A Simple, Accurate and Cost-Effective Capillary Electrophoresis Test with Computational Methods to Aid in Universal Microsatellite Instability Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. Patient Samples
2.3. Immunohistochemistry Analysis
2.4. DNA Extraction and Quantitation
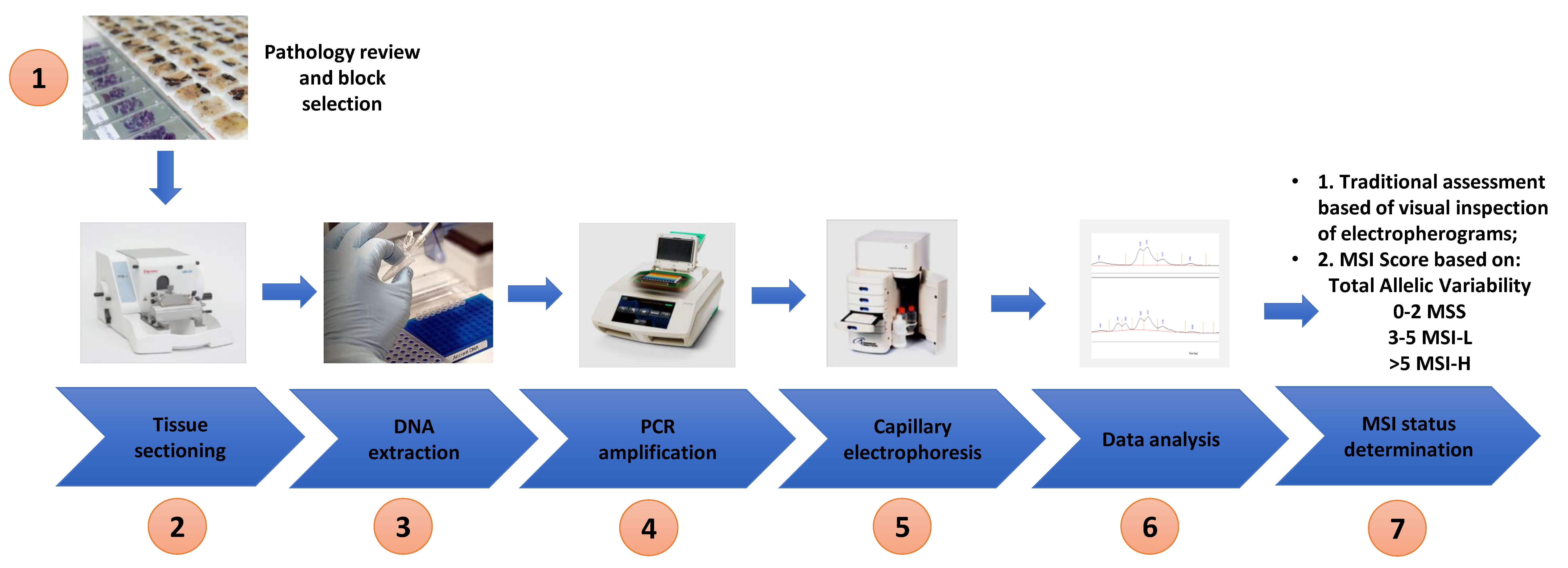
2.5. PCR Amplification and Capillary Electrophoresis Detection of MSI
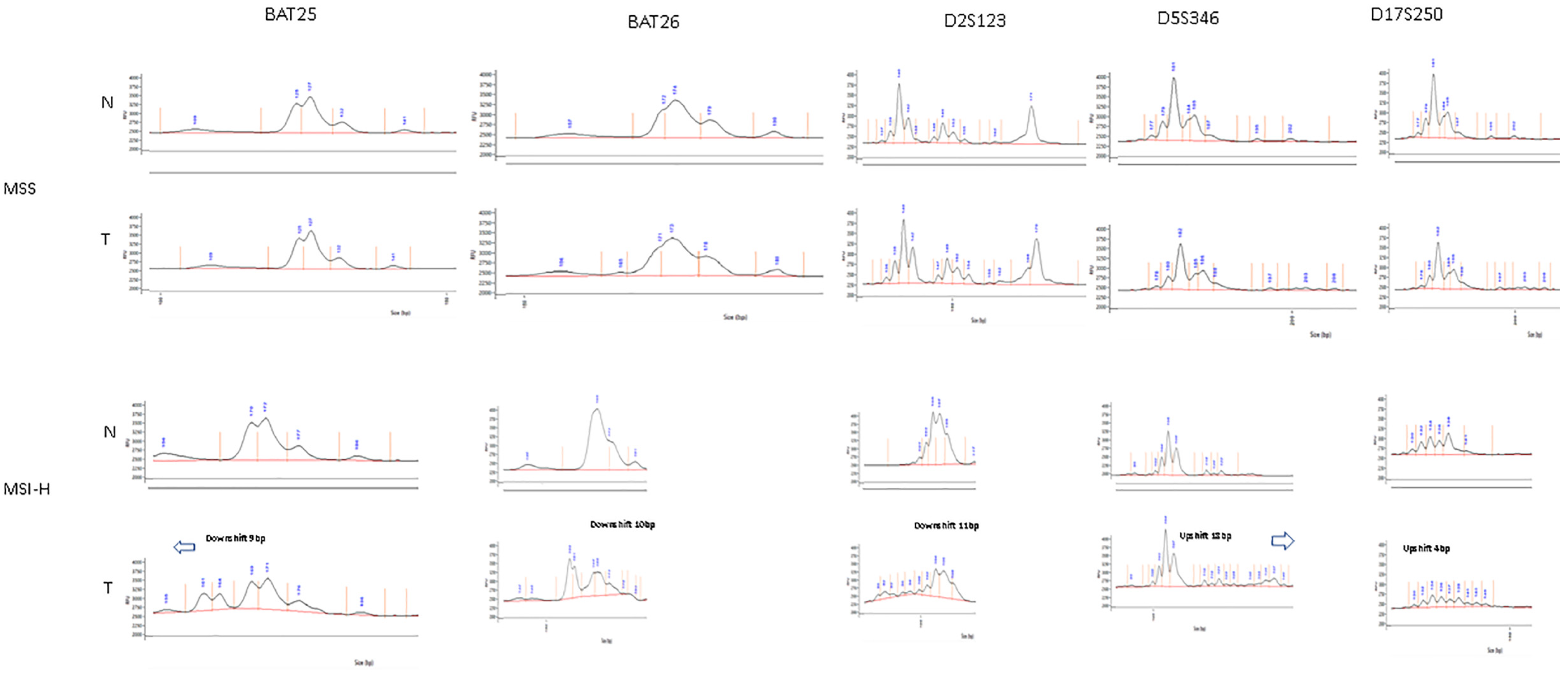
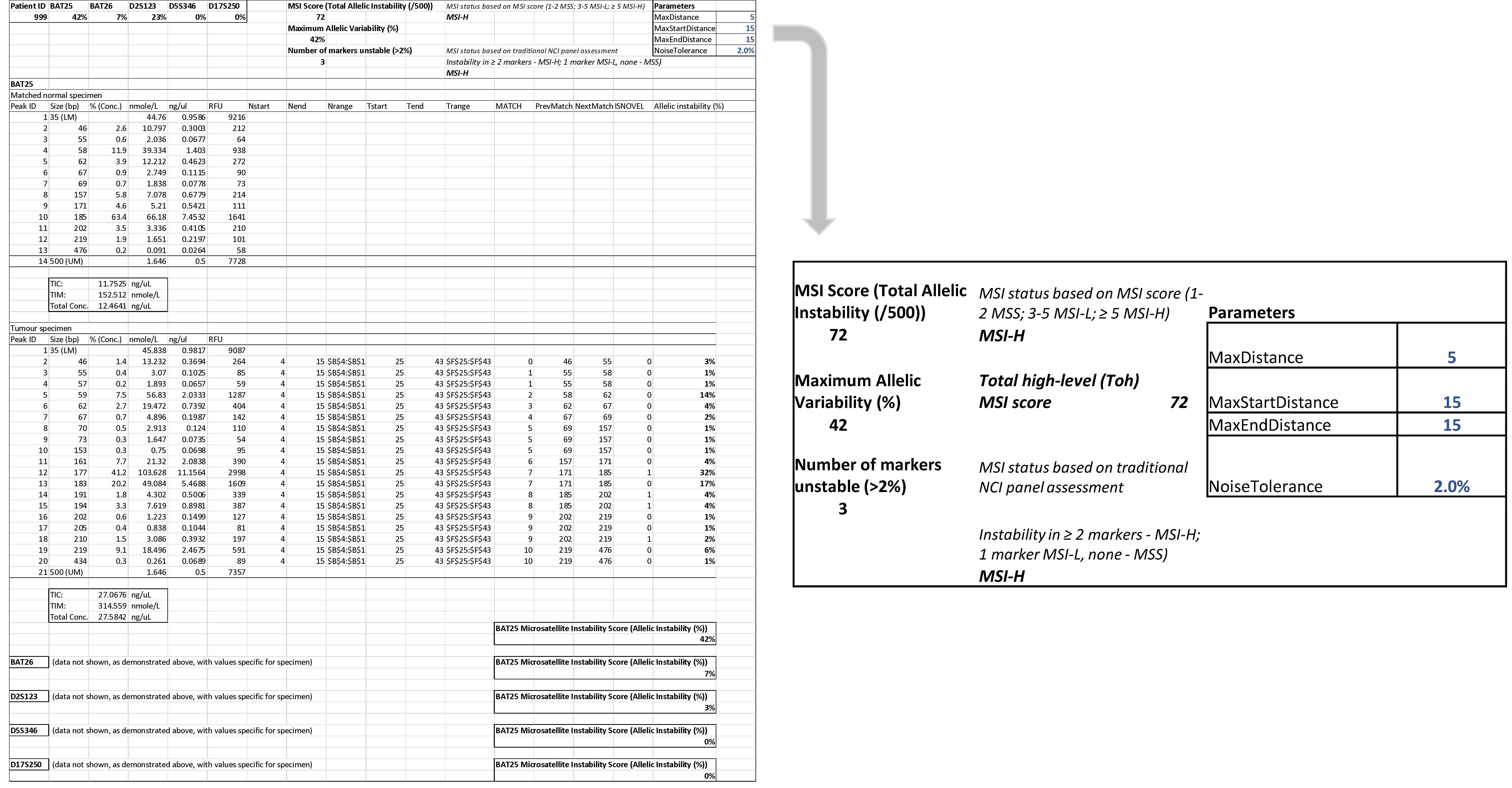
2.6. Microsatellite Analysis
2.7. Statistical Analysis
3. Results
3.1. MSI Assessment of Colorectal Cancer Cell Lines
3.2. Limit of Detection of MSI
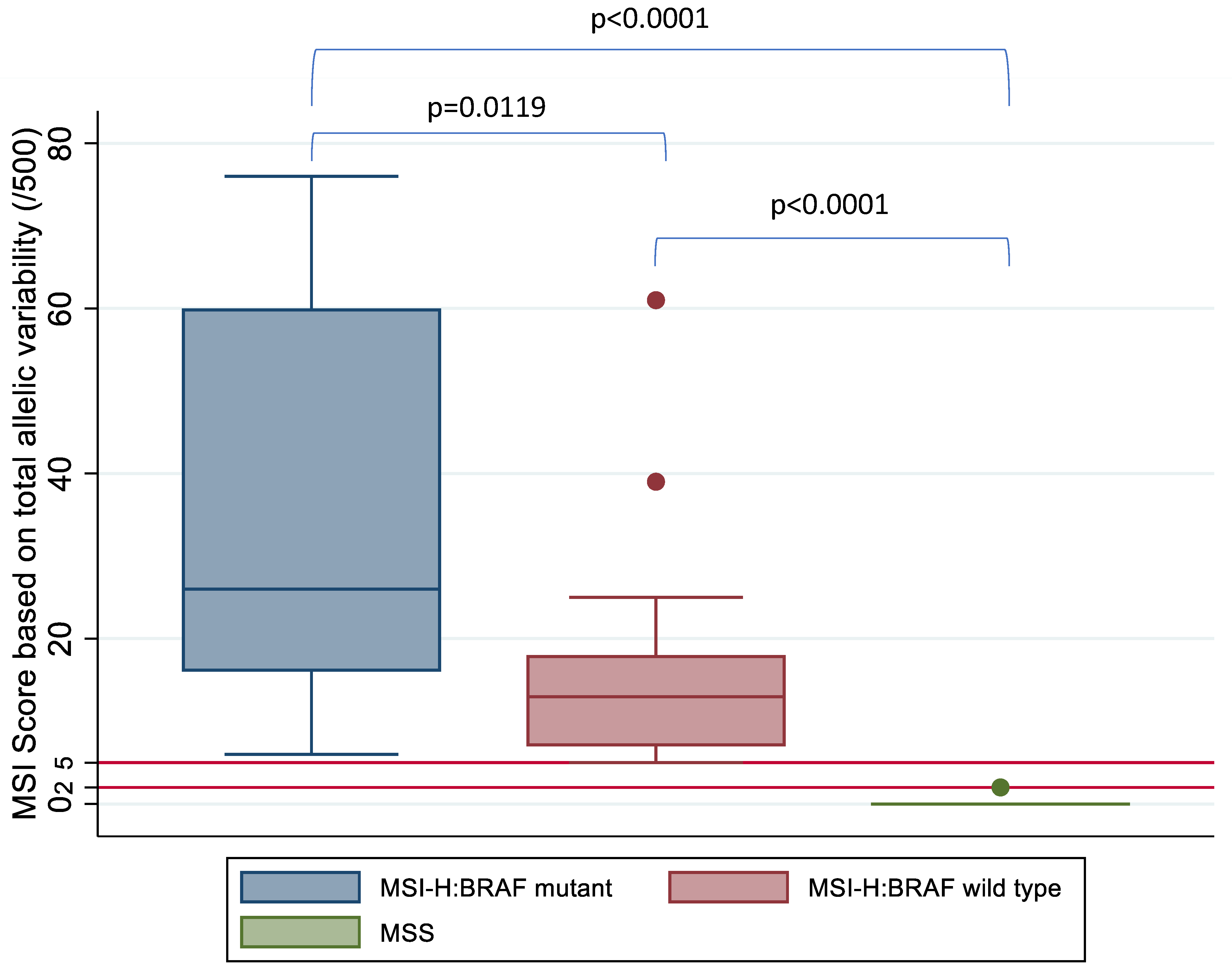
3.3. Patient Tumour Specimens
3.4. Analysis of Discordant IHC and DNA Based MSI Status
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaltonen, L.A.; Salovaara, R.; Kristo, P.; Canzian, F.; Hemminki, A.; Peltomaki, P.; Chadwick, R.B.; Kaariainen, H.; Eskelinen, M.; Jarvinen, H.; et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N. Engl. J. Med. 1998, 338, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. NICE Recommends Wider Use of Tests to Detect Cancer-Causing Genetic Condition; National Institute for Health and Care Excellence: London, UK, 2016. [Google Scholar]
- Koenig, J.L.; Toesca, D.A.S.; Harris, J.P.; Tsai, C.J.; Haraldsdottir, S.; Lin, A.Y.; Pollom, E.L.; Chang, D.T. Microsatellite Instability and Adjuvant Chemotherapy in Stage II Colon Cancer. Am. J. Clin. Oncol. 2019, 42, 573–580. [Google Scholar] [CrossRef]
- Des Guetz, G.; Schischmanoff, O.; Nicolas, P.; Perret, G.Y.; Morere, J.F.; Uzzan, B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur. J. Cancer (Oxf. Engl. 1990) 2009, 45, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Mahajan, H.; Chapuis, P.; Spring, K. Current status on microsatellite instability, prognosis and adjuvant therapy in colon cancer: A nationwide survey of medical oncologists, colorectal surgeons and gastrointestinal pathologists. Cancer Rep. 2021, 4, e1297. [Google Scholar] [CrossRef]
- Webber, E.M.; Kauffman, T.L.; O’Connor, E.; Goddard, K.A. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 2015, 15, 156. [Google Scholar] [CrossRef] [Green Version]
