Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Sampling and Tissue Collection
2.3. Assessment of Endothelium-Dependent Vasodilation by MRI In Vivo
2.4. Assessment of NO Production in the Aorta Using EPR
2.5. Assessment of Biomarkers of Endothelial Dysfunction in Plasma by microLC/MS-MRM
2.6. Immunohistochemical Characteristics of PVAT and Endothelium of the TA and AA
2.7. Oxidant Properties of PVAT by EPR Spectroscopy
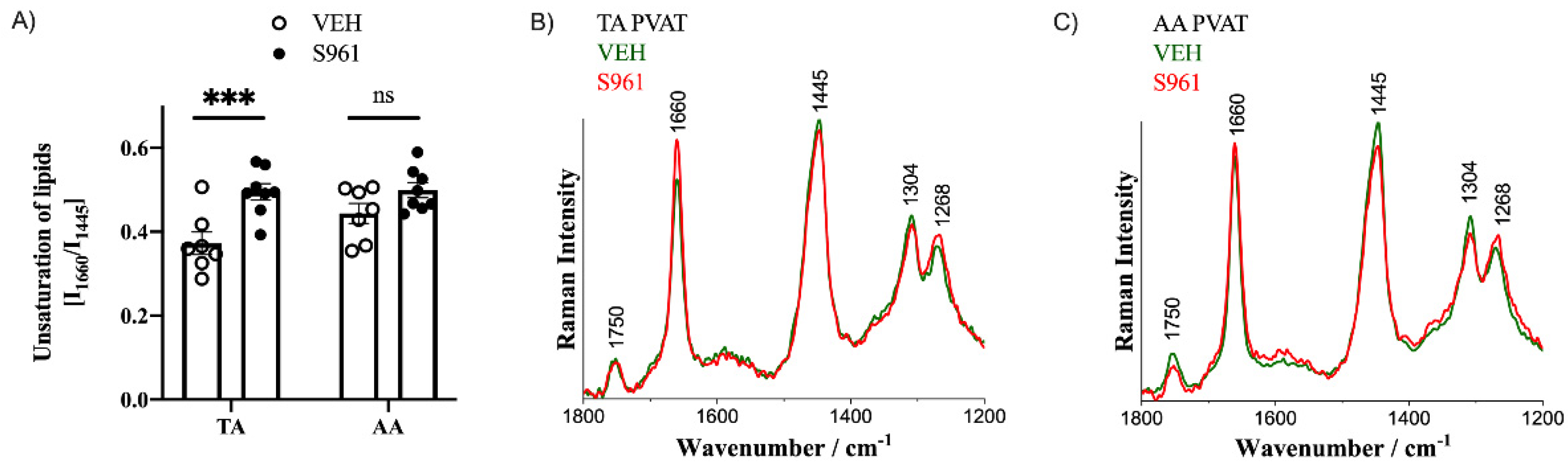
2.8. Lipid Characteristic of Adipose Tissue by Raman Spectroscopy
2.9. Statistical Analysis
3. Results
3.1. Effects of Short-Term Administration of IRA in Mice—Basic Characteristics
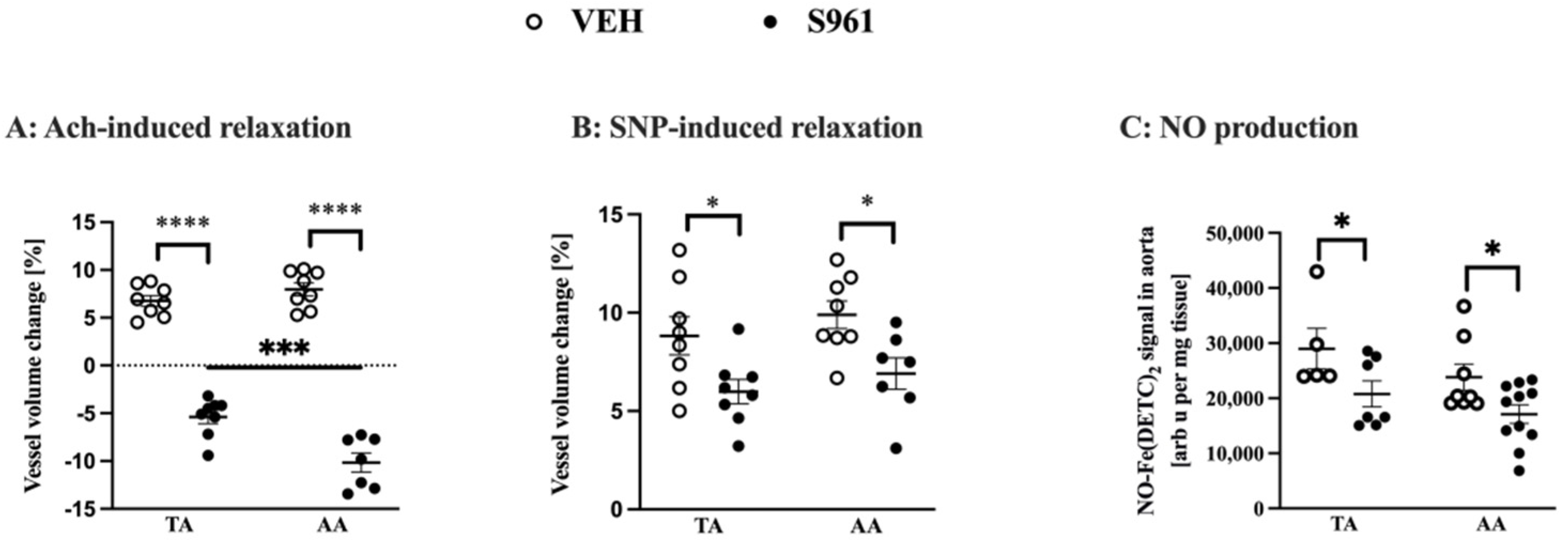
3.2. Effects of Short-Term Administration of IRA in Mice on Vascular Function and NO Production
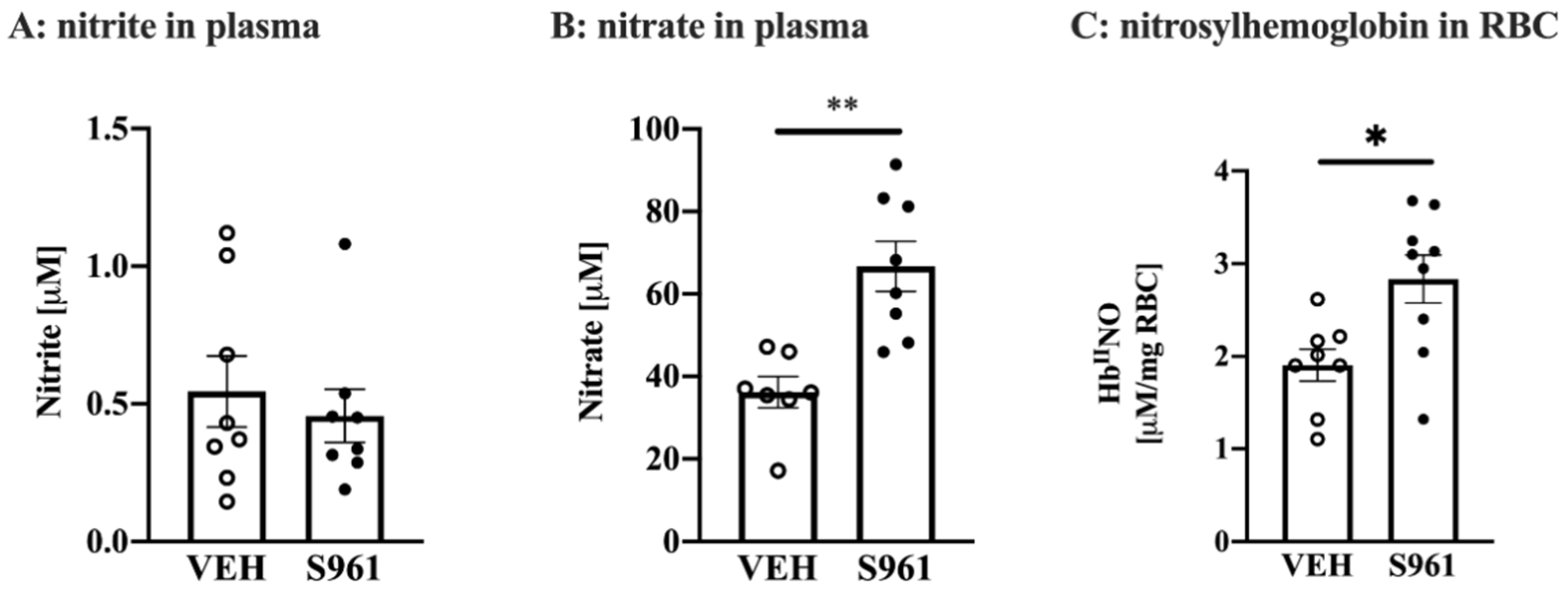
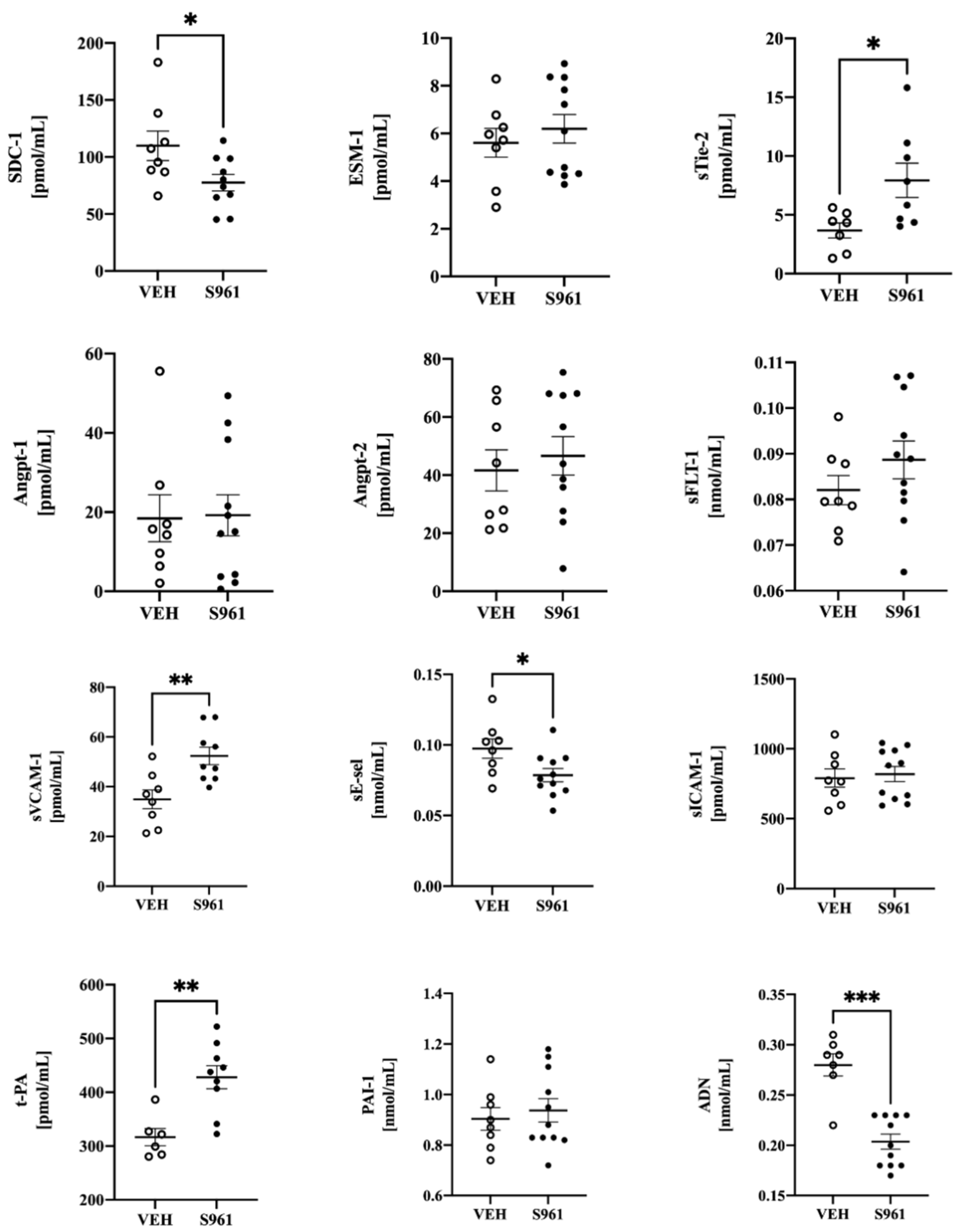
3.3. Effects of Short-Term Administration of IRA in Mice on Systemic NO Bioavailability and Protein Biomarkers of Endothelial Dysfunction
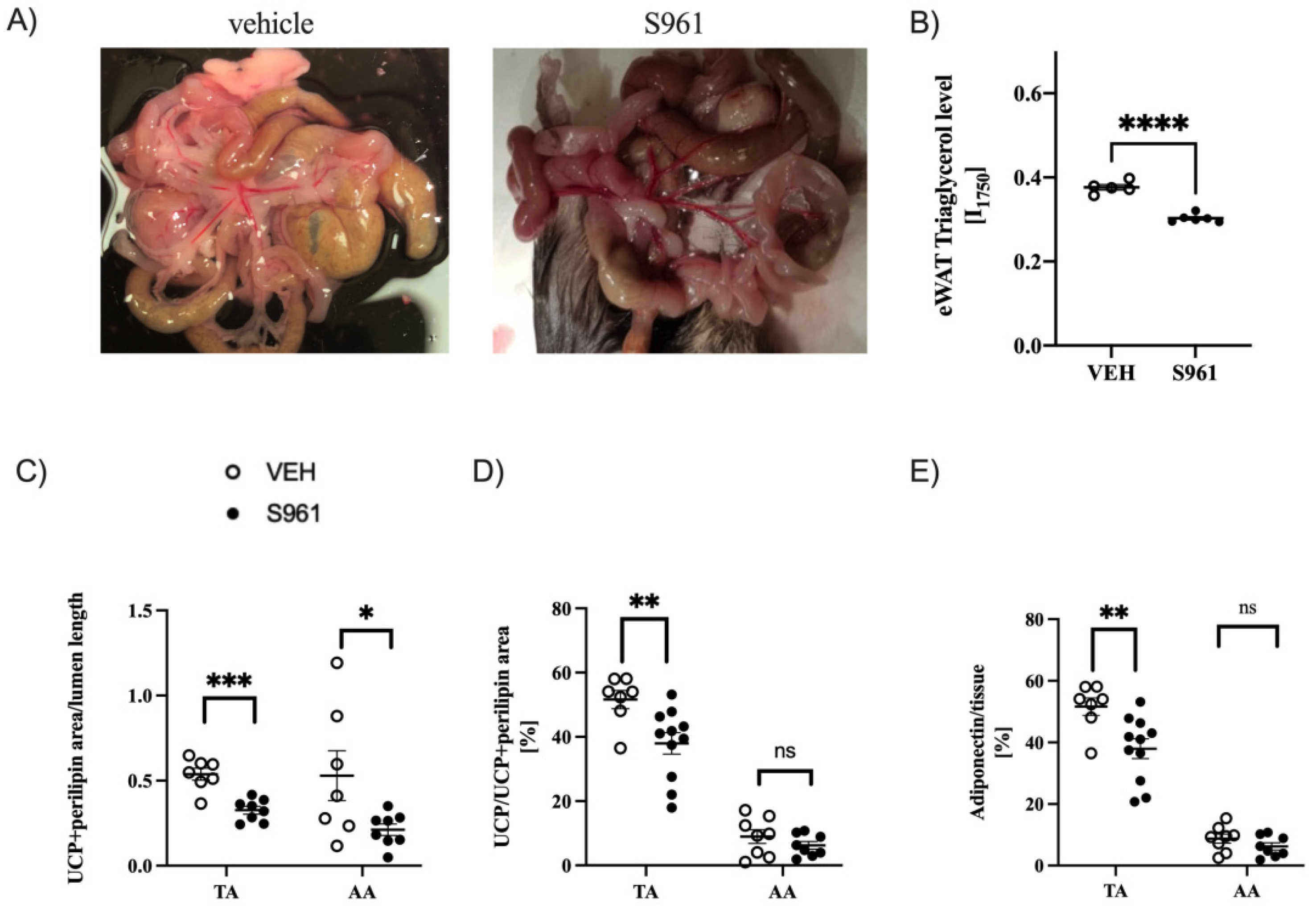
3.4. Effects of Short-Term Administration of IRA in Mice on PVAT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chlopicki, S. Perspectives in pharmacology of endothelium: From bench to bedside. Pharmacol. Rep. 2015, 67, vi–ix. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity. Cold Spring Harb. Perspect. Med. 2011, 2, a006429. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostallari, E.; Shah, V.H. Angiocrine signaling in the hepatic sinusoids in health and disease. Am. J. Physiol. Liver Physiol. 2016, 311, G246–G251. [Google Scholar] [CrossRef] [Green Version]
