Abstract
The Hippo pathway is pervasively activated and has been well recognized to play critical roles in human cancer. The deregulation of Hippo signaling involved in cancer development, progression, and resistance to cancer treatment have been confirmed in several human cancers. Its biological significance and deregulation in cancer have drawn increasing interest in the past few years. A fundamental understanding of the complexity of the Hippo pathway in cancer is crucial for improving future clinical interventions and therapy for cancers. In this review, we try to clarify the complex regulation and function of the Hippo signaling network in cancer development, including its role in signal transduction, metabolic regulation, and tumor development, as well as tumor therapies targeting the Hippo pathway.
1. Introduction
During tumor development, cancer cells are exposed to dynamic changes in the tumor microenvironment and availability of nutrients [1,2,3]. Hence, cancer cells need to adapt their physiological processes and metabolism to these changes so as to maintain, survive, proliferate, and even undergo behavioral changes such as invasion and metastasis [4,5]. This is a complex signaling cascade, including cellular perception and transduction of stimuli in the microenvironment, as well as intracellular metabolic reprogramming to support the high demand for energy and building blocks [6]. The involved signaling cascades are affected by some transcription factors and associated pathways. One such pathway is the Hippo signaling pathway, which regulates gene expression in response to changes in extracellular and intracellular cues, leading to changes in cell behavior [7].
The Hippo pathway is a unique signaling module that regulates cell-specific transcriptional responses and responds to a wide range of intrinsic and extrinsic cues [8,9,10]. Based on the ability of the yes-associated protein/transcriptional coactivator with a PDZ-binding motif (YAP/TAZ) to regulate signal transduction, metabolism adaptation, and phenotypic changes, it is expected that the Hippo signaling pathway will function as a central hub for cancer development. In this review, we will introduce the regulatory role of the Hippo pathway in tumor development, signal transduction, and metabolism, and discuss possible cancer treatment strategies targeting the Hippo pathway.
2. Role of Hippo Signaling in the Development of Cancer
Hippo signaling, first discovered in Drosophila, has been implicated as a key regulator of organ size based on its important roles in regulating cell proliferation and apoptosis [11,12,13], and in regulating tissue-specific stem cells. The Drosophila Homolog of YAP, Yorkie (Yki), was found to act as a critical target of the Wts/Lats protein kinase as well as a potential oncogene, and the Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yki [14]. Evidence also demonstrates the critical role of the Hippo pathway in cancer stem cell biology, including EMT, drug resistance, self-renewal, and differentiation [15,16]. The role of the Hippo pathway in inhibiting cell growth, proliferation, promoting apoptosis, and regulating stem cell biology is the key to tumor inhibition [17].
Excessive activation or deletion of the Hippo signaling pathway will lead to abnormal cell growth and dysregulation of tissue and organ homeostasis, which will further lead to abnormal tissue and organ development, and impaired regeneration or tumorigenesis [18,19,20,21]. In fact, studies have demonstrated the important role of the Hippo pathway in the development of many kinds of cancer [22,23]. Numerous correlations between aberrant Hippo pathway protein expression and the cancer’s clinical stage (evaluated comprehensively according to tumor size, lymph node status, and distant metastasis) have been reported (as reviewed in [24]). Consistently, studies have reported that inhibition of the Hippo pathway promoted cell proliferation, migration, invasion, and the development of hepatocellular carcinoma [25,26]. As reviewed recently, the high expression of YAP/TAZ could promote breast cancer metastasis, and targeted therapy against YAP/TAZ can effectively block breast cancer metastasis [27]. These results establish a clear role for the Hippo signaling pathway in cancer progression.
As profiled by The Cancer Genome Atlas (TCGA), Hippo pathway genes such as large tumor suppressor1/2 (LATS1/2) and YAP were somatically mutated in 10% of 9125 tumors across 33 cancers [28]. The defect of mammalian sterile 20 like1/2 (MST1/2), an important component of the Hippo pathway, would lead to YAP activation, sustained liver overgrowth, and the eventual development of hepatocellular carcinoma and cholangiocarcinoma [29]. Studies have focused on how upstream inputs affect the activity of the Hippo signaling pathway, how it functions, and the contribution of its dysregulation to cancer development. Research on the mechanism of the Hippo regulatory network in cancer development has made some achievements, and its role in signal transduction and metabolism regulation is the most understood at present.
3. Role of Hippo Signaling in Mechanotransduction
During tumorigenesis or metastasis, cells are in a complex microenvironment and constantly respond to biochemical cues and mechanical stress from the microenvironment. These biochemical and mechanical signals are converted into intracellular signals through signal transduction to regulate the biological behavior of cells [30]. In recent years, studies have shown that during tumor development and growth, Hippo signaling plays an important role in signal transduction, particularly in mechanotransduction [31]. The key components of the Hippo cascade, including MST1/2, LATS1/2, and YAP/TAZ, constitute a phosphokinase axis, which regulate the downstream effectors to maintain homeostasis and prevent tumor growth [32].
