Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis
Abstract
1. Introduction
2. Wound Healing
3. Fibroblasts and Myofibroblasts