- Chłopicki, S.; Gryglewski, R.J. Angiotensin Converting Enzyme (ACE) and HydroxyMethylGlutaryl-CoA (HMG-CoA) Reductase Inhibitors in the Forefront of Pharmacology of Endothelium. Pharmacol. Rep. 2005, 57, 86–96. [Google Scholar]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Gruss, S.M.; Nhim, K.; Gregg, E.; Bell, M.; Luman, E.; Albright, A. Public Health Approaches to Type 2 Diabetes Prevention: The US National Diabetes Prevention Program and Beyond. Curr. Diabetes Rep. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, G.M.; Meier, D.A.; Hager, S.R. Endothelial dysfunction in a model of hyperglycemia and hyperinsulinemia. Am. J. Physiol. Circ. Physiol. 1995, 269, H845–H850. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Bar, A.; Kieronska-Rudek, A.; Proniewski, B.; Suraj-Prażmowska, J.; Czamara, K.; Marczyk, B.; Matyjaszczyk-Gwarda, K.; Jasztal, A.; Kuś, E.; Majka, Z.; et al. In Vivo Magnetic Resonance Imaging-Based Detection of Heterogeneous Endothelial Response in Thoracic and Abdominal Aorta to Short-Term High-Fat Diet Ascribed to Differences in Perivascular Adipose Tissue in Mice. J. Am. Heart Assoc. 2020, 9, e016929. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, R.; Bitton-Worms, K.; Caspi, A.; Shen-Orr, Z.; Leroith, D. Investigating New Therapeutic Strategies Targeting Hyperinsulinemia’s Mitogenic Effects in a Female Mouse Breast Cancer Model. Endocrinology 2013, 154, 1701–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikram, A.; Jena, G. S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem. Biophys. Res. Commun. 2010, 398, 260–265. [Google Scholar] [CrossRef]
- Galougahi, K.K.; Liu, C.; Garcia, A.; Gentile, C.; Fry, N.A.; Hamilton, E.J.; Hawkins, C.L.; Figtree, G.A. β3 Adrenergic Stimulation Restores Nitric Oxide/Redox Balance and Enhances Endothelial Function in Hyperglycemia. J. Am. Heart Assoc. 2016, 5, e002824. [Google Scholar] [CrossRef] [Green Version]
