The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era
Abstract
1. Introduction
1.1. Standard Therapeutic Options in Hematological Malignancies
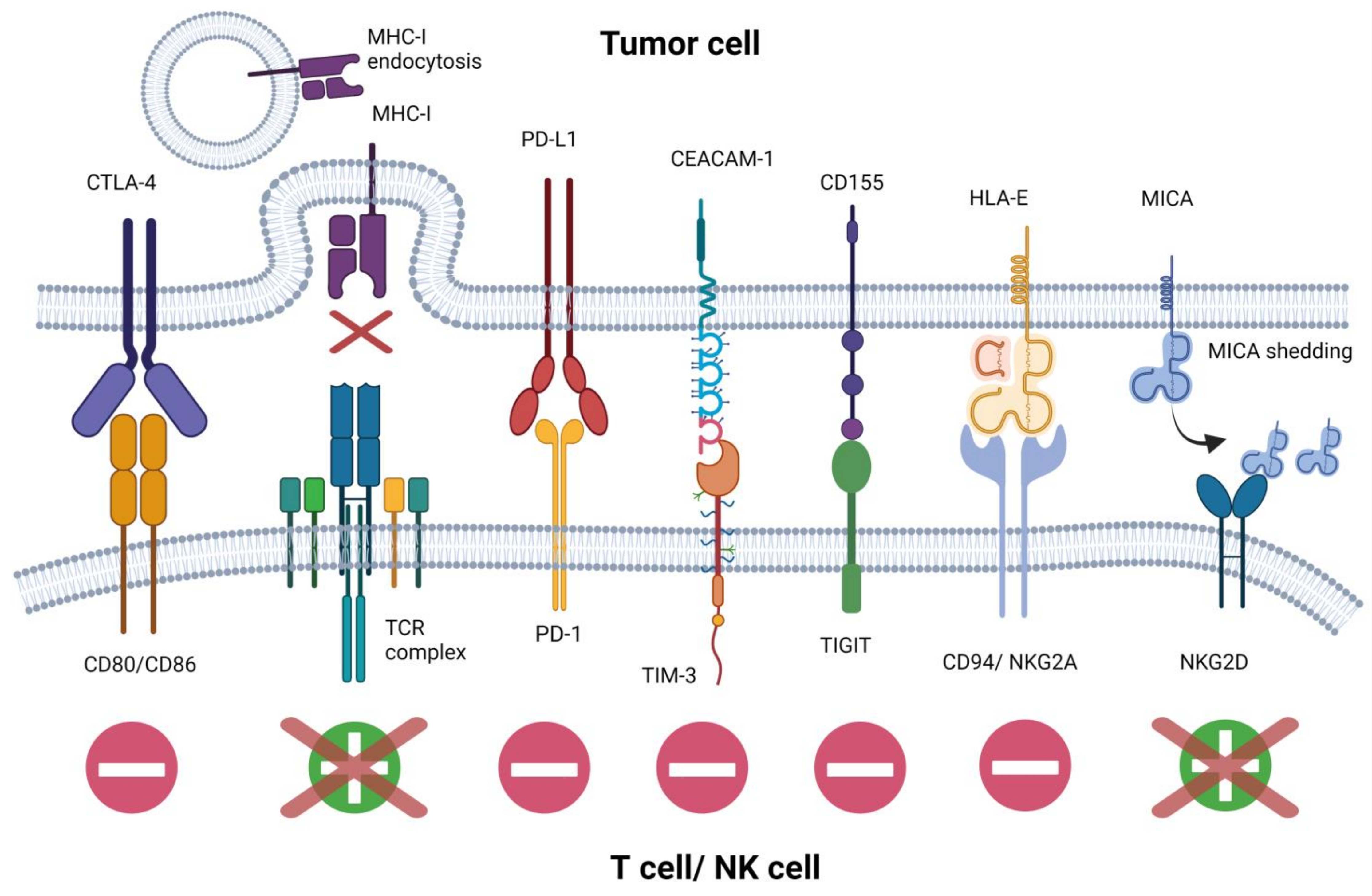
1.2. Mechanisms of Immune Evasion
2. CAR-T in Onco-Hematology
2.1. Registered CAR-T Approaches
2.2. Experimental CAR-T Approaches Targeting Other Antigens
2.3. Limitations
2.3.1. Toxicities
2.3.2. Strategies to Overcome CAR-T Limitations
2.3.3. Financial Aspects
3. NK Cell-Based Immunotherapies
3.1. NK Cell Harnessing Strategies
3.2. Checkpoint Blockade and ADCC Enhancement Strategy
3.3. Off-the-Shelf NK Cell Transfer
3.4. Specific Killer Engagers
3.5. CAR-NK Concept Outsmarts CAR-T Cells
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lake, R.A.; Robinson, B.W.S. Immunotherapy and Chemotherapy—A Practical Partnership. Nat. Rev. Cancer 2005, 5, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Im, A.; Pavletic, S.Z. Immunotherapy in Hematologic Malignancies: Past, Present, and Future. J. Hematol. Oncol. 2017, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-Y.; Seo, H.; Lee, J.; Jung, H. Immunotherapy in Hematologic Malignancies: Emerging Therapies and Novel Approaches. Int. J. Mol. Sci. 2020, 21, 8000. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Relapsed or Refractory Acute Lymphoblastic Leukemia in Adults—UpToDate. Available online: https://www.uptodate.com/contents/treatment-of-relapsed-or-refractory-acute-lymphoblastic-leukemia-in-adults (accessed on 8 June 2021).
- Braig, F.; Brandt, A.; Goebeler, M.; Tony, H.-P.; Kurze, A.-K.; Nollau, P.; Bumm, T.; Böttcher, S.; Bargou, R.C.; Binder, M. Resistance to Anti-CD19/CD3 BiTE in Acute Lymphoblastic Leukemia May Be Mediated by Disrupted CD19 Membrane Trafficking. Blood 2017, 129, 100–104. [Google Scholar] [CrossRef]
- Feugier, P.; Van Hoof, A.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Fermé, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients With Diffuse Large B-Cell Lymphoma: A Study by the Groupe d’Etude Des Lymphomes de l’Adulte. J. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trümper, L.; Österborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1708566 (accessed on 7 June 2021).