As reported by Sansores-Garcia, the Hippo pathway was first linked to the cytoskeleton, as the activity of Yap is modulated by changes in F-actin [33]. Since then, cytoskeletal rearrangement and intracellular signal transduction have attracted extensive attention in the study of the mechanisms of cell regulation by mechanical factors [34]. According to previous research, mechanical forces from the microenvironment are transmitted through membrane receptors, actin cytoskeleton, and the nuclear membrane, and then affect gene transcription in the nucleus [35,36], ultimately determining cell fate and influencing tumor progression. Mechanical stimuli such as extracellular matrix (ECM) stiffness, cell morphology, and cell density would cause changes in cell geometry and cytoskeleton tension [37,38]. Studies have shown that changes in the activity or state of the cytoskeleton are involved in the regulation of the Hippo signaling pathway; knocking down or interfering with the distribution of the cytoskeleton leads to Hippo changes, thus revealing the relationship between Hippo signaling and the cytoskeleton [39,40,41,42]. At present, it is still controversial whether mechanical factors regulate YAP/TAZ through the non-classical Hippo pathway (actin cytoskeleton -YAP) or the classical Hippo pathway (MST- Lats -YAP) [43,44]. Earlier studies have found that the mechanotransduction mediated by YAP/TAZ required Rho GTPase activity and tension of the actomyosin cytoskeleton but was independent of the Hippo-LATS cascade [38]. In addition, it was reported that the depletion of LATS1/2 did not rescue YAP/TAZ inhibition through a physically soft environment [33,45]. These studies demonstrated that mechanical cues can affect YAP/TAZ activity independent of LATS (non-classical Hippo pathway). Inconsistently, the LATS1/2-dependent regulation of YAP/TAZ activity by stress fiber (F-actin) formation has been reported [46], demonstrating that mechanical cues can also affect YAP/TAZ activity in a LATS-dependent way (classical Hippo pathway). In addition, when cells are exposed to energy stress or certain biochemical stimuli, the signal transduction pattern is similar. These research results unraveled how external environmental signals control related gene expression through classical and actin cytoskeleton-regulated Hippo signaling, helping to more clearly depict the mechanism of Hippo pathway mediated signal transduction (Figure 1).
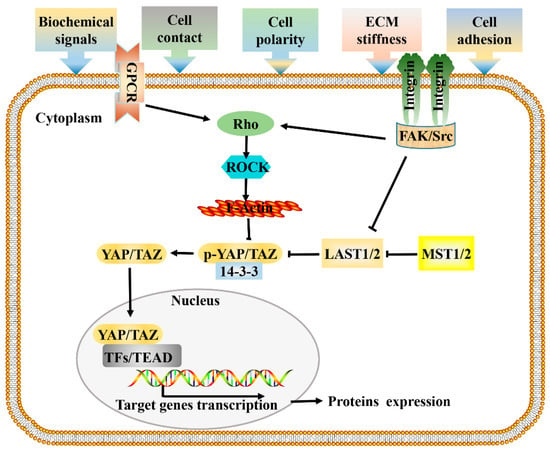
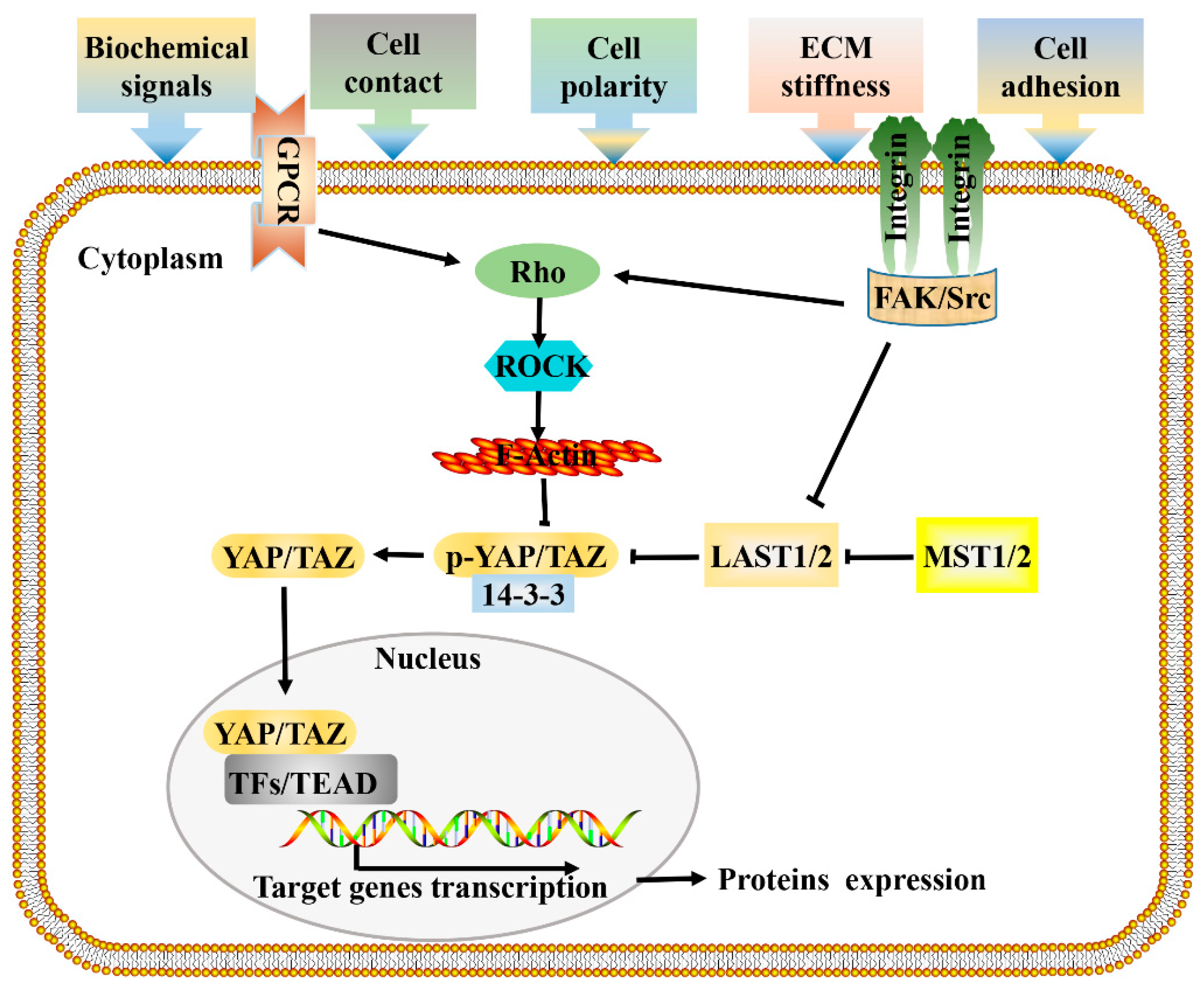
Figure 1.