- Bubb, K.J.; Ritchie, R.H.; Figtree, G.A. Modified redox signaling in vasculature after chronic infusion of the insulin receptor antagonist, S961. Microcirculation 2018, 26, e12501. [Google Scholar] [CrossRef] [PubMed]
- Henrichot, E.; Juge-Aubry, C.E.; Pernin, A.; Pache, J.-C.; Velebit, V.; Dayer, J.-M.; Meda, P.; Chizzolini, C.; Meier, C.A. Production of Chemokines by Perivascular Adipose Tissue. Arter. Thromb. Vasc. Biol. 2005, 25, 2594–2599. [Google Scholar] [CrossRef] [Green Version]
- Schäffer, L.; Brand, C.L.; Hansen, B.F.; Ribel, U.; Shaw, A.C.; Slaaby, R.; Sturis, J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 2008, 376, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hempe, J.M.; Ory-Ascani, J. Simultaneous analysis of reduced glutathione and glutathione disulfide by capillary zone electrophoresis. Electrophoresis 2014, 35, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Kramkowski, K.; Leszczynska, A.; Przyborowski, K.; Kaminski, T.; Rykaczewska, U.; Sitek, B.; Zakrzewska, A.; Proniewski, B.; Smolenski, R.; Chabielska, E.; et al. Role of xanthine oxidoreductase in the anti-thrombotic effects of nitrite in rats in vivo. Platelets 2015, 27, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Skórka, T.; Jasiński, K.; Sternak, M.; Bartel, Ż.; Tyrankiewicz, U.; Chlopicki, S. Retrospectively gated MRI forin vivoassessment of endothelium-dependent vasodilatation and endothelial permeability in murine models of endothelial dysfunction. NMR Biomed. 2016, 29, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Sternak, M.; Bar, A.; Adamski, M.G.; Mohaissen, T.; Marczyk, B.; Kieronska, A.; Stojak, M.; Kus, K.; Tarjus, A.; Jaisser, F.; et al. The Deletion of Endothelial Sodium Channel α (αENaC) Impairs Endothelium-Dependent Vasodilation and Endothelial Barrier Integrity in Endotoxemia in Vivo. Front. Pharmacol. 2018, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybyło, M.; Kurpinska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [Green Version]