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J. Clin. Oncol. 2015, 33, 2516–2522. [Google Scholar] [CrossRef]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in Refractory Diffuse Large B-Cell Lymphoma: Results from the International SCHOLAR-1 Study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Berendsen, M.R.; Stevens, W.B.C.; van den Brand, M.; van Krieken, J.H.; Scheijen, B. Molecular Genetics of Relapsed Diffuse Large B-Cell Lymphoma: Insight into Mechanisms of Therapy Resistance. Cancers 2020, 12, 3553. [Google Scholar] [CrossRef]
- Teoh, P.J.; Chng, W.J. CAR T-Cell Therapy in Multiple Myeloma: More Room for Improvement. Blood Cancer J. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J. Treatment Options for Triple-Class Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic Aberrations and Survival in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef]
- Thompson, P.A.; O’Brien, S.M.; Wierda, W.G.; Ferrajoli, A.; Stingo, F.; Smith, S.C.; Burger, J.A.; Estrov, Z.; Jain, N.; Kantarjian, H.M.; et al. Complex Karyotype Is a Stronger Predictor than Del (17p) for an Inferior Outcome in Relapsed or Refractory Chronic Lymphocytic Leukemia Patients Treated with Ibrutinib-Based Regimens. Cancer 2015, 121, 3612–3621. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Schnaiter, A.; Paschka, P.; Zenz, T.; Rossi, M.; Döhner, K.; Bühler, A.; Böttcher, S.; Ritgen, M.; Kneba, M.; et al. Gene Mutations and Treatment Outcome in Chronic Lymphocytic Leukemia: Results from the CLL8 Trial. Blood 2014, 123, 3247–3254. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients with Chronic Lymphocytic Leukaemia: A Randomised, Open-Label, Phase 3 Trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Bair, S.M.; Porter, D.L. Accelerating Chimeric Antigen Receptor Therapy in Chronic Lymphocytic Leukemia: The Development and Challenges of Chimeric Antigen Receptor T-Cell Therapy for Chronic Lymphocytic Leukemia. Am. J. Hematol. 2019, 94, S10–S17. [Google Scholar] [CrossRef]
- Lemal, R.; Tournilhac, O. State-of-the-Art for CAR T-Cell Therapy for Chronic Lymphocytic Leukemia in 2019. J. Immunother. Cancer 2019, 7, 202. [Google Scholar] [CrossRef]
- Schetelig, J.; Dreger, P. Chronic Lymphocytic Leukemia. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Carreras, E., Dufour, C., Mohty, M., Kröger, N., Eds.; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-02277-8. [Google Scholar]
- Smith, S.K.; Zimmerman, S.; Williams, C.S.; Zebrack, B.J. Health Status and Quality of Life among Non-Hodgkin Lymphoma Survivors. Cancer 2009, 115. [Google Scholar] [CrossRef]
- Oerlemans, S.; Mols, F.; Nijziel, M.R.; Lybeert, M.; van de Poll-Franse, L.V. The Impact of Treatment, Socio-Demographic and Clinical Characteristics on Health-Related Quality of Life among Hodgkin’s and Non-Hodgkin’s Lymphoma Survivors: A Systematic Review. Ann. Hematol. 2011, 90. [Google Scholar] [CrossRef]
- Tward, J.D.; Wendland, M.M.; Shrieve, D.C.; Szabo, A.; Gaffney, D.K. The Risk of Secondary Malignancies over 30 Years after the Treatment of Non-Hodgkin Lymphoma. Cancer 2006, 107. [Google Scholar] [CrossRef]
- Travis, L.B.; Curtis, R.E.; Glimelius, B.; Holowaty, E.; Leeuwen, F.E.V.; Lynch, C.F.; Adami, J.; Gospodarowicz, M.; Wacholder, S.; Inskip, P. Second Cancers among Long-Term Survivors of Non-Hodgkin’s Lymphoma. J. Natl. Cancer Inst. 1993, 85. [Google Scholar] [CrossRef] [PubMed]
- Hequet, O.; Le, Q.H.; Moullet, I.; Pauli, E.; Salles, G.; Espinouse, D.; Dumontet, C.; Thieblemont, C.; Arnaud, P.; Antal, D.; et al. Subclinical Late Cardiomyopathy after Doxorubicin Therapy for Lymphoma in Adults. J. Clin. Oncol. 2004, 22. [Google Scholar] [CrossRef]
- Moser, E.C.; Noordijk, E.M.; van Leeuwen, F.E.; le Cessie, S.; Baars, J.W.; Thomas, J.; Carde, P.; Meerwaldt, J.H.; van Glabbeke, M.; Kluin-Nelemans, H.C. Long-Term Risk of Cardiovascular Disease after Treatment for Aggressive Non-Hodgkin Lymphoma. Blood 2006, 107. [Google Scholar] [CrossRef] [PubMed]
- Murbraech, K.; Smeland, K.B.; Holte, H.; Loge, J.H.; Lund, M.B.; Wethal, T.; Holte, E.; Rösner, A.; Dalen, H.; Kvaløy, S.; et al. Heart Failure and Asymptomatic Left Ventricular Systolic Dysfunction in Lymphoma Survivors Treated With Autologous Stem-Cell Transplantation: A National Cross-Sectional Study. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Burnet, M. Cancer—A Biological Approach: I. The Processes of Control. II. The significance of somatic mutation. Br. Med. J. 1957, 1, 779–786. [Google Scholar] [CrossRef]
- Burnet, F.M. Immunological Aspects of Malignant Disease. Lancet 1967, 1, 1171–1174. [Google Scholar] [CrossRef]
- Lichtman, M.A. Battling the Hematological Malignancies: The 200 Years’ War. Oncologist 2008, 13, 126–138. [Google Scholar] [CrossRef]
- Sun, C.; Dotti, G.; Savoldo, B. Utilizing Cell-Based Therapeutics to Overcome Immune Evasion in Hematologic Malignancies. Blood 2016, 127, 3350–3359. [Google Scholar] [CrossRef]
- Tang, S.; Ning, Q.; Yang, L.; Mo, Z.; Tang, S. Mechanisms of Immune Escape in the Cancer Immune Cycle. Int. Immunopharmacol. 2020, 86, 106700. [Google Scholar] [CrossRef]
- Curran, E.K.; Godfrey, J.; Kline, J. Mechanisms of Immune Tolerance in Leukemia and Lymphoma. Trends Immunol. 2017, 38, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H. The Targeting of Immunosuppressive Mechanisms in Hematological Malignancies. Leukemia 2014, 28, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Petty, A.J.; Yang, Y. Tumor-Associated Macrophages in Hematologic Malignancies: New Insights and Targeted Therapies. Cells 2019, 8, 1526. [Google Scholar] [CrossRef]
- Komohara, Y.; Niino, D.; Ohnishi, K.; Ohshima, K.; Takeya, M. Role of Tumor-Associated Macrophages in Hematological Malignancies. Pathol. Int. 2015, 65, 170–176. [Google Scholar] [CrossRef]