Signal transduction network of Hippo pathway. Cells respond to mechanical forces, cell polarity, and adhesion signals by adjusting their tensional state and actin dynamics to regulate the activity of Hippo pathway components. When a cell adheres to a larger area or grows on a harder ECM, causes the activation of integrin signaling, and promotes the assembly of FAK/Src complex, which inactivate LATS1/2 and facilitate the polymerization of F-actin cytoskeleton via Rho-GTPases, F-actin then induces the dephosphorylation and guides the nuclear translocation of YAP/TAZ. GPCR signaling responds to a variety of activators (energy, proteins, lipids, sugars), and performs the same function by acting on RHO-GTPases. Hypo-phosphorylated YAP and TAZ accumulate in the nucleus where they can bind to various TFs, most notably the TEAD family, to direct gene expression changes that control a range of biological events. Pointed and blunt arrowheads indicate activating and inhibitory interactions, respectively. FAK, Focal adhesion kinase; GPCRs, G-protein-coupled receptors; LATS, Large tumor suppressor; MST, Mammalian sterile 20 like; Src, steroid receptor coactivator; TEAD, TEA domain protein; TFs, transcription factors; YAP/TAZ, yes-associated protein/transcriptional coactivator with PDZ-binding motif.
4. Hippo Signaling in Cancer Metabolic Reprogramming
Metabolism is a fundamental function of cells that can be reprogrammed to meet the energy and material needs of cells through the regulation of signaling pathways. In turn, metabolic pathways or metabolites can modulate a network of signaling pathways, allowing cells to coordinate their metabolism and behavior in an integrated manner [47]. In the past several years, increasing studies have provided appreciation and understanding of how Hippo signaling controls cellular and organismal metabolism, and the diverse mechanisms through which metabolites and metabolic signals, in turn, influence Hippo signaling [8,47]. The Hippo signaling pathway is a highly conserved tumor suppressor pathway, which was identified as emerging nodes in the coordination of nutrient availability with cancer development and tissue homeostasis (Table 1).

Table 1.
Integration of Hippo-YAP signaling with metabolism.
4.1. Regulation of Metabolism by Hippo Signaling
The development and growth of tumors increase the demands for energy and macromolecules and is often accompanied by the activation of Hippo signaling. Consistent with this, the Hippo signaling pathway and its downstream effectors, YAP and TAZ, have been identified as important regulators of many cellular metabolic pathways of tumor cells, including glucose metabolism, lipid metabolism, amino acid metabolism, and mitochondrial homeostasis [8,60]. The Hippo signaling pathway regulates multiple metabolic pathways (Figure 2), which enables it to coordinate the availability of energy and metabolites to regulate cancer development.
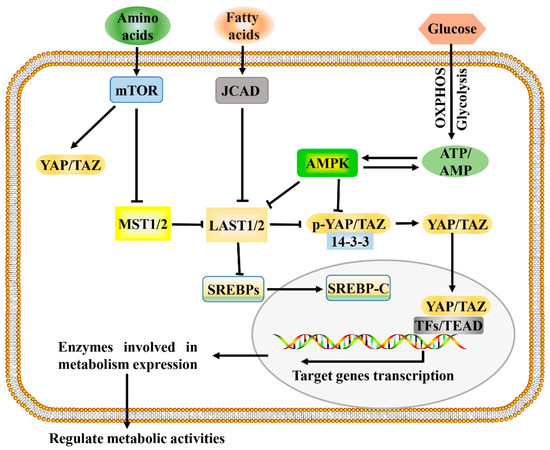
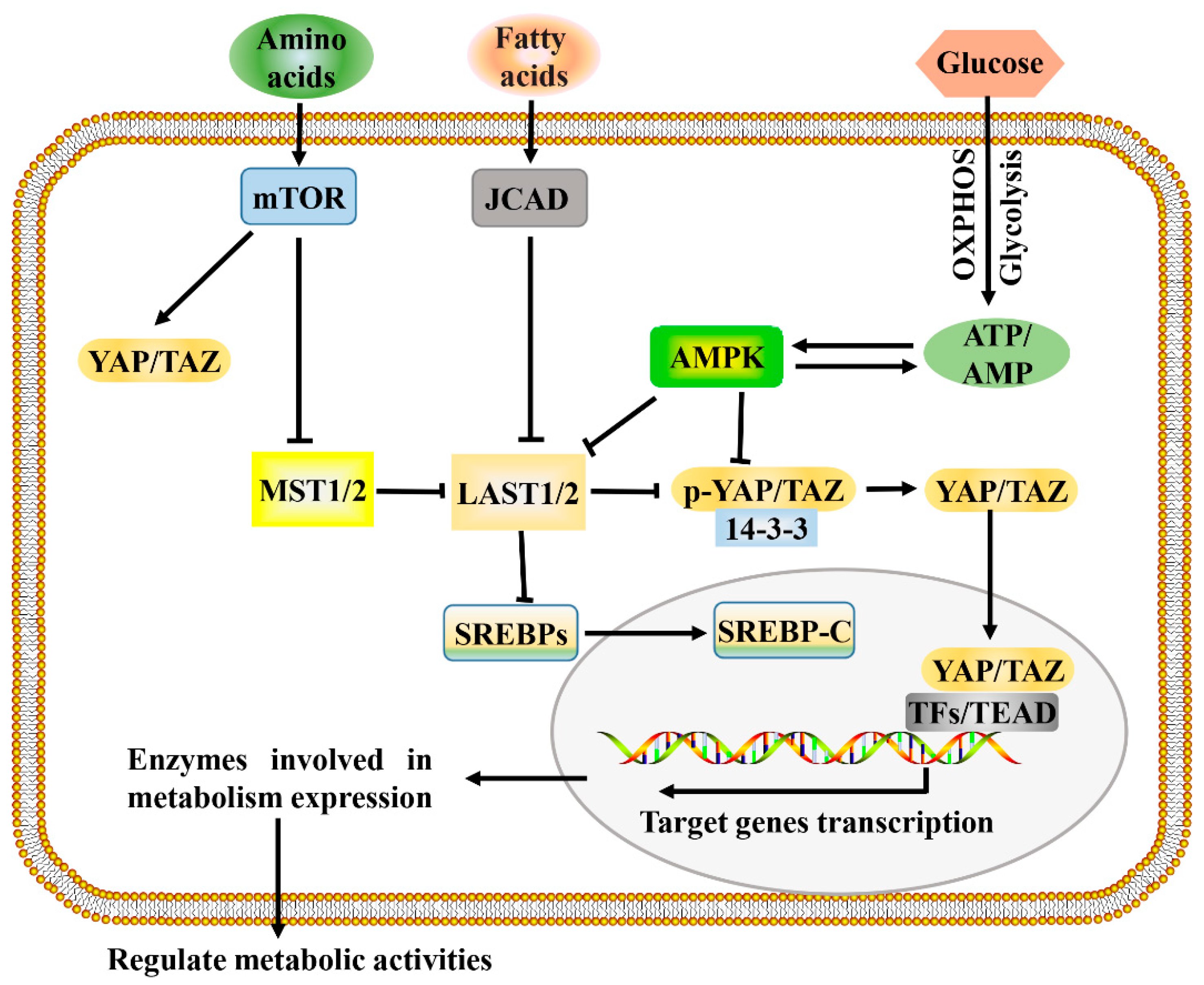
Figure 2.
