells10061297
Chicago/Turabian StyleTung, Chih-Kuan, and Susan S. Suarez. 2021. "Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization" Cells 10, no. 6: 1297. https://doi.org/10.3390/cells10061297
APA StyleTung, C.-K., & Suarez, S. S. (2021). Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells, 10(6), 1297. https://doi.org/10.3390/cells10061297