- De Veirman, K.; Van Valckenborgh, E.; Lahmar, Q.; Geeraerts, X.; De Bruyne, E.; Menu, E.; Van Riet, I.; Vanderkerken, K.; Van Ginderachter, J.A. Myeloid-Derived Suppressor Cells as Therapeutic Target in Hematological Malignancies. Front. Oncol. 2014, 4, 349. [Google Scholar] [CrossRef] [PubMed]
- Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Riviere, I. The Basic Principles of Chimeric Antigen Receptor (CAR) Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Tran, E.; Longo, D.L.; Urba, W.J. A Milestone for CAR T Cells. N. Engl. J. Med. 2017, 377, 2593–2596. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, D. T Cell Antigen Receptor Signal Transduction Pathways. Annu. Rev. Immunol. 1996, 14, 259–274. [Google Scholar] [CrossRef]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Haji-Fatahaliha, M.; Hosseini, M.; Akbarian, A.; Sadreddini, S.; Jadidi-Niaragh, F.; Yousefi, M. CAR-Modified T-Cell Therapy for Cancer: An Updated Review. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1339–1349. [Google Scholar] [CrossRef]
- Salter, A.I.; Ivey, R.G.; Kennedy, J.J.; Voillet, V.; Rajan, A.; Alderman, E.J.; Voytovich, U.J.; Lin, C.; Sommermeyer, D.; Liu, L.; et al. Phosphoproteomic Analysis of Chimeric Antigen Receptor Signaling Reveals Kinetic and Quantitative Differences That Affect Cell Function. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Harris, D.T.; Kranz, D.M. Adoptive T Cell Therapies: A Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol. Sci. 2016, 37, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T Cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef]
- Shank, B.R.; Do, B.; Sevin, A.; Chen, S.E.; Neelapu, S.S.; Horowitz, S.B. Chimeric Antigen Receptor T Cells in Hematologic Malignancies. Pharmacotherapy 2017, 37, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Brocker, T.; Karjalainen, K. Signals through T Cell Receptor-Zeta Chain Alone Are Insufficient to Prime Resting T Lymphocytes. J. Exp. Med. 1995, 181, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.A.; Allison, J.P. Co-Stimulation in T Cell Responses. Curr. Opin. Immunol. 1997, 9, 396–404. [Google Scholar] [CrossRef]
- Pulè, M.A.; Straathof, K.C.; Dotti, G.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K. A Chimeric T Cell Antigen Receptor That Augments Cytokine Release and Supports Clonal Expansion of Primary Human T Cells. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 12, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhu, Y.-M.; Zheng, L.-L.; Shen, H.-J.; Ou, R.-M.; Liu, Z.; She, Y.-L.; Chen, R.; Li, C.; Huang, J.; et al. Chimeric Antigen Receptor-T Cells with 4-1BB Co-Stimulatory Domain Present a Superior Treatment Outcome than Those with CD28 Domain Based on Bioinformatics. Acta Haematol. 2018, 140, 131–140. [Google Scholar] [CrossRef]
- Yi, Z.; Prinzing, B.L.; Cao, F.; Gottschalk, S.; Krenciute, G. Optimizing EphA2-CAR T Cells for the Adoptive Immunotherapy of Glioma. Mol. Ther. Methods Clin. Dev. 2018, 9, 70–80. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKS, the Fourth-Generation CAR T Cells: Current Developments and Clinical Translation. Adv. Cell Gene Ther. 2020, 3, e84. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Yu, Z.; Frasheri, D.; Restifo, N.P.; Rosenberg, S.A. Adoptive Transfer of Syngeneic T Cells Transduced with a Chimeric Antigen Receptor That Recognizes Murine CD19 Can Eradicate Lymphoma and Normal B Cells. Blood 2010, 116, 3875–3886. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Yang, J.C.; Sherry, R.M.; Feldman, S.A.; McIntyre, L.; Bot, A.; Rossi, J.; Lam, N.; et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD19 Chimeric Antigen Receptor T Cell Therapy. Mol. Ther. 2017, 25, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S. CAR-T Efficacy: Is Conditioning the Key? Blood 2019, 133, 1799–1800. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Finkelstein, S.E.; Klebanoff, C.A.; Antony, P.A.; Palmer, D.C.; Spiess, P.J.; Hwang, L.N.; Yu, Z.; Wrzesinski, C.; Heimann, D.M.; et al. Removal of Homeostatic Cytokine Sinks by Lymphodepletion Enhances the Efficacy of Adoptively Transferred Tumor-Specific CD8+ T Cells. J. Exp. Med. 2005, 202, 907–912. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Khong, H.T.; Antony, P.A.; Palmer, D.C.; Restifo, N.P. Sinks, Suppressors and Antigen Presenters: How Lymphodepletion Enhances T Cell-Mediated Tumor Immunotherapy. Trends Immunol. 2005, 26, 111–117. [Google Scholar] [CrossRef]
- North, R.J. Cyclophosphamide-Facilitated Adoptive Immunotherapy of an Established Tumor Depends on Elimination of Tumor-Induced Suppressor T Cells. J. Exp. Med. 1982, 155, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Maude, S.L.; Rives, S.; Baruchel, A.; Boyer, M.; Bittencourt, H.; Bader, P.; Buchner, J.; Laetsch, T.W.; Stefanski, H.; et al. Tisagenlecleucel for the Treatment of Pediatric and Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia: Updated Analysis of the ELIANA Clinical Trial. Biol. Blood Marrow Transplant. 2019, 25, S126–S127. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Halford, Z.; Anderson, M.K.; Bennett, L.L. Axicabtagene Ciloleucel: Clinical Data for the Use of CAR T-Cell Therapy in Relapsed and Refractory Large B-Cell Lymphoma. Ann. Pharmacother. 2021, 55, 390–405. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- FDA. FDA Approval Brings First Gene Therapy to the United States. Available online: https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states (accessed on 26 June 2020).
- FDA. FDA Approves Tisagenlecleucel for Adults with Relapsed or Refractory Large B-Cell Lymphoma; FDA: Silver Spring, MA, USA, 2019.