- Romiti, A.; Rulli, E.; Pilozzi, E.; Gerardi, C.; Roberto, M.; Legramandi, L.; Falcone, R.; Pacchetti, I.; Marchetti, P.; Floriani, I. Exploring the Prognostic Role of Microsatellite Instability in Patients with Stage II Colorectal Cancer: A Systematic Review and Meta-Analysis. Available online: http://www.journals.elsevier.com/clinical-colorectal-cancer; http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emex&NEWS=N&AN=613354522 (accessed on 30 April 2021).
- Elsaleh, H.; Joseph, D.; Grieu, F.; Zeps, N.; Spry, N.; Iacopetta, B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000, 355, 1745–1750. [Google Scholar] [CrossRef]
- Chan, G.H.J.; Chee, C.E. Making sense of adjuvant chemotherapy in colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1183–1192. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Niedzwiecki, D.; Compton, C.C.; Hahn, H.P.; Hall, M.; Damas, B.; Jewell, S.D.; Mayer, R.J.; Goldberg, R.M.; Saltz, L.B.; et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Kim, J.Y.; Kim, Y.H.; Chang, D.K.; Rhee, P.L.; et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother. Pharmacol. 2010, 66, 659–667. [Google Scholar] [CrossRef]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandala, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. ESMO 2013, 24 (Suppl. 6), 64–72. [Google Scholar] [CrossRef]
- ESMO Guidelines Committee eUpdate Early Colon Cancer Treatment Recommendations. 2019. Available online: https://www.esmo.org/guidelines/gastrointestinal-cancers/early-colon-cancer/eupdate-early-colon-cancer-treatment-recommendations (accessed on 30 April 2021).
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Guastadisegni, C.; Colafranceschi, M.; Ottini, L.; Dogliotti, E. Microsatellite instability as a marker of prognosis and response to therapy: A meta-analysis of colorectal cancer survival data. Eur. J. Cancer 2010, 46, 2788–2798. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Phan, K.; Reza, F.; Chapuis, P.; Spring, K.J. Rate of dissemination and prognosis in early and advanced stage colorectal cancer based on microsatellite instability status: Systematic review and meta-analysis. Int. J. Colorectal Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Lim, S.H.; MacKenzie, S.; de Souza, P.; Bokey, L.; Chapuis, P.; Spring, K.J. Association Between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer. Cells 2020, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Toh, J.W.; de Souza, P.; Lim, S.H.; Singh, P.; Chua, W.; Ng, W.; Spring, K.J. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin. Colorectal Cancer 2016, 15, 285–291. [Google Scholar] [CrossRef]