A proposed model for Hippo pathway regulation by metabolism and Hippo pathway targets for metabolism. Metabolism can regulate or be regulated by the Hippo pathway through different mechanisms. In a nutrient-rich environment, glucose through glycolysis and mitochondrial OXPHOS inactivates AMPK by increasing the ATP:AMP ratio; in an energy stress environment, active AMPK inhibits YAP/TAZ by direct phosphorylation and/or activating LATS1/2. Amino acids induce the activation of mTOR, which also activates YAP/TAZ through various mechanisms. Fatty acids increase the expression of JCAD, which inhibits LATS2. Hippo core kinases MST and LATS regulate lipogenesis, as LATS2 can directly bind to SREBP precursors (SREBPs) and inhibit its processing to mature cleaved SREBP (SREBP-C), thus blocking its transcriptional activity. One possible mode of the Hippo pathway regulating metabolism is as follows: Hypo-phosphorylated YAP and TAZ accumulate in the nucleus, where they can bind to various TFs (such as HIF1α and SREBP) or TEAD family, to direct transcription and expression of enzymes involved in complex metabolic pathways. AMPK, AMP-activated protein kinase; JCAD, junctional protein associated with coronary artery disease; LATS, large tumor suppressor; MST, mammalian sterile 20 like; OXPHOS, oxidative phosphorylation; SREBP, sterol regulatory element-binding protein; TEAD, TEA domain protein; TFs, transcription factors; YAP/TAZ, yes-associated protein/transcriptional coactivator with PDZ-binding motif.
Researchers found that the knockout of MST1/2 and LATS1/2 cells lead to the decrease of glucose levels in the culture medium and the increase of medium acidity, suggesting the increase of glucose uptake and the glycolysis rate, which is consistent with the result of increased YAP/TAZ transcriptional activity [48]. Although there are still limitations at the cellular level, the Hippo signaling pathway has gradually shown its potential as a regulator of intracellular glucose metabolism. As transcriptional coactivators of Hippo signaling, YAP/TAZ were proved to promote glucose uptake and glycolysis by upregulating the expression of glucose transporters and glycolytic enzymes. Studies have identified glucose transporter 3 (GLUT3) and GLUT1 as targets of YAP, with their expression and glucose uptake being regulated by YAP [49,50]. Moreover, our recent study indicated that the activation of YAP promoted the expression of GLUT1 and the glucose uptake of hepatocellular carcinoma (HCC) cells [51]. These studies demonstrate that YAP can promote glucose uptake and glycolysis by upregulating the expression of the glucose transporter. Concurrently, emerging evidences have shown that Hippo signaling regulates glycolysis by regulating the expression of the key enzymes of this pathway [61]. Studies have reported that the deletion of YAP/TAZ in cancer cells could downregulate the expression of a variety of key enzymes involved in glycolysis, including HK1, HK2, PFKFB4, PFKP, GAPDH, PGK1, PGAM1, LDHA, PDHA1, and PDHB, leading to the inhibition of glycolysis activity [52,53]. Consistently, our study also indicated that the knockdown of YAP/TAZ downregulated the transcription and expression of HK2 and LDHA, leading to the decrease of glycolysis activity in HCC cells [51]. In addition to direct transcriptional and expression regulation, YAP/TAZ can also regulate the expression of key glycolysis enzymes by interacting with transcription factors. Hypoxia-inducible factor 1α (HIF1α) and c-Myc as the main transcription factors regulating glycolysis [62,63] were also found to be associated with YAP and to regulate the expression of key glycolysis enzymes and glycolysis. As studies reported, YAP binds to these transcription factors in the nucleus and regulates cell glycolysis [62,64,65,66]. Altogether, Hippo signaling can regulate cell glucose metabolism in a variety of ways.
In addition to glucose metabolism, lipid metabolism and amino acid metabolism are also dysregulated in cancer and can be regulated by Hippo signaling. Sterol regulatory element binding protein (SREBP) is a transcription factor that mainly regulates the biosynthesis of cholesterol, fatty acids, and triacylglycerol, and is involved in the regulation of the expression of key genes in lipid synthesis and absorption [67,68]. It has been demonstrated that YAP is a co-factor of SREBP, and the activation of LATS1 or the inhibition of YAP reduce hepatocyte lipogenesis by inhibiting the function of YAP–SREBP complexes [54]. In addition, as reviewed by Ibar et al., multiple Hippo signaling components (including MST1, LAST2, and YAP) are involved in regulating the activity of SREBPs, thereby controlling lipogenesis and cholesterol synthesis in hepatocyte [8]. Bile acid is an important component of bile and plays an important role in lipid metabolism. A study reported that the activation of Hippo signaling suppressed bile acid metabolism, liver overgrowth, and tumorigenesis [69], suggesting it is involved in the regulation of lipid metabolism. Studies have shown that YAP/TAZ upregulates the expression of amino acid transporter carrier family 38 member 1 (SLC38A1) and solute carrier family 7 member 5 (SLC7A5), resulting in increased amino acid uptake and the regulation of amino acid metabolism in HCC [6]. In addition, the expression of amino acid transporters SLC1A5 and glutaminase1 (GLS1) was also reported to be positively correlated with the expression of YAP/TAZ in human breast cancer samples [55]. Therefore, Hippo signaling is involved in regulating the metabolic network of tumors, but the regulatory mechanism in cancer cells remains to be further explored.