- Otáhal, P.; Průková, D.; Král, V.; Fabry, M.; Vočková, P.; Latečková, L.; Trněný, M.; Klener, P. Lenalidomide Enhances Antitumor Functions of Chimeric Antigen Receptor Modified T Cells. Oncoimmunology 2016, 5, e1115940. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kenderian, S.S.; Shestova, O.; Fraietta, J.A.; Qayyum, S.; Zhang, Q.; Maus, M.V.; Liu, X.; Nunez-Cruz, S.; Klichinsky, M.; et al. The Addition of the BTK Inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses against Mantle Cell Lymphoma. Clin. Cancer Res. 2016, 22, 2684–2696. [Google Scholar] [CrossRef] [PubMed]
- Randomized, Phase II Dose Optimization Study of Chimeric Antigen Receptor (CAR) Modified T Cells Directed against CD19 in Patients (Pts) with Relapsed, Refractory (R/R) CLL. J. Clin. Oncol. 2014. [CrossRef]
- Turtle, C.J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef]
- Griggio, V.; Perutelli, F.; Salvetti, C.; Boccellato, E.; Boccadoro, M.; Vitale, C.; Coscia, M. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 594556. [Google Scholar] [CrossRef]
- Gill, S.; Frey, N.V.; Hexner, E.O.; Lacey, S.F.; Melenhorst, J.J.; Byrd, J.C.; Metzger, S.; Marcus, T.; Gladney, W.; Marcucci, K.; et al. CD19 CAR-T Cells Combined with Ibrutinib to Induce Complete Remission in CLL. J. Clin. Oncol. 2017, 35, 7509. [Google Scholar] [CrossRef]
- Geyer, M.B.; Rivière, I.; Sénéchal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Palomba, M.L.; Halton, E.; et al. Safety and Tolerability of Conditioning Chemotherapy Followed by CD19-Targeted CAR T Cells for Relapsed/Refractory CLL. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B Cell Maturation Antigen-Specific CAR T Cells Are Clinically Active in Multiple Myeloma. J. Clin. Invest. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Update of CARTITUDE-1: A Phase Ib/II Study of JNJ-4528, a B-Cell Maturation Antigen (BCMA)-Directed CAR-T-Cell Therapy, in Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2020. [CrossRef]
- Janssen Research and Development, LLC. A Phase 1b-2, Open-Label Study of JNJ-68284528, a Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Directed against BCMA in Subjects with Relapsed or Refractory Multiple Myeloma; Janssen Research and Development, LLC: Raritan, NJ, USA, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03548207 (accessed on 8 June 2021).
- Madduri, D.; Usmani, S.Z.; Jagannath, S.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Banerjee, A.; Goldberg, J.D.; Schecter, J.M.; et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM). Blood 2019, 134, 577. [Google Scholar] [CrossRef]
- Mardiana, S.; Gill, S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 Antibodies in Multiple Myeloma: Back to the Future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Rodríguez-Otero, P.; Prósper, F.; Alfonso, A.; Paiva, B.; San Miguel, J.F. CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. J. Clin. Med. 2020, 9, 3577. [Google Scholar] [CrossRef]
- Mihara, K.; Bhattacharyya, J.; Kitanaka, A.; Yanagihara, K.; Kubo, T.; Takei, Y.; Asaoku, H.; Takihara, Y.; Kimura, A. T-Cell Immunotherapy with a Chimeric Receptor against CD38 Is Effective in Eliminating Myeloma Cells. Leukemia 2012, 26, 365–367. [Google Scholar] [CrossRef]
- Mihara, K.; Yanagihara, K.; Takigahira, M.; Imai, C.; Kitanaka, A.; Takihara, Y.; Kimura, A. Activated T-Cell-Mediated Immunotherapy with a Chimeric Receptor against CD38 in B-Cell Non-Hodgkin Lymphoma. J. Immunother. 2009, 32, 737–743. [Google Scholar] [CrossRef]
- Holthof, L.C.; van der Schans, J.J.; Katsarou, A.; Poels, R.; Gelderloos, A.T.; Drent, E.; van Hal-van Veen, S.E.; Li, F.; Zweegman, S.; van de Donk, N.W.C.J.; et al. Bone Marrow Mesenchymal Stromal Cells Can Render Multiple Myeloma Cells Resistant to Cytotoxic Machinery of CAR T Cells through Inhibition of Apoptosis. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mei, H.; Hu, Y.; Guo, T.; Liu, L.; Jiang, H.; Tang, L.; Wu, Y.; Ai, L.; Deng, J.; et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 2019, 134, 930. [Google Scholar] [CrossRef]
- Poels, R.; Drent, E.; Lameris, R.; Katsarou, A.; Themeli, M.; van der Vliet, H.J.; de Gruijl, T.D.; van de Donk, N.W.C.J.; Mutis, T. Preclinical Evaluation of Invariant Natural Killer T Cells Modified with CD38 or BCMA Chimeric Antigen Receptors for Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.W.C.J.; Mutis, T.; Szegezdi, E.; et al. CD38 Knockout Natural Killer Cells Expressing an Affinity Optimized CD38 Chimeric Antigen Receptor Successfully Target Acute Myeloid Leukemia with Reduced Effector Cell Fratricide. Haematologica 2020. [Google Scholar] [CrossRef]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-CAR T Cells Induce Remissions in CD19-CAR Naïve and Resistant B-ALL. Nat. Med. 2018, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Meerten, T.; Hagenbeek, A. CD20-Targeted Therapy: A Breakthrough in the Treatment of Non-Hodgkin’s Lymphoma. Neth. J. Med. 2009, 67, 251–259. [Google Scholar]
- Doraiswamy, A.; Shah, M.R.; Bannerji, R. Immunotherapies Old and New: Hematopoietic Stem Cell Transplant, Chimeric Antigen Receptor T Cells, and Bispecific Antibodies for the Treatment of Relapsed/Refractory Diffuse Large B Cell Lymphoma. Curr. Hematol. Malig. Rep. 2021, 16, 72–81. [Google Scholar] [CrossRef]
- Till, B.G.; Jensen, M.C.; Wang, J.; Chen, E.Y.; Wood, B.L.; Greisman, H.A.; Qian, X.; James, S.E.; Raubitschek, A.; Forman, S.J.; et al. Adoptive Immunotherapy for Indolent Non-Hodgkin Lymphoma and Mantle Cell Lymphoma Using Genetically Modified Autologous CD20-Specific T Cells. Blood 2008, 112, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Han, P.; Qi, X.; Li, F.; Li, M.; Fan, L.; Zhang, H.; Zhang, X.; Yang, X. 4-1BB Signaling Boosts the Anti-Tumor Activity of CD28-Incorporated 2nd Generation Chimeric Antigen Receptor-Modified T Cells. Front. Immunol. 2020, 11, 539654. [Google Scholar] [CrossRef]
- Chen, F.; Fan, C.; Gu, X.; Zhang, H.; Liu, Q.; Gao, X.; Lu, J.; He, B.; Lai, X. Construction of Anti-CD20 Single-Chain Antibody-CD28-CD137-TCRζ Recombinant Genetic Modified T Cells and Its Treatment Effect on B Cell Lymphoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 2110–2115. [Google Scholar] [CrossRef]