- Michael, J.; Overman, S.K.; Raymond, S.M.; Leach, J.; Lonardi, S.; Lenz, H.; Michael, A.; Morse, J.D.; Andrew, H.; Michael, D.; et al. A Study of Nivolumab and Nivolumab Plus Ipilimumab in Recurrent and Metastatic Colon Cancer (CheckMate 142). J. Clin. Oncol. 2016, 34, 2016. [Google Scholar]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, R.; Sidransky, D.; Eshleman, R.; Burt, W.; Meltzer, J.; Rodriguez-Bigas, A.; Fodde, R.; Ranzani, N.; et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; McClain, M.R.; Melillo, S.; Hampel, H.L.; Thibodeau, S.N. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet. Med. Off. J. Am. Coll. Med Genet. 2009, 11, 42–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacher, J.W.; Flanagan, L.A.; Smalley, R.L.; Nassif, N.A.; Burgart, L.J.; Halberg, R.B.; Megid, W.M.; Thibodeau, S.N. Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis. Markers 2004, 20, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Cheah, P.L.; Li, J.; Looi, L.M.; Koh, C.C.; Lau, T.P.; Chang, S.W.; Teoh, K.H.; Mun, K.S.; Nazarina, A.R. Screening for microsatellite instability in colorectal carcinoma: Practical utility of immunohistochemistry and PCR with fragment analysis in a diagnostic histopathology setting. Malays. J. Pathol. 2019, 41, 91–100. [Google Scholar] [PubMed]
- Arulananda, S.; Thapa, B.; Walkiewicz, M.; Zapparoli, G.V.; Williams, D.S.; Dobrovic, A.; John, T. Mismatch Repair Protein Defects and Microsatellite Instability in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, A.I.; Haghighi, M.M.; Zadeh, S.M.; Fatemi, S.R.; Taleghani, M.Y.; Zali, N.; Akbari, Z.; Kashfi, S.M.; Zali, M.R. Simplified MSI marker panel for diagnosis of colorectal cancer. Asian Pac. J. Cancer Prev. APJCP 2011, 12, 2101–2104. [Google Scholar]
- Murphy, K.M.; Zhang, S.; Geiger, T.; Hafez, M.J.; Bacher, J.; Berg, K.D.; Eshleman, J.R. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J. Mol. Diagn. JMD 2006, 8, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Nagasaka, T.; Hamelin, R.; Boland, C.R. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS ONE 2010, 5, e9393. [Google Scholar] [CrossRef]