- Phinikaridou, A.; Andia, M.E.; Protti, A.; Indermuehle, A.; Shah, A.; Smith, A.; Warley, A.; Botnar, R.M. Noninvasive Magnetic Resonance Imaging Evaluation of Endothelial Permeability in Murine Atherosclerosis Using an Albumin-Binding Contrast Agent. Circulation 2012, 126, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Proniewski, B.; Kij, A.; Sitek, B.; Kelley, E.E.; Chlopicki, S. Multiorgan Development of Oxidative and Nitrosative Stress in LPS-Induced Endotoxemia in C57Bl/6 Mice: DHE-BasedIn VivoApproach. Oxid. Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Przyborowski, K.; Proniewski, B.; Czarny, J.; Smeda, M.; Sitek, B.; Zakrzewska, A.; Zoladz, J.A.; Chlopicki, S. Vascular Nitric Oxide–Superoxide Balance and Thrombus Formation after Acute Exercise. Med. Sci. Sports Exerc. 2018, 50, 1405–1412. [Google Scholar] [CrossRef]
- Suraj, J.; Kurpinska, A.; Olkowicz, M.; Niedzielska–Andres, E.; Smolik, M.; Zakrzewska, A.; Jasztal, A.; Sitek, B.; Chlopicki, S.; Walczak, M. Development, validation and application of a micro–liquid chromatography–tandem mass spectrometry based method for simultaneous quantification of selected protein biomarkers of endothelial dysfunction in murine plasma. J. Pharm. Biomed. Anal. 2018, 149, 465–474. [Google Scholar] [CrossRef]
- Suraj, J.; Kurpińska, A.; Zakrzewska, A.; Sternak, M.; Stojak, M.; Jasztal, A.; Walczak, M.; Chlopicki, S. Early and late endothelial response in breast cancer metastasis in mice: Simultaneous quantification of endothelial biomarkers using mass spectrometry-based method. Dis. Model. Mech. 2019, 12, dmm036269. [Google Scholar] [CrossRef] [Green Version]