- Lee, S.Y.; Olsen, P.; Lee, D.H.; Kenoyer, A.L.; Budde, L.E.; O’Steen, S.; Green, D.J.; Heimfeld, S.; Jensen, M.C.; Riddell, S.R.; et al. Preclinical Optimization of a CD20-Specific Chimeric Antigen Receptor Vector and Culture Conditions. J. Immunother. 2018, 41, 19–31. [Google Scholar] [CrossRef]
- Watanabe, K.; Terakura, S.; Martens, A.C.; van Meerten, T.; Uchiyama, S.; Imai, M.; Sakemura, R.; Goto, T.; Hanajiri, R.; Imahashi, N.; et al. Target Antigen Density Governs the Efficacy of Anti-CD20-CD28-CD3 ζ Chimeric Antigen Receptor-Modified Effector CD8+ T Cells. J. Immunol. 2015, 194, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Rufener, G.A.; Press, O.W.; Olsen, P.; Lee, S.Y.; Jensen, M.C.; Gopal, A.K.; Pender, B.; Budde, L.E.; Rossow, J.K.; Green, D.J.; et al. Preserved Activity of CD20-Specific Chimeric Antigen Receptor-Expressing T Cells in the Presence of Rituximab. Cancer Immunol. Res. 2016, 4, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Han, Q.; Liu, Y.; Dai, H.; Guo, Y.; Bo, J.; Fan, H.; Zhang, Y.; Zhang, Y.; et al. Effective Response and Delayed Toxicities of Refractory Advanced Diffuse Large B-Cell Lymphoma Treated by CD20-Directed Chimeric Antigen Receptor-Modified T Cells. Clin. Immunol. 2014, 155, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Guo, Y.; Dai, H.; Yang, Q.; Zhang, Y.; Zhang, Y.; Chen, M.; Wang, C.; Feng, K.; et al. Treatment of CD20-Directed Chimeric Antigen Receptor-Modified T Cells in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma: An Early Phase IIa Trial Report. Signal Transduct. Target. Ther. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Shalabi, H.; Kraft, I.L.; Wang, H.-W.; Yuan, C.M.; Yates, B.; Delbrook, C.; Zimbelman, J.D.; Giller, R.; Stetler-Stevenson, M.; Jaffe, E.S.; et al. Sequential Loss of Tumor Surface Antigens Following Chimeric Antigen Receptor T-Cell Therapies in Diffuse Large B-Cell Lymphoma. Haematologica 2018, 103, e215–e218. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Q.; Liang, X.; Chen, Z.; Zhang, X.; Zhou, X.; Li, M.; Tu, H.; Liu, Y.; Tu, S.; et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Shah, N.N.; Maatman, T.; Hari, P.; Johnson, B. Multi Targeted CAR-T Cell Therapies for B-Cell Malignancies. Front. Oncol. 2019, 9, 146. [Google Scholar] [CrossRef]
- Fousek, K.; Watanabe, J.; Joseph, S.K.; George, A.; An, X.; Byrd, T.T.; Morris, J.S.; Luong, A.; Martínez-Paniagua, M.A.; Sanber, K.; et al. CAR T-Cells That Target Acute B-Lineage Leukemia Irrespective of CD19 Expression. Leukemia 2021, 35, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Xiong, Y.; Wu, D.; Hu, P.; Alabanza, L.; Steimle, B.; Mahmud, H.; Anthony-Gonda, K.; Krueger, W.; Zhu, Z.; et al. Trispecific CD19-CD20-CD22-Targeting DuoCAR-T Cells Eliminate Antigen-Heterogeneous B Cell Tumors in Preclinical Models. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Martyniszyn, A.; Krahl, A.-C.; André, M.C.; Hombach, A.A.; Abken, H. CD20-CD19 Bispecific CAR T Cells for the Treatment of B-Cell Malignancies. Hum. Gene Ther. 2017, 28, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Zah, E.; Lin, M.-Y.; Silva-Benedict, A.; Jensen, M.C.; Chen, Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016, 4, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific Anti-CD20, Anti-CD19 CAR T Cells for Relapsed B Cell Malignancies: A Phase 1 Dose Escalation and Expansion Trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, Z.; Jia, H.; Tong, C.; Guo, Y.; Ti, D.; Han, X.; Liu, Y.; Zhang, W.; Wang, C.; et al. Bispecific CAR-T Cells Targeting Both CD19 and CD22 for Therapy of Adults with Relapsed or Refractory B Cell Acute Lymphoblastic Leukemia. J. Hematol. Oncol. 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Bobrowicz, M.; Kubacz, M.; Slusarczyk, A.; Winiarska, M. CD37 in B Cell Derived Tumors-More than Just a Docking Point for Monoclonal Antibodies. Int. J. Mol. Sci. 2020, 21, 9531. [Google Scholar] [CrossRef]
- Scarfò, I.; Ormhøj, M.; Frigault, M.J.; Castano, A.P.; Lorrey, S.; Bouffard, A.A.; van Scoyk, A.; Rodig, S.J.; Shay, A.J.; Aster, J.C.; et al. Anti-CD37 Chimeric Antigen Receptor T Cells Are Active against B- and T-Cell Lymphomas. Blood 2018, 132, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Köksal, H.; Dillard, P.; Josefsson, S.E.; Maggadottir, S.M.; Pollmann, S.; Fåne, A.; Blaker, Y.N.; Beiske, K.; Huse, K.; Kolstad, A.; et al. Preclinical Development of CD37CAR T-Cell Therapy for Treatment of B-Cell Lymphoma. Blood Adv. 2019, 3, 1230–1243. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Zhou, H.; Li, F.; Valentine, M.; Sun, J.; Berahovich, R.; Xu, S.; Quintanilla, M.; Ma, M.C.; Sienkiewicz, J.; et al. Novel CD37, Humanized CD37 and Bi-Specific Humanized CD37-CD19 CAR-T Cells Specifically Target Lymphoma. Cancers 2021, 13, 981. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kemler, I.; Dingli, D. Preclinical Development of CD126 CAR-T Cells with Broad Antitumor Activity. Blood Cancer J. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T Cell–Induced Cytokine Release Syndrome Is Mediated by Macrophages and Abated by IL-1 Blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Song, Y.; Liu, D. CD19 CAR-T Cell Therapy for Relapsed/Refractory Acute Lymphoblastic Leukemia: Factors Affecting Toxicities and Long-Term Efficacies. J. Hematol. Oncol. 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-Cell Therapy in Patients with B-Cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, J.; Wu, Z.; Yu, J.; Cui, Q.; Pu, C.; Liang, B.; Luo, Y.; Shi, J.; Jin, A.; et al. Predominant Cerebral Cytokine Release Syndrome in CD19-Directed Chimeric Antigen Receptor-Modified T Cell Therapy. J. Hematol. Oncol. 2016, 9, 70. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet Lond. Engl. 2015, 385, 517–528. [Google Scholar] [CrossRef]
- CAR T-Cell Leukemia Trial Put on Hold after Two Deaths. Available online: http://www.medscape.com/viewarticle/865878 (accessed on 18 July 2020).