- Buhard, O.; Suraweera, N.; Lectard, A.; Duval, A.; Hamelin, R. Quasimonomorphic mononucleotide repeats for high-level microsatellite instability analysis. Dis. Markers 2004, 20, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umetani, N.; Sasaki, S.; Watanabe, T.; Ishigami, H.; Ueda, E.; Nagawa, H. Diagnostic primer sets for microsatellite instability optimized for a minimal amount of damaged DNA from colorectal tissue samples. Ann. Surg. Oncol. 2000, 7, 276–280. [Google Scholar] [CrossRef]
- Toh, J.; Chapuis, P.H.; Bokey, L.; Chan, C.; Spring, K.J.; Dent, O.F. Competing risks analysis of microsatellite instability as a prognostic factor in colorectal cancer. Br. J. Surg. 2017, 104, 1250–1259. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Hain, E.; Buhard, O.; Guilloux, A.; Bardier, A.; Kaci, R.; Bertheau, P.; Renaud, F.; Bibeau, F.; Fléjou, J.F.; et al. Association of Primary Resistance to Immune Checkpoint Inhibitors in Metastatic Colorectal Cancer with Misdiagnosis of Microsatellite Instability or Mismatch Repair Deficiency Status. JAMA Oncol. 2019, 5, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Analytical, A. Impact of Accurate Qualification and Quantification of Fragments; MipTec: Basel, Switzerland, 2013. [Google Scholar]
- Cheng, D.T.; Prasad, M.; Chekaluk, Y.; Benayed, R.; Sadowska, J.; Zehir, A.; Syed, A.; Wang, Y.E.; Somar, J.; Li, Y.; et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med. Genom. 2017, 10, 33. [Google Scholar] [CrossRef]
- Waalkes, A.; Smith, N.; Penewit, K.; Hempelmann, J.; Konnick, E.Q.; Hause, R.J.; Pritchard, C.C.; Salipante, S.J. Accurate Pan-Cancer Molecular Diagnosis of Microsatellite Instability by Single-Molecule Molecular Inversion Probe Capture and High-Throughput Sequencing. Clin. Chem. 2018, 64, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Kautto, E.A.; Bonneville, R.; Miya, J.; Yu, L.; Krook, M.A.; Reeser, J.W.; Roychowdhury, S. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget 2017, 8, 7452–7463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]

| MSS | % | MSI-H BRAF Mutant | % | MSI-H BRAF Normal | % | |
|---|---|---|---|---|---|---|
| n | 30 | 19 | 23 | |||
| F | 5 | 17% | 9 | 47% | 12 | 52% |
| M | 25 | 83% | 10 | 53% | 11 | 48% |
| ASA (mean, SD) | 2.2, 0.9 | 3, 1 | 2, 1 | |||
| Age (mean, SD) | 68, 14 | 80, 7 | 67, 16 | |||
| Age (median) | 66 | 81 | 66 | |||
| Liver metastases | 3 | 10% | 2 | 11% | 2 | 9% |
| Lung metastases | 3 | 10% | 2 | 11% | 2 | 9% |
| Brain metastases | 3 | 10% | 2 | 11% | 2 | 9% |
| Nodal metastases | 3 | 10% | 2 | 11% | 2 | 9% |
| Stage I | 10 | 33% | 2 | 11% | 8 | 35% |