4.2. Metabolic Cues That Control Hippo Signaling
It is easy to accept that metabolism is regulated by Hippo signaling, and in fact, studies have made a strong case that Hippo signaling can in turn be regulated by metabolites or metabolic pathways (Figure 2) [70,71]. It is well known that the metabolic reprogramming of cancer cells tends to enhance aerobic glycolysis, and glucose is one of the major sources of cancer cell metabolism. Studies have reported that Hippo signaling is regulated by aerobic glycolysis, and the reduction of glycolysis leads to the inhibition of YAP/TAZ transcriptional activity [8,60,72]. A 2-DG (a glycolysis inhibitor) treatment downregulates the overall levels of the YAP/TAZ gene signature in MCF10A and MDA-MB-231 mammary cells, illustrating that glycolysis regulates YAP/TAZ transcriptional activity [56]. In the absence of glucose, the AMP-activated protein kinase (AMPK) pathway is activated, and AMPK acts as a regulator of the Hippo pathway in response to energy stress, leading to phosphorylation of YAP and promoting its inactivation [49]. These results suggest that glucose and glycolysis are involved in the regulation of the Hippo pathway.
In addition to glucose and glycolysis mentioned above, some other metabolic pathways were also found involved in regulating the activity of Hippo signaling, such as lipids, hormones, and other metabolites [60,73]. As reviewed, alterations on lipid metabolism contribute to the activation of several important oncogenic signaling pathways, including Hippo signaling [73,74]. Research findings have indicated that YAP/TAZ activity is regulated by the SREBP/mevalonate pathway in many cancer cells [54,58]. Oncogenic mutant p53, acting as a positive transcriptional cofactor for SREBPs, leads to increased mevalonic acid and promotes YAP activity in tumor cells [58]. The mevalonate pathway, involved in the synthesis of cholesterol, bile acids, steroid hormones, and statins used to inhibit this pathway, was found to efficiently suppress YAP/TAZ nuclear translocation [58]. Palmitic acid is a common saturated fatty acid in organisms. It has been reported that palmitic acid inhibits YAP by upregulating MST1, thereby inhibiting endothelial cell proliferation, migration, and angiogenesis [75]. These results reveal a tight connection between YAP/TAZ activity and metabolic substances and pathways.
5. Target the Hippo Signaling Pathway for Cancer Therapy
As mentioned above, the Hippo pathway is a major signaling pathway that is responsible for human cancer development. The Hippo pathway’s contribution to cancer has sparked interest in the development of potential therapeutics [76]. Given the association of elevated and hyperactive YAP/TAZ with many cancers, the anti-cancer therapeutic strategies targeting the Hippo pathway would aim at inhibiting the activities and functions of YAP and TAZ directly or indirectly [77]. Several Hippo pathway-targeted strategies have been reviewed, such as development of drugs targeting MST and LATS activation, YAP/TAZ activation or YAP/TAZ–TEAD interaction [78,79,80]. The study indicated that YAP activates the DNA damage response pathway, and by targeting YAP, dasatinib acts as a chemosensitizer for a subset of molecular targeted drugs [81]. Apigenin was reported to decrease the expression of YAP/TAZ and disrupt the YAP/TAZ–TEAD interaction in TNBC cells, suggesting a promising therapeutic agent for the treatment of TNBC patients [82]. In addition, statins were identified as potent YAP inhibitors, verteporfin and vestigial like family member 4 (VGLL4) were identified as inhibitors of the YAP–TEAD interaction (as reviewed in [23]). Therefore, some progress has been made in inhibiting upstream Hippo kinase as a strategy for inhibiting tumor progression. However, due to the ambiguous regulatory mechanism of the Hippo pathway, tumor therapies targeting the Hippo pathway still face great challenges.
There are many signal pathways involved in the regulation of cancer development. The regulation of the Hippo pathway by the tumor-related signal pathways is not surprising given its important role in tumorigenesis [83]. The powerful pluripotency of the Hippo pathway in the development of cancer is inseparable from its interaction with a variety of tumor-related signal pathways [84]. Some tumor-related signals, including the Wnt pathway, Notch pathway, Src signal, p53 signal, PI3K/Akt, RAS signaling pathway, TGFβ signaling, among others, were reported to interact with the Hippo pathway and synergistically promote cancer development [83,85,86,87,88,89,90]. They can affect YAP/TAZ dependently/independently of the Hippo pathway, which in turn affects the biological behavior of cancer cells and cancer development. This suggests that the study of the effect of the Hippo pathway on cancer development needs to shift from the concept of the simple linear pathway to the perspective of a network connection composed of multiple signaling pathways. In addition to the direct targeting of Hippo pathway components, pharmacologically regulated signal pathways that interact with the Hippo pathway or combined therapies that inhibit YAP/TAZ target genes may be promising approaches for targeting the Hippo pathway in cancer cells.
6. Conclusions
Extensive research studies have provided tremendous insight into the regulation and role of Hippo signaling in cancer development. In response to tissue-level mechanical forces and a variety of biochemical factors, F-actin cytoskeleton acts as the main determinant of the regulation of Hippo–YAP/TAZ activity; through feedback and crosstalk mechanisms, YAP/TAZ influences a variety of cellular events, from metabolism to biological behaviors. At present, some progress has been made in the study of the molecular mechanism by which the cytoskeleton regulates the Hippo signaling pathway, but some key questions remain unanswered [42,91]. The Hippo pathway regulatory network is complex and diverse, and its regulatory mechanism is still poorly understood. A key challenge for the future will be to explore the mechanisms by which the Hippo signaling pathway plays regulatory roles in different environments, and to develop targeted cancer treatments. We believe that targeting the Hippo pathway will lead to fruitful therapies in the near future.