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric Antigen Receptor T-Cell Therapy—Assessment and Management of Toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Amrolia, P.J.; Pule, M. Chimeric Antigen Receptor T Cells for ALL. Lancet Lond. Engl. 2015, 385, 488–490. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Boyiadzis, M.M.; Dhodapkar, M.V.; Brentjens, R.J.; Kochenderfer, J.N.; Neelapu, S.S.; Maus, M.V.; Porter, D.L.; Maloney, D.G.; Grupp, S.A.; Mackall, C.L.; et al. Chimeric Antigen Receptor (CAR) T Therapies for the Treatment of Hematologic Malignancies: Clinical Perspective and Significance. J. Immunother. Cancer 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Kansagra, A.J.; Frey, N.V.; Bar, M.; Laetsch, T.W.; Carpenter, P.A.; Savani, B.N.; Heslop, H.E.; Bollard, C.M.; Komanduri, K.V.; Gastineau, D.A.; et al. Clinical Utilization of Chimeric Antigen Receptor T-Cells (CAR-T) in B-Cell Acute Lymphoblastic Leukemia (ALL)-an Expert Opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant. 2019, 54, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Caulier, B.; Enserink, J.M.; Wälchli, S. Pharmacologic Control of CAR T Cells. Int. J. Mol. Sci. 2021, 22, 4320. [Google Scholar] [CrossRef]
- Philip, B.; Kokalaki, E.; Mekkaoui, L.; Thomas, S.; Straathof, K.; Flutter, B.; Marin, V.; Marafioti, T.; Chakraverty, R.; Linch, D.; et al. A Highly Compact Epitope-Based Marker/Suicide Gene for Easier and Safer T-Cell Therapy. Blood 2014, 124, 1277–1287. [Google Scholar] [CrossRef]
- Wang, X.; Chang, W.-C.; Wong, C.W.; Colcher, D.; Sherman, M.; Ostberg, J.R.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. A Transgene-Encoded Cell Surface Polypeptide for Selection, in Vivo Tracking, and Ablation of Engineered Cells. Blood 2011, 118, 1255–1263. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.-K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef]
- Gargett, T.; Brown, M.P. The Inducible Caspase-9 Suicide Gene System as a “Safety Switch” to Limit on-Target, off-Tumor Toxicities of Chimeric Antigen Receptor T Cells. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Amatya, C.; Pegues, M.A.; Lam, N.; Vanasse, D.; Geldres, C.; Choi, S.; Hewitt, S.M.; Feldman, S.A.; Kochenderfer, J.N. Development of CAR T Cells Expressing a Suicide Gene Plus a Chimeric Antigen Receptor Targeting Signaling Lymphocytic-Activation Molecule F7. Mol. Ther. 2021, 29, 702–717. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11. [Google Scholar] [CrossRef]
- Mestermann, K.; Giavridis, T.; Weber, J.; Rydzek, J.; Frenz, S.; Nerreter, T.; Mades, A.; Sadelain, M.; Einsele, H.; Hudecek, M. The Tyrosine Kinase Inhibitor Dasatinib Acts as a Pharmacologic on/off Switch for CAR T Cells. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Yu, K.-R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.-H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef] [PubMed]
- Zhylko, A.; Winiarska, M.; Graczyk-Jarzynka, A. The Great War of Today: Modifications of CAR-T Cells to Effectively Combat Malignancies. Cancers 2020, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Marks, I.; Srinivasarao, M.; Kanduluru, A.K.; Mahalingam, S.M.; Liu, X.; Chu, H.; Low, P.S. Use of a Single CAR T Cell and Several Bispecific Adapters Facilitates Eradication of Multiple Antigenically Different Solid Tumors. Cancer Res. 2019, 79, 387–396. [Google Scholar] [CrossRef]
- Urbanska, K.; Lanitis, E.; Poussin, M.; Lynn, R.C.; Gavin, B.P.; Kelderman, S.; Yu, J.; Scholler, N.; Powell, D.J. A Universal Strategy for Adoptive Immunotherapy of Cancer through Use of a Novel T-Cell Antigen Receptor. Cancer Res. 2012, 72, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Fasslrinner, F.; Loureiro, L.R.; Koristka, S.; Feldmann, A.; Bachmann, M. Adaptor CAR Platforms-Next Generation of T Cell-Based Cancer Immunotherapy. Cancers 2020, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Mittelstaet, J.; Baden, C.; Chan, K.C.-H.; Seitz, C.; Schlegel, P.; Kaiser, A.; Handgretinger, R.; Schleicher, S. Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells: An off-the-Shelf Cellular Therapeutic for Universal Tumor Targeting. Oncoimmunology 2020, 9, 1825177. [Google Scholar] [CrossRef]
- Seitz, C.M.; Schlegel, P.; Hau, J.; Krahl, A.-C.; Schroeder, S.; Bender, G.; Reiter, S.; Schleicher, S.; Schilbach, K.; Ebinger, M.; et al. Novel Adapter Chimeric Antigen Receptor (ACAR) T Cells for Temporally Controllable Targeting of Single and Multiple Tumor Antigens. Blood 2017, 130, 1912. [Google Scholar] [CrossRef]
- Hernandez, I.; Prasad, V.; Gellad, W.F. Accounting for All Costs in the Total Cost of Chimeric Antigen Receptor T-Cell Immunotherapy-Reply. JAMA Oncol. 2018, 4, 1785–1786. [Google Scholar] [CrossRef]
- Whittington, M.D.; Ollendorf, D.A.; Campbell, J.D. Accounting for All Costs in the Total Cost of Chimeric Antigen Receptor T-Cell Immunotherapy. JAMA Oncol. 2018, 4, 1784–1785. [Google Scholar] [CrossRef] [PubMed]
- CAR-T Therapies: Final Evidence Report. Available online: https://icer-review.org/material/car-t-final-report/ (accessed on 21 July 2020).