| Stage II | 12 | 40% | 10 | 53% | 9 | 39% |
| Stage III | 5 | 17% | 6 | 32% | 4 | 17% |
| Stage IV | 3 | 10% | 1 | 5% | 2 | 9% |
| Caecum | 2 | 7% | 4 | 21% | 2 | 9% |
| Ascending Colon | 1 | 3% | 1 | 5% | 5 | 22% |
| Hepatic Flexure | 0 | 0% | 1 | 5% | 1 | 4% |
| Transverse Colon | 4 | 13% | 6 | 32% | 3 | 13% |
| Splenic Flexure | 1 | 3% | 0 | 0% | 1 | 4% |
| Descending Colon | 0 | 0% | 2 | 11% | 1 | 4% |
| Sigmoid Colon | 12 | 40% | 3 | 16% | 1 | 4% |
| Rectum | 10 | 33% | 2 | 11% | 9 | 39% |
| Total Right | 7 | 23% | 12 | 63% | 11 | 48% |
| Total Left | 23 | 77% | 7 | 37% | 12 | 52% |
| TIL present | 1 | 3% | 12 | 63% | 8 | 35% |
| TIL inconspicuous | 29 | 97% | 7 | 37% | 15 | 65% |
| Tumour size (mean, cm) | 4.6 | 4.6 | 4.4 | |||
| Low grade | 1 | 3% | 0 | 0% | 0 | 0% |
| Moderate grade | 26 | 87% | 13 | 68% | 18 | 78% |
| High grade | 3 | 10% | 6 | 32% | 5 | 22% |
| Poorly differentiated | 3 | 10% | 5 | 26% | 5 | 22% |
| Name | Primer Sequence 5′ to 3′ | TM (°C) |
|---|---|---|
| BAT-25 | 5′-TCGCCTCCAAGAATGTAAGT-3′ (F) | 57.1 |
| 5′-TCTGCATTTTAACTATGGCTC-3′ (R) | 54.5 | |
| BAT-26 | 5′-TGACTACTTTTGACTTCAGCC-3′ (F) | 54.4 |
| 5′-AACCATTCAACATTTTTAACCC-3′ (R) | 56.8 | |
| D5S346 | 5′-TACTCACTCTAGTGATAAATCGG-3 (F) | 56.3 |
| 5′-TTCAGGGAATTGAGAGTTACAG-3′ (R) | 52.2 | |
| D2S123 | 5′-GCCAGAGAAATTAGACACAGTG-3′ (F) | 52.8 |
| 5′-CTGACTTGGATACCATCTATCTA-3′ (R) | 55.8 | |
| D17S250 | 5′-AATAGACAATAAAAATATGTGTGTG-3′ (F) | 52 |
| 5′-TATATATTTAAACCATTTGAAAGTG-3′ (R) | 51.7 |
| Colorectal Cancer Cell Lines | Expected MSI Status | Experimental MSI Status | ||
|---|---|---|---|---|
| SW480 | MSS | MSS | Sensitivity | 100% |
| SW620 | MSS | MSS | Specificity | 100% |
| SW1222 | MSS | MSS | PPV | 100% |
| HT29 | MSS | MSS | NPV | 100% |
| LS174T | MSI-H | MSI-H | ||
| RKO | MSI-H | MSI-H | ||
| LISP1 | MSI-H | MSI-H | ||
| DLD1 | MSI-H | MSI-H | ||
| LOVO | MSI-H | MSI-H | ||
| HT116 | MSI-H | MSI-H | ||
| LIM1215 | MSI-H | MSI-H | ||
| LIM2033 | MSI-H | MSI-H |
| ID | MSI Status Based on IHC | BAT-25 | BAT-26 | D5S346 | D2S123 | D17S250 | † No. of NCI (/5) Markers >2% Instability in ≥2 Markers: MSI-H; 1 Marker MSI-L; None: MSS | MSI Status | Maximum Allelic Variability (/100) | MSI Score Based on Total Allelic Variability (/500) | ‡ MSI Status Based on Total Allelic Variability (MSI Score = 1–2 MSS; 3–5 MSI-L; ≥5 MSI-H) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% RKO | MSI-H 100% | 7% | 0% | 36% | 0% | 13% | 3 | MSI-H | 36% | 56 | MSI-H |