Author Contributions
Q.L. conducted the literature review and drafted the original manuscript. X.L. and G.S. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Key Program from the National Natural Science Foundation of China (11832008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMPK | AMP-activated protein kinase |
| ECM | Extracellular matrix |
| FAK | Focal adhesion kinase |
| GLUT3 | Glucose transporter 3 |
| GPCRs | G-protein-coupled receptors |
| HCC | Hepatocellular carcinoma |
| HIF1α | Hypoxia-inducible factor 1α |
| LATS | Large tumor suppressor |
| MST | Mammalian sterile 20 like |
| PFK1 | Phosphofructokinase 1 |
| SCD1 | Stearoyl-CoA-desaturase 1 |
| SLC38A1 | Solute carrier family 38 member 1 |
| SLC7A5 | Solute carrier family 7 member 5 |
| Src | Steroid receptor coactivator |
| SREBP | Sterol regulatory element binding protein |
| TCGA | The Cancer Genome Atlas |
| TEAD | TEA domain protein |
| TFs | Transcription factors |
| VGLL4 | Vestigial like family member 4 |
| YAP | Yes-associated protein |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
References
- Harachi, M.; Masui, K.; Okamura, Y.; Tsukui, R.; Mischel, P.S.; Shibata, N. mTOR complexes as a nutrient sensor for driving cancer progression. Int. J. Mol. Sci. 2018, 19, 3267. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz López, K.G.; Toledo Guzmán, M.E.; Sánchez, E.O.; García Carrancá, A. mTORC1 as a regulator of mitochondrial functions and a therapeutic target in cancer. Front. Oncol. 2019, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.T.; Vennin, C.; Timpson, P.; Pajic, M. Effective modulation of stromal signaling through ROCK inhibition: Is it all in the timing? Mol. Cell. Oncol. 2017, 4, e1333973. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef]
- Kajanova, I.; Zatovicova, M.; Jelenska, L.; Sedlakova, O.; Barathova, M.; Csaderova, L.; Debreova, M.; Lukacikova, L.; Grossmannova, K.; Labudova, M.; et al. Impairment of carbonic anhydrase IX ectodomain cleavage reinforces tumorigenic and metastatic phenotype of cancer cells. Br. J. Cancer 2020, 122, 1590–1603. [Google Scholar] [CrossRef]
- Park, Y.-Y.; Sohn, B.H.; Johnson, R.L.; Kang, M.-H.; Kim, S.B.; Shim, J.-J.; Mangala, L.S.; Kim, J.H.; Yoo, J.E.; Rodriguez-Aguayo, C.; et al. YAP1 and TAZ activates mTORC1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology 2016, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, Y.; Guo, G.; Liu, Y.; Zhang, Z.; Dong, S.; Nan, Y.; Zhao, Z.; Zhong, Y.; Huang, Q. IKBKE regulates cell proliferation and epithelial-mesenchymal transition of human malignant glioma via the Hippo pathway. Oncotarget 2017, 8, 49502–49514. [Google Scholar] [CrossRef]
- Ibar, C.; Irvine, K.D. Integration of Hippo-YAP signaling with metabolism. Dev. Cell 2020, 54, 256–267. [Google Scholar] [CrossRef]
- Pennarossa, G.; Gandolfi, F.; Brevini, T.A.L. Biomechanical signaling in oocytes and parthenogenetic cells. Front. Cell Dev. Biol. 2021, 9, 646945. [Google Scholar] [CrossRef]
- Yamauchi, T.; Moroishi, T. Hippo pathway in mammalian adaptive immune system. Cells 2019, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Tapon, N.; Harvey, K.F.; Bell, D.W.; Wahrer, D.C.; Schiripo, T.A.; Haber, D.; Hariharan, I.K. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002, 23, 467–478. [Google Scholar] [CrossRef]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 22, 457–467. [Google Scholar] [CrossRef]
- Pantalacci, S.; Tapon, N.; Léopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003, 5, 921–927. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 12, 421–434. [Google Scholar] [CrossRef]
- Mo, J.S.; Park, H.W.; Guan, K.L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014, 15, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, D.; Chi, F.; Zhao, B. The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell 2012, 3, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ham, K.; Hoque, M.O. A time for YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int. J. Cancer 2018, 143, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Lo Sardo, F.; Strano, S.; Blandino, G. YAP and TAZ in lung cancer: Oncogenic role and clinical targeting. Cancers 2018, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, K.; Ma, Y.; Zhou, M.; Fan, S. The TAZ-miR-224-SMAD4 axis promotes tumorigenesis in osteosarcoma. Cell Death Dis. 2017, 8, e2539. [Google Scholar] [CrossRef]
- Wang, S.; Ma, K.; Chen, L.; Zhu, H.; Liang, S.; Liu, M.; Xu, N. TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis. Biosci. Rep. 2016, 36, e00386. [Google Scholar] [CrossRef]
- Stein, C.; Bardet, A.F.; Roma, G.; Bergling, S.; Clay, I.; Ruchti, A.; Agarinis, C.; Schmelzle, T.; Bouwmeester, T.; Schübeler, D.; et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 2015, 11, e1005465. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Liu, M.; Chong, R.; Ding, S.-J.; Chen, Y.; Dong, J. CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res. 2013, 73, 6722–6733. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, J.E.; Park, H.W. The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol. Cells 2018, 41, 83–92. [Google Scholar]
- van Rensburg, H.J.J.; Yang, X. The roles of the Hippo pathway in cancer metastasis. Cell. Signal. 2016, 28, 1761–1772. [Google Scholar] [CrossRef]