- Kim, D.W.; Cho, J.-Y. Recent Advances in Allogeneic CAR-T Cells. Biomolecules 2020, 10, 263. [Google Scholar] [CrossRef]
- Perez, C.; Gruber, I.; Arber, C. Off-the-Shelf Allogeneic T Cell Therapies for Cancer: Opportunities and Challenges Using Naturally Occurring “Universal” Donor T Cells. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human Natural Killer Cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK Cells and Cancer: You Can Teach Innate Cells New Tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Cohen, A.D.; Jagannath, S.; Munshi, N.C.; Spitzer, G.; Hofmeister, C.C.; Efebera, Y.A.; Andre, P.; Zerbib, R.; Caligiuri, M.A. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2015, 21, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Vey, N.; Bourhis, J.-H.; Boissel, N.; Bordessoule, D.; Prebet, T.; Charbonnier, A.; Etienne, A.; Andre, P.; Romagne, F.; Benson, D.; et al. A Phase 1 Trial of the Anti-Inhibitory KIR MAb IPH2101 for AML in Complete Remission. Blood 2012, 120, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Plonquet, A.; Haioun, C.; Jais, J.-P.; Debard, A.-L.; Salles, G.; Bene, M.-C.; Feugier, P.; Rabian, C.; Casasnovas, O.; Labalette, M.; et al. Peripheral Blood Natural Killer Cell Count Is Associated with Clinical Outcome in Patients with AaIPI 2-3 Diffuse Large B-Cell Lymphoma. Ann. Oncol. 2007, 18, 1209–1215. [Google Scholar] [CrossRef]
- Carlsten, M.; Järås, M. Natural Killer Cells in Myeloid Malignancies: Immune Surveillance, NK Cell Dysfunction, and Pharmacological Opportunities to Bolster the Endogenous NK Cells. Front. Immunol. 2019, 10, 2357. [Google Scholar] [CrossRef] [PubMed]
- Scoville, S.D.; Nalin, A.P.; Chen, L.; Chen, L.; Zhang, M.H.; McConnell, K.; Beceiro Casas, S.; Ernst, G.; Traboulsi, A.A.-R.; Hashi, N.; et al. Human AML Activates the Aryl Hydrocarbon Receptor Pathway to Impair NK Cell Development and Function. Blood 2018, 132, 1792–1804. [Google Scholar] [CrossRef]
- Reiners, K.S.; Kessler, J.; Sauer, M.; Rothe, A.; Hansen, H.P.; Reusch, U.; Hucke, C.; Köhl, U.; Dürkop, H.; Engert, A.; et al. Rescue of Impaired NK Cell Activity in Hodgkin Lymphoma with Bispecific Antibodies in Vitro and in Patients. Mol. Ther. 2013, 21, 895–903. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor Regression. Clin. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Björklund, A.T.; Carlsten, M.; Sohlberg, E.; Liu, L.L.; Clancy, T.; Karimi, M.; Cooley, S.; Miller, J.S.; Klimkowska, M.; Schaffer, M.; et al. Complete Remission with Reduction of High-Risk Clones Following Haploidentical NK-Cell Therapy against MDS and AML. Clin. Cancer Res. 2018, 24, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Miller, J.S.; Stuart, R.K.; Cooley, S.; Salhotra, A.; Curtsinger, J.; Westervelt, P.; DiPersio, J.F.; Hillman, T.M.; Silver, N.; et al. A Phase 1 Trial of CNDO-109-Activated Natural Killer Cells in Patients with High-Risk Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 1581–1589. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting Natural Killer Cells in Cancer Immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Schlom, J.; Hodge, J.W. IL-15 Superagonist/IL-15RαSushi-Fc Fusion Complex (IL-15SA/IL-15RαSu-Fc; ALT-803) Markedly Enhances Specific Subpopulations of NK and Memory CD8+ T Cells, and Mediates Potent Anti-Tumor Activity against Murine Breast and Colon Carcinomas. Oncotarget 2016, 7, 16130–16145. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse after Transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.; Ullrich, E. Novel Immune Modulators Used in Hematology: Impact on NK Cells. Front. Immunol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Parameswaran, R.; Ramakrishnan, P.; Moreton, S.A.; Xia, Z.; Hou, Y.; Lee, D.A.; Gupta, K.; deLima, M.; Beck, R.C.; Wald, D.N. Repression of GSK3 Restores NK Cell Cytotoxicity in AML Patients. Nat. Commun. 2016, 7, 11154. [Google Scholar] [CrossRef]
- Lagrue, K.; Carisey, A.; Morgan, D.J.; Chopra, R.; Davis, D.M. Lenalidomide Augments Actin Remodeling and Lowers NK-Cell Activation Thresholds. Blood 2015, 126, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR Antibody Enhancement of Anti-Lymphoma Activity of Natural Killer Cells as Monotherapy and in Combination with Anti-CD20 Antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Tinker, A.V.; Hirte, H.W.; Provencher, D.; Butler, M.; Ritter, H.; Tu, D.; Azim, H.A.; Paralejas, P.; Grenier, N.; Hahn, S.-A.; et al. Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Urbani, E.; André, P.; Mancusi, A.; Tosti, A.; Topini, F.; Bléry, M.; Animobono, L.; Romagné, F.; Wagtmann, N.; et al. Effects of Anti-NKG2A Antibody Administration on Leukemia and Normal Hematopoietic Cells. Haematologica 2016, 101, 626–633. [Google Scholar] [CrossRef]
- McWilliams, E.M.; Mele, J.M.; Cheney, C.; Timmerman, E.A.; Fiazuddin, F.; Strattan, E.J.; Mo, X.; Byrd, J.C.; Muthusamy, N.; Awan, F.T. Therapeutic CD94/NKG2A Blockade Improves Natural Killer Cell Dysfunction in Chronic Lymphocytic Leukemia. Oncoimmunology 2016, 5, e1226720. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.-C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Invest. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-Dependent Cell Cytotoxicity: Immunotherapy Strategies Enhancing Effector NK Cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.F.; Tay, R.E.; Pan, D.; Luoma, A.M.; Ito, Y.; Badrinath, S.; Tsoucas, D.; Franz, B.; May, K.F.; Harvey, C.J.; et al. Antibody-Mediated Inhibition of MICA and MICB Shedding Promotes NK Cell–Driven Tumor Immunity. Science 2018, 359, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Koerner, S.P.; André, M.C.; Leibold, J.S.; Kousis, P.C.; Kübler, A.; Pal, M.; Haen, S.P.; Bühring, H.-J.; Grosse-Hovest, L.; Jung, G.; et al. An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against Myeloid Leukemia. Leukemia 2017, 31, 459–469. [Google Scholar] [CrossRef]