- Suraj, J.; Kurpinska, A.; Sternak, M.; Smolik, M.; Niedzielska-Andres, E.; Zakrzewska, A.; Sacha, T.; Kania, A.; Chlopicki, S.; Walczak, M. Quantitative measurement of selected protein biomarkers of endothelial dysfunction in plasma by micro-liquid chromatography-tandem mass spectrometry based on stable isotope dilution method. Talanta 2019, 194, 1005–1016. [Google Scholar] [CrossRef]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elajaili, H.B.; Hernandez-Lagunas, L.; Ranguelova, K.; Dikalov, S.; Nozik-Grayck, E. Use of Electron Paramagnetic Resonance in Biological Samples at Ambient Temperature and 77 K. J. Vis. Exp. 2019, e58461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czamara, K.; Majka, Z.; Fus, A.; Matjasik, K.; Pacia, M.Z.; Sternak, M.; Chlopicki, S.; Kaczor, A. Raman spectroscopy as a novel tool for fast characterization of the chemical composition of perivascular adipose tissue. Analyst 2018, 143, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Zhang, P.; Ma, X.; Che, K.; Wang, Y. The Differences in Homeostasis Model Assessment Values in Type 2 Diabetic Patients with Different Lengths of History of Diabetes. Arch. Endocrinol. Metab. 2019, 63, 222–227. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992, 35, 771–776. [Google Scholar]
- Standley, P.R.; Bakir, M.H.; Sowers, J.R. Vascular Insulin Abnormalities, Hypertension, and Accelerated Atherosclerosis. Am. J. Kidney Dis. 1993, 21, S39–S46. [Google Scholar] [CrossRef]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Münzel, T.; Daiber, A.; Förstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Victorio, J.A.; Fontes, M.T.; Rossoni, L.V.; Davel, A.P. Different Anti-Contractile Function and Nitric Oxide Production of Thoracic and Abdominal Perivascular Adipose Tissues. Front. Physiol. 