| 80% RKO | MSI-H 80% | 42% | 7% | 23% | 0% | 0% | 3 | MSI-H | 42% | 73 | MSI-H |
| 60% RKO | MSI-H 60% | 11% | 0% | 21% | 6% | 8% | 4 | MSI-H | 21% | 46 | MSI-H |
| 40% RKO | MSI-H 40% | 11% | 0% | 18% | 0% | 22% | 3 | MSI-H | 22% | 51 | MSI-H |
| 20% RKO | MSI-H 20% | 6% | 0% | 17% | 0% | 35% | 3 | MSI-H | 35% | 58 | MSI-H |
| 10% RKO | MSI-H 10% | 0% | 5% | 3% | 0% | 17% | 3 | MSI-H | 17% | 25 | MSI-H |
| 5% RKO | MSI-H 5% | 10% | 0% | 0% | 0% | 18% | 2 | MSI-H | 18% | 29 | MSI-H |
| ID | MSI Status Based on IHC | BAT-25 | BAT-26 | D5S346 | D2S123 | D17S250 | † No. of NCI (/5) Markers >2% Instability in ≥2 Markers: MSI-H; 1 Marker MSI-L; None: MSS | MSI Status | Maximum Allelic Variability (/100) | ‡ MSI Score Based on Total Allelic Variability (/500) | MSI Status Based on Total Allelic Variability (MSI Score = 1–2 MSS; 3–5 MSI-L; ≥5 MSI-H) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID 14 | MSI-H BRAF mutant | 4% | 14% | 0% | 10% | 3% | 4 | MSI-H | 14% | 31 | MSI-H |
| ID 24 | MSI-H BRAF mutant | 24% | 36% | 0% | 0% | 0% | 2 | MSI-H | 36% | 61 | MSI-H |
| ID 25 | MSI-H BRAF mutant | 7% | 20% | 0% | 0% | 0% | 2 | MSI-H | 20% | 26 | MSI-H |
| ID 29 | MSI-H BRAF mutant | 2% | 4% | 0% | 0% | 0% | 1 | MSI-L * | 4% | 6 | MSI-H |
| ID 31 | MSI-H BRAF mutant | 16% | 41% | 0% | 0% | 3% | 3 | MSI-H | 41% | 60 | MSI-H |
| ID 32 | MSI-H BRAF mutant | 5% | 0% | 3% | 0% | 0% | 2 | MSI-H | 5% | 8 | MSI-H |
| ID 34 | MSI-H BRAF mutant | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 35 | MSI-H BRAF mutant | 11% | 0% | 9% | 0% | 0% | 2 | MSI-H | 11% | 20 | MSI-H |
| ID 38 | MSI-H BRAF mutant | 13% | 34% | 12% | 0% | 0% | 3 | MSI-H | 34% | 59 | MSI-H |
| ID 42 | MSI-H BRAF mutant | 16% | 28% | 9% | 5% | 0% | 4 | MSI-H | 28% | 58 | MSI-H |
| ID 47 | MSI-H BRAF mutant | 25% | 38% | 9% | 0% | 4% | 4 | MSI-H | 38% | 76 | MSI-H |
| ID 52 | MSI-H BRAF mutant | 0% | 11% | 0% | 0% | 5% | 2 | MSI-H | 11% | 16 | MSI-H |
| ID 55 | MSI-H BRAF mutant | 7% | 54% | 3% | 0% | 8% | 4 | MSI-H | 54% | 71 | MSI-H |
| ID 45 | MSI-H BRAF mutant | 0% | 5% | 0% | 2% | 0% | 1 | MSI-L * | 5% | 7 | MSI-H |
| ID 142 | MSI-H BRAF mutant | 3% | 7% | 5% | 0% | 7% | 3 | MSI-H | 7% | 21 | MSI-H |
| ID 252 | MSI-H BRAF mutant | 0% | 8% | 7% | 0% | 0% | 2 | MSI-H | 8% | 14 | MSI-H |
| ID 422 | MSI-H BRAF mutant | 0% | 0% | 0% | 67% | 0% | 1 | MSI-L * | 67% | 67 | MSI-H |