- Akkapeddi, P.; Fragoso, R.; Hixon, J.A.; Ramalho, A.S.; Oliveira, M.L.; Carvalho, T.; Gloger, A.; Matasci, M.; Corzana, F.; Durum, S.K.; et al. A Fully Human Anti-IL-7Rα Antibody Promotes Antitumor Activity against T-Cell Acute Lymphoblastic Leukemia. Leukemia 2019, 33, 2155–2168. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 Clinical Trial of Adoptive Immunotherapy Using “off-the-Shelf” Activated Natural Killer Cells in Patients with Refractory and Relapsed Acute Myeloid Leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of Patients with Advanced Cancer with the Natural Killer Cell Line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Solocinski, K.; Padget, M.R.; Fabian, K.P.; Wolfson, B.; Cecchi, F.; Hembrough, T.; Benz, S.C.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. Overcoming Hypoxia-Induced Functional Suppression of NK Cells. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Fabian, K.P.; Padget, M.R.; Donahue, R.N.; Solocinski, K.; Robbins, Y.; Allen, C.T.; Lee, J.H.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. PD-L1 Targeting High-Affinity NK (t-HaNK) Cells Induce Direct Antitumor Effects and Target Suppressive MDSC Populations. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Robbins, Y.; Sievers, C.; Friedman, J.; Abdul Sater, H.; Clavijo, P.E.; Judd, N.; Tsong, E.; Silvin, C.; Soon-Shiong, P.; et al. Chimeric Antigen Receptor Engineered NK Cellular Immunotherapy Overcomes the Selection of T-Cell Escape Variant Cancer Cells. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lee, D.-H.; Choi, W.S.; Yi, E.; Kim, H.; Kim, J.M.; Jin, H.-S.; Kim, H.S. Harnessing NK Cells for Cancer Immunotherapy: Immune Checkpoint Receptors and Chimeric Antigen Receptors. BMB Rep. 2021, 54, 44–58. [Google Scholar] [CrossRef]
- Robbins, Y.; Greene, S.; Friedman, J.; Clavijo, P.E.; Van Waes, C.; Fabian, K.P.; Padget, M.R.; Abdul Sater, H.; Lee, J.H.; Soon-Shiong, P.; et al. Tumor Control via Targeting PD-L1 with Chimeric Antigen Receptor Modified NK Cells. eLife 2020, 9. [Google Scholar] [CrossRef]
- Davis, Z.B.; Vallera, D.A.; Miller, J.S.; Felices, M. Natural Killer Cells Unleashed: Checkpoint Receptor Blockade and BiKE/TriKE Utilization in NK-Mediated Anti-Tumor Immunotherapy. Semin. Immunol. 2017, 31, 64–75. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord Blood NK Cells Engineered to Express IL-15 and a CD19-Targeted CAR Show Long-Term Persistence and Potent Antitumor Activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-Man Clinical Trial of CAR NK-92 Cells: Safety Test of CD33-CAR NK-92 Cells in Patients with Relapsed and Refractory Acute Myeloid Leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- Oelsner, S.; Waldmann, A.; Billmeier, A.; Röder, J.; Lindner, A.; Ullrich, E.; Marschalek, R.; Dotti, G.; Jung, G.; Große-Hovest, L.; et al. Genetically Engineered CAR NK Cells Display Selective Cytotoxicity against FLT3-Positive B-ALL and Inhibit in Vivo Leukemia Growth. Int. J. Cancer 2019, 145, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]

| Name | Molecular Target | Intracellular Activation Domain | Indication | Registration Date | Registration Basis |
|---|---|---|---|---|---|
| tisagenlecleucel (Kymriah) | CD19 | 41BB-CD3ζ | B-ALL, DLBCL | Aug 2017 |
|
| axicabtagene ciloleucel (Yescarta) | CD19 | CD28-CD3ζ | DLBCL, FL | Oct 2017 | ZUMA-1 trial (NCT02348216) [67,68] |
| brexucabtagene autoleucel (Tecartus) | CD19 | CD28-CD3ζ | MCL | Jul 2020 | ZUMA-2 trial (NCT02601313) [69] |
| lisocabtagene maraleucel (Breyanzi) | CD19 | 41BB-CD3ζ, also contains a nonfunctional truncated epidermals growth factor receptor (EGFRt) | DLBCL | May 2021 | TRANSCEND trial (NCT02631044) [70] |
| idecabtagene vicleucel (Abecma) | BCMA | 41BB-CD3ζ | MM | Mar 2021 | NCT02658929, NCT02546167 [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miazek-Zapala, N.; Slusarczyk, A.; Kusowska, A.; Zapala, P.; Kubacz, M.; Winiarska, M.; Bobrowicz, M. The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era. Cells 2021, 10, 1511. https://doi.org/10.3390/cells10061511
Miazek-Zapala N, Slusarczyk A, Kusowska A, Zapala P, Kubacz M, Winiarska M, Bobrowicz M. The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era. Cells. 2021; 10(6):1511. https://doi.org/10.3390/cells10061511
Chicago/Turabian StyleMiazek-Zapala, Nina, Aleksander Slusarczyk, Aleksandra Kusowska, Piotr Zapala, Matylda Kubacz, Magdalena Winiarska, and Malgorzata Bobrowicz. 2021. "The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era" Cells 10, no. 6: 1511. https://doi.org/10.3390/cells10061511
APA StyleMiazek-Zapala, N., Slusarczyk, A., Kusowska, A., Zapala, P., Kubacz, M., Winiarska, M., & Bobrowicz, M. (2021). The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era. Cells, 10(6), 1511. https://doi.org/10.3390/cells10061511