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Targosz-Korecka, M.; Jaglarz, M.; Malek-Zietek, K.E.; Gregorius, A.; Zakrzewska, A.; Sitek, B.; Rajfur, Z.; Chlopicki, S.; Szymonski, M. AFM-based detection of glycocalyx degradation and endothelial stiffening in the db/db mouse model of diabetes. Sci. Rep. 2017, 7, 15951. [Google Scholar] [CrossRef] [Green Version]
- Fedorowicz, A.; Buczek, E.; Mateuszuk, Ł.; Czarnowska, E.; Sitek, B.; Jasztal, A.; Chmura-Skirlinska, A.; Dib, M.; Steven, S.; Daiber, A.; et al. Comparison of Pulmonary and Systemic NO- and PGI2-Dependent Endothelial Function in Diabetic Mice. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, A.; Tengbom, J.; Alvarsson, M.; Wernly, B.; Zhou, Z.; Pernow, J. Red Blood Cell Peroxynitrite Causes Endothelial Dysfunction in Type 2 Diabetes Mellitus via Arginase. Cells 2020, 9, 1712. [Google Scholar] [CrossRef]
- Vicent, D.; Ilany, J.; Kondo, T.; Naruse, K.; Fisher, S.J.; Kisanuki, Y.Y.; Bursell, S.; Yanagisawa, M.; King, G.L.; Kahn, C.R. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Investig. 2003, 111, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Fujisaka, S.; Cai, W.; Winnay, J.N.; Konishi, M.; O’Neill, B.T.; Li, M.; García-Martín, R.; Takahashi, H.; Hu, J.; et al. Adipocyte Dynamics and Reversible Metabolic Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab. 2017, 25, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, T.; O’Hare, J.; Diggs-Andrews, K.; Schweiger, M.; Cheng, B.; Lindtner, C.; Zielinski, E.; Vempati, P.; Su, K.; Dighe, S.; et al. Brain Insulin Controls Adipose Tissue Lipolysis and Lipogenesis. Cell Metab. 2011, 13, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Ohishi, M.