- Li, J.; Xue, J.; Ling, M.; Sun, J.; Xiao, T.; Dai, X.; Sun, Q.; Cheng, C.; Xia, H.; Wei, Y.; et al. MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway. Cancer Lett. 2021, 497, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Yang, Y. Hepatic Hippo signaling inhibits development of hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, M.; Cai, M.; Zhang, C.; Qiu, Y.; Wang, X.; Zhang, T.; Zhou, H.; Wang, J.; Zhao, W.; et al. Transcriptional co-activators YAP/TAZ: Potential therapeutic targets for metastatic breast cancer. Biomed. Pharmacother. 2021, 133, 110956. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [PubMed]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.-S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, M.; Shao, J. Effects of verapamil on the immediate-early gene expression of bone marrow mesenchymal stem cells stimulated by mechanical strain in vitro. Med. Sci. Monit. Basic Res. 2013, 19, 68–75. [Google Scholar] [CrossRef][Green Version]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS. Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tumaneng, K.; Guan, K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Sansores-Garcia, L.; Bossuyt, W.; Wada, K.; Yonemura, S.; Tao, C.; Sasaki, H.; Halder, G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011, 10, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wu, J.W.; Wang, C.W.; Jang, A.C.C. Hippo signaling-mediated mechanotransduction in cell movement and cancer metastasis. Front. Mol. Biosci. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Oh, S.; Chung, H.; Chang, S.; Lee, S.-H.; Seok, S.H.; Lee, H. Effect of Mechanical Stretch on the DNCB-induced proinflammatory cytokine secretion in human keratinocytes. Sci. Rep. 2019, 9, 5156. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Cosgrove, B.D.; Heo, S.-J.; Shurden, Z.E.; Mauck, R.L. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 2015, 108, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef]
- Mo, J.-S.; Yu, F.-X.; Gong, R.; Brown, J.H.; Guan, K.-L. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012, 26, 2138–2143. [Google Scholar] [CrossRef]
- Seo, J.; Kim, J. Regulation of Hippo signaling by actin remodeling. BMB Rep. 2018, 51, 151–156. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Gao, Z.; Zhang, P.; Gao, J.; Wu, X. Regulation of Hippo signaling by mechanical signals and the cytoskeleton. DNA Cell Biol. 2020, 39, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Irvine, K.D. Cellular organization and cytoskeletal regulation of the Hippo signaling network. Trends Cell Biol. 2016, 26, 694–704. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.-L.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, A.; Khudiakov, A.; Vaz, R.; Muravyev, A.; Sukhareva, K.; Sejersen, T.; Kostareva, A. FLNC expression level influences the activity of TEAD-YAP/TAZ signaling. Genes 2020, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef]
- Santinon, G.; Pocaterra, A.; Dupont, S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016, 26, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, S.W.; Meng, Z.; Lin, K.C.; Lin, B.; Hong, A.W.; Chun, J.V.; Guan, K.-L. Characterization of Hippo pathway components by gene inactivation. Mol. Cell 2016, 64, 993–1008. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.-D.; Li, X.; Aziz, K.E.; Gan, B.; Johnson, R.L.; Chen, J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015, 17, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.G.; Tsomides, A.; Yimlamai, D.; Hwang, K.L.; Miesfeld, J.; Galli, G.G.; Fowl, B.H.; Fort, M.; Ma, K.Y.; Sullivan, M.R.; et al. Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. EMBO J. 2018, 37, e100294. [Google Scholar] [CrossRef]
- Liu, Q.P.; Luo, Q.; Deng, B.; Ju, Y.; Song, G.B. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling. Cancers 2020, 12, 490. [Google Scholar] [CrossRef]
- White, S.M.; Avantaggiati, M.L.; Nemazanyy, I.; Di Poto, C.; Yang, Y.; Pende, M.; Gibney, G.T.; Ressom, H.W.; Field, J.; Atkins, M.B.; et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev. Cell 2019, 49, 425–443. [Google Scholar] [CrossRef]
- Murakami, S.; Nemazanyy, I.; White, S.M.; Chen, H.; Nguyen, C.D.K.; Graham, G.T.; Saur, D.; Pende, M.; Yi, C. A Yap-Myc-Sox2-p53 regulatory network dictates metabolic homeostasis and differentiation in Kras-driven pancreatic ductal adenocarcinomas. Dev. Cell 2019, 51, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Gao, Y.; Zhang, G.; Zhou, Y.; Cao, J.; Wan, D.; Zhu, X.; Xiong, W. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J. Cell. Mol. Med. 2019, 23, 3616–3628. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.N.; Ngwa, V.M.; Wang, S.; Shiuan, E.; Brantley-Sieders, D.M.; Kim, L.C.; Reynolds, A.B.; Chen, J. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 2017, 10, eaan4667. [Google Scholar] [CrossRef] [PubMed]
- Enzo, E.; Santinon, G.; Pocaterra, A.; Aragona, M.; Bresolin, S.; Forcato, M.; Grifoni, D.; Pession, A.; Zanconato, F.; Guzzo, G.; et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015, 34, 1349–1370. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Ruggeri, N.; Specchia, V.; Cordenonsi, M.; Mano, M.; Dupont, S.; Manfrin, A.; Ingallina, E.; Sommaggio, R.; Piazza, S.; et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014, 16, 357–366. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, H.; Zhang, Y.; Mei, L.; Fang, X.; Zhang, X.; Zhang, F.; Chen, H.; Liu, Y.; et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl. Acad. Sci. USA 2014, 111, E89–E98. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Taouk, G.M. A potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front. Oncol. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, S.; Fan, X.-G.; Wang, H.; Lotze, M.T.; Zeh, H.J., 3rd; Billiar, T.R.; Kang, R.; Tang, D. High mobility group protein B1 controls liver cancer initiation through yes-associated protein-dependent aerobic glycolysis. Hepatology 2018, 67, 1823–1841. [Google Scholar] [CrossRef]