| ID 242 | MSI-H BRAF mutant | 2% | 12% | 6% | 0% | 0% | 3 | MSI-H | 12% | 20 | MSI-H |
| ID | MSI Status Based on IHC | BAT-25 | BAT-26 | D5S346 | D2S123 | D17S250 | † No. of NCI (/5) Markers >2% Instability in ≥2 Markers: MSI-H; 1 Marker MSI-L; None: MSS | MSI Status | Maximum Allelic Variability (/100) | ‡ MSI Score Based on Total Allelic Variability (/500) | MSI Status Based on Total Allelic Variability (MSI Score = 1–2 MSS; 3–5 MSI-L; >5 MSI-H) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID 1 | MSI-H BRAF wild type | 12% | 5% | 4% | 0% | 5% | 4 | MSI-H | 12% | 25 | MSI-H |
| ID 2 | MSI-H BRAF wild type | 5% | 6% | 0% | 0% | 2% | 2 | MSI-H | 6% | 13 | MSI-H |
| ID 8 | MSI-H BRAF wild type | 24% | 22% | 9% | 0% | 6% | 4 | MSI-H | 24% | 61 | MSI-H |
| ID 9 | MSI-H BRAF wild type | 4% | 0% | 0% | 0% | 9% | 2 | MSI-H | 9% | 13 | MSI-H |
| ID 16 | MSI-H BRAF wild type | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 17 | MSI-H BRAF wild type | 0% | 0% | 0% | 6% | 0% | 1 | MSI-L * | 6% | 6 | MSI-H |
| ID 18 | MSI-H BRAF wild type | 0% | 4% | 3% | 0% | 0% | 2 | MSI-H | 4% | 7 | MSI-H |
| ID 27 | MSI-H BRAF wild type | 0% | 5% | 0% | 0% | 0% | 1 | MSI-L | 5% | 5 | MSI-L |
| ID 40 | MSI-H BRAF wild type | 2% | 0% | 3% | 0% | 0% | 1 | MSI-L * | 3% | 5 | MSI-L |
| ID 46 | MSI-H BRAF wild type | 4% | 0% | 0% | 0% | 4% | 2 | MSI-H | 4% | 8 | MSI-H |
| ID 56 | MSI-H BRAF wild type | 3% | 0% | 2% | 0% | 0% | 1 | MSI-L * | 3% | 5 | MSI-L |
| ID 58 | MSI-H BRAF wild type | 0% | 5% | 0% | 4% | 5% | 3 | MSI-H | 5% | 13 | MSI-H |
| ID 63 | MSI-H BRAF wild type | 10% | 2% | 0% | 0% | 5% | 2 | MSI-H | 10% | 17 | MSI-H |
| ID 64 | MSI-H BRAF wild type | 5% | 26% | 8% | 0% | 0% | 3 | MSI-H | 26% | 39 | MSI-H |
| ID 72 | MSI-H BRAF wild type | 0% | 0% | 0% | 4% | 0% | 1 | MSI-L * | 4% | 4 | MSI-L |
| ID 152 | MSI-H BRAF wild type | 2% | 2% | 8% | 0% | 0% | 1 | MSI-L * | 8% | 12 | MSI-H |
| ID 172 | MSI-H BRAF wild type | 14% | 0% | 4% | 0% | 0% | 2 | MSI-H | 14% | 18 | MSI-H |
| ID 232 | MSI-H BRAF wild type | 0% | 0% | 4% | 0% | 9% | 2 | MSI-H | 9% | 13 | MSI-H |
| ID 272 | MSI-H BRAF wild type | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID | MSI Status Based on IHC | BAT-25 | BAT-26 | D5S346 | D2S123 | D17S250 | † No. of NCI (/5) Markers >2% Instability in ≥2 Markers: MSI-H; 1 Marker MSI-L; None: MSS | MSI Status | Maximum Allelic Variability (/100) | ‡ MSI Score Based on Total Allelic Variability (/500) | MSI Status Based on Total Allelic Variability (MSI Score = 1–2 MSS; 3–5 MSI-L; >5 MSI-H) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID 3 | MSS | 8% | 11% | 0% | 4% | 0% | 3 | MSI-H | 11% | 24 | MSI-H |