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nagaretani, H.; Kumada, M.; Ohashi, K.; Okamoto, Y.; Nishizawa, H.; et al. Association of Hypoadiponectinemia With Impaired Vasoreactivity. Hypertension 2003, 42, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Westphal, S.; Taneva, E.; Kästner, S.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Kropf, S.; Dierkes, J.; Luley, C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis 2006, 185, 313–319. [Google Scholar] [CrossRef]
- Van Dam, A.D.; Boon, M.R.; Berbée, J.F.; Rensen, P.C.; van Harmelen, V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur. J. Pharmacol. 2017, 816, 82–92. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Vehicle | S961 | p |
|---|---|---|---|
| Creatinine [µmol/L] | 18.8 (16.1–20.0) | 11.5 (8.1–16.4) * | 0.033 |
| TG [mmol/L] | 0.91 (0.74–1.10) | 1.34 (0.93–1.64) * | 0.029 |
| TC [mmol/L] | 2.40 ± 0.18 | 2.32 ± 0.09 | 0.67 |
| HDL [mmol/L] | 1.309 ± 0.039 | 1.175 ± 0.026 ** | 0.0001 |
| LDL [mmol/L] | 0.21 (0.19–0.28) | 0.15 (0.13–0.23) | 0.058 |
| ALT [U/L] | 39.89 ± 4.65 | 57.54 ± 2.82 ** | 0.003 |
| AST [U/L] | 84.18 ± 8.96 | 94.74 ± 6.64 | 0.35 |
| LDH [U/L] | 754 ± 76 | 693 ± 65 | 0.55 |
| HbA1c [%] | 5.6 (5.5–5.8) | 10.0 (9.1–10.5) **** | <0.0001 |
| Plasma glucose [mmol/L] | 12.2 ± 0.8 | 33.7 ± 1.2 **** | <0.0001 |
| Blood glucose [mmol/L] | 6.8 ± 0.6 | 29.6 ± 0.6 **** | <0.0001 |
| C-peptide 2 [nmol/L] | 0.6 (0.4–1.4) | 15.5 (14.5–17.8) **** | <0.0001 |
| HOMA-IRC-peptide | 0.4 (0.2–0.6) | 23.1 (19.6–25.7) **** | <0.0001 |
| HOMA-β | 1.1 (0.7–5.0) | 9.8 (9.3–11.9) **** | <0.0001 |
| GSH:GSSH ratio | 30.7 ± 4.5 | 16.8 ± 2.5 * | 0.013 |
| Blood Count | Vehicle | S961 | p |
|---|---|---|---|
| White blood cells [103/µL] | 2.83 ± 0.18 | 2.47 ± 0.18 | 0.19 |
| Red blood cells [106/µL] | 9.62 ± 0.24 | 10.96 ± 0.15 **** | 0.000151 |