- Yeung, S.J.; Pan, J.; Lee, M.-H. Roles of p53, MYC and HIF-1 in regulating glycolysis–the seventh hallmark of cancer. Cell. Mol. Life Sci. 2008, 65, 3981–3999. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Wang, S.; Pan, X.; Zhang, Y.; Xu, J.; Jiang, Y.; Li, H.; Zhang, Q.; Gao, J.; et al. S1P/S1PR3 axis promotes aerobic glycolysis by YAP/c-MYC/PGAM1 axis in osteosarcoma. EBioMedicine 2019, 40, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, H.-Y.; Wang, J.; Wang, Y.; Zhang, P.; Ma, N.; Mo, S.-J. Phosphorylation of 14-3-3ζ links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D. Sterol regulatory element-binding proteins: Transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 2002, 30, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Stack, M.S. Lipid regulatory proteins as potential therapeutic targets for ovarian cancer in obese women. Cancers 2020, 12, 3469. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Q.; Zhang, S.; Chen, Q.; Wang, C.; Zhang, W.; Xiao, C.; Li, Y.; Nian, C.; Li, J.; et al. FGF15 activates Hippo signaling to suppress bile acid metabolism and liver tumorigenesis. Dev. Cell 2019, 48, 460–474. [Google Scholar] [CrossRef]
- Ardestani, A.; Lupse, B.; Maedler, K. Hippo signaling: Key emerging pathway in cellular and whole-body metabolism. Trends Endocrinol. Metab. 2018, 29, 492–509. [Google Scholar] [CrossRef]
- Rozengurt, E.; Eibl, G. Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development. World J. Gastroenterol. 2019, 25, 1797–1816. [Google Scholar] [CrossRef]
- Liu, H.; Du, S.; Lei, T.; Wang, H.; He, X.; Tong, R.; Wang, Y. Multifaceted regulation and functions of YAP/TAZ in tumors. Oncol. Rep. 2018, 40, 16–28. [Google Scholar]
- Di Benedetto, G.; Parisi, S.; Russo, T.; Passaro, F. YAP and TAZ mediators at the crossroad between metabolic and cellular reprogramming. Metabolites 2021, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, J.; Chen, S.; Cai, J.; Ban, Y.; Peng, Q.; Zhou, Y.; Zeng, Z.; Peng, S.; Li, X.; et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 118. [Google Scholar] [CrossRef]
- Yuan, L.; Mao, Y.; Luo, W.; Wu, W.; Xu, H.; Wang, X.L.; Shen, Y.H. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J. Biol. Chem. 2017, 292, 15002–15015. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.A.; Kagey, J.D. The role of the Hippo pathway in human disease and tumorigenesis. Clin. Transl. Med. 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Halder, G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014, 13, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Maehama, T.; Nishio, M.; Goto, H.; Kato, W.; Omori, H.; Miyachi, Y.; Togashi, H.; Shimono, Y.; Suzuki, A. Targeting the Hippo signalling pathway for cancer treatment. J. Biochem. 2017, 161, 237–244. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Han, Y. Analysis of the role of the Hippo pathway in cancer. J. Transl. Med. 2019, 17, 1–17. [Google Scholar] [CrossRef]
- Oku, Y.; Nishiya, N.; Tazawa, T.; Kobayashi, T.; Umezawa, N.; Sugawara, Y.; Uehara, Y. Augmentation of the therapeutic efficacy of WEE1 kinase inhibitor AZD1775 by inhibiting the YAP-E2F1-DNA damage response pathway axis. FEBS Open Bio 2018, 8, 1001–1012. [Google Scholar] [CrossRef]
- Li, Y.-W.; Xu, J.; Zhu, G.-Y.; Huang, Z.-J.; Lu, Y.; Li, X.-Q.; Wang, N.; Zhang, F.-X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Yang, C.-T.; Jablons, D.M.; You, L. The crosstalk between Src and Hippo/YAP signaling pathways in non-small cell lung cancer (NSCLC). Cancers 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Robitaille, M.; Remke, M.; Maier, C.; Malhotra, A.; Gregorieff, A.; Wrana, J.L.; Taylor, M.D.; Angers, S.; Kenney, A.M. YB-1 is elevated in medulloblastoma and drives proliferation in Sonic hedgehog-dependent cerebellar granule neuron progenitor cells and medulloblastoma cells. Oncogene 2016, 35, 4256–4268. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, M.; Verduci, L.; Blandino, G.; Strano, S. Mutant p53 protein and the Hippo transducers YAP and TAZ: A critical oncogenic node in human cancers. Int. J. Mol. Sci. 2017, 18, 961. [Google Scholar] [CrossRef]
- Molina-Castro, S.E.; Tiffon, C.; Giraud, J.; Boeuf, H.; Sifre, E.; Giese, A.; Belleannée, G.; Lehours, P.; Bessède, E.; Mégraud, F.; et al. The Hippo kinase LATS2 controls helicobacter pylori-induced epithelial-mesenchymal transition and intestinal metaplasia in gastric mucosa. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 257–276. [Google Scholar] [CrossRef]
- Kim, W.; Khan, S.K.; Gvozdenovic-Jeremic, J.; Kim, Y.; Dahlman, J.; Kim, H.; Park, O.; Ishitani, T.; Jho, E.; Gao, B. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Investig. 2017, 127, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Coggins, G.E.; Farrel, A.; Rathi, K.S.; Hayes, C.M.; Scolaro, L.; Rokita, J.L.; Maris, J.M. YAP1 mediates resistance to MEK1/2 inhibition in neuroblastomas with hyperactivated RAS signaling. Cancer Res. 2019, 79, 6204–6214. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Kim, H.-B.; Kim, M.-C.; Lee, J.; Lee, J.H.; Kim, J.-H.; Kim, J.-W.; Park, W.-Y.; Kim, S.-Y.; Kim, J.B.; et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J. Clin. Investig. 2018, 128, 1010–1025. [Google Scholar] [CrossRef]
- Pascual, J.; Jacobs, J.; Sansores-Garcia, L.; Natarajan, M.; Zeitlinger, J.; Aerts, S.; Halder, G.; Hamaratoglu, F. Hippo Reprograms the Transcriptional Response to Ras Signaling. Dev. Cell 2017, 25, 667–680. [Google Scholar] [CrossRef]
- Misra, J.R.; Irvine, K.D. The Hippo signaling network and its biological functions. Annu. Rev. Genet. 2018, 52, 65–87. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).