| ID 4 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 5 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 11 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 15 | MSS | 0% | 2% | 0% | 0% | 0% | 0 | MSS | 2% | 2 | MSS |
| ID 19 | MSS | 4% | 0% | 0% | 0% | 0% | 1 | MSI-L * | 4% | 4 | MSI-L |
| ID 21 | MSS | 0% | 0% | 0% | 0% | 5% | 1 | MSI-L * | 5% | 5 | MSI-L |
| ID 28 | MSS | 1% | 0% | 0% | 1% | 1% | 0 | MSS | 1% | 4 | MSI-L |
| ID 33 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 48 | MSS | 0% | 0% | 0% | 0% | 3% | 1 | MSI-L * | 3% | 3 | MSI-L |
| ID 50 | MSS | 0% | 0% | 4% | 0% | 0% | 1 | MSI-L * | 4% | 4 | MSI-L |
| ID 61 | MSS | 0% | 3% | 0% | 0% | 0% | 1 | MSI-L * | 3% | 3 | MSI-L |
| ID 65 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 67 | MSS | 15% | 10% | 0% | 0% | 4% | 3 | MSI-H | 15% | 28 | MSI-H |
| ID 69 | MSS | 6% | 0% | 0% | 0% | 4% | 2 | MSI-H | 6% | 9 | MSI-H |
| ID 70 | MSS | 0% | 2% | 0% | 0% | 0% | 0 | MSS | 2% | 2 | MSS |
| ID 71 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 522 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
| ID 712 | MSS | 0% | 0% | 0% | 0% | 0% | 0 | MSS | 0% | 0 | MSS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toh, J.W.T.; Singh, P.; Tangirala, V.A.A.S.K.; Limmer, A.; Spring, K.J. A Simple, Accurate and Cost-Effective Capillary Electrophoresis Test with Computational Methods to Aid in Universal Microsatellite Instability Testing. Cells 2021, 10, 1401. https://doi.org/10.3390/cells10061401
Toh JWT, Singh P, Tangirala VAASK, Limmer A, Spring KJ. A Simple, Accurate and Cost-Effective Capillary Electrophoresis Test with Computational Methods to Aid in Universal Microsatellite Instability Testing. Cells. 2021; 10(6):1401. https://doi.org/10.3390/cells10061401
Chicago/Turabian StyleToh, James Wei Tatt, Puneet Singh, Venkata A. A. S. K. Tangirala, Alex Limmer, and Kevin J. Spring. 2021. "A Simple, Accurate and Cost-Effective Capillary Electrophoresis Test with Computational Methods to Aid in Universal Microsatellite Instability Testing" Cells 10, no. 6: 1401. https://doi.org/10.3390/cells10061401