| Hemoglobin [103/µL] | 13.97 ± 0.41 | 15.96 ± 0.21 **** | 0.000248 |
| Hematocrit [%] | 50.3 ± 1.50 | 59.1 ± 0.80 **** | 0.000046 |
| Mean corpuscular volume [fL] | 52.1 ± 0.50 | 53.9 ± 0.50 * | 0.031 |
| Mean corpuscular hemoglobin [pg] | 14.5 ± 0.20 | 14.6 ± 0.10 | 0.87 |
| Mean corpuscular hemoglobin concentration [g/dL] | 27.8 ± 0.20 | 27.0 ± 0.20 * | 0.028 |
| Platelets [103/µL] | 1280 ± 52 | 1331 ± 58 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proniewski, B.; Bar, A.; Kieronska-Rudek, A.; Suraj-Prażmowska, J.; Buczek, E.; Czamara, K.; Majka, Z.; Czyzynska-Cichon, I.; Kwiatkowski, G.; Matyjaszczyk-Gwarda, K.; et al. Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice. Cells 2021, 10, 1448. https://doi.org/10.3390/cells10061448
Proniewski B, Bar A, Kieronska-Rudek A, Suraj-Prażmowska J, Buczek E, Czamara K, Majka Z, Czyzynska-Cichon I, Kwiatkowski G, Matyjaszczyk-Gwarda K, et al. Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice. Cells. 2021; 10(6):1448. https://doi.org/10.3390/cells10061448
Chicago/Turabian StyleProniewski, Bartosz, Anna Bar, Anna Kieronska-Rudek, Joanna Suraj-Prażmowska, Elżbieta Buczek, Krzysztof Czamara, Zuzanna Majka, Izabela Czyzynska-Cichon, Grzegorz Kwiatkowski, Karolina Matyjaszczyk-Gwarda, and et al. 2021. "Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice" Cells 10, no. 6: 1448. https://doi.org/10.3390/cells10061448
APA StyleProniewski, B., Bar, A., Kieronska-Rudek, A., Suraj-Prażmowska, J., Buczek, E., Czamara, K., Majka, Z., Czyzynska-Cichon, I., Kwiatkowski, G., Matyjaszczyk-Gwarda, K., & Chlopicki, S. (2021). Systemic Administration of Insulin Receptor Antagonist Results in Endothelial and Perivascular Adipose Tissue Dysfunction in Mice. Cells, 10(6), 1448. https://doi.org/10.3390/cells10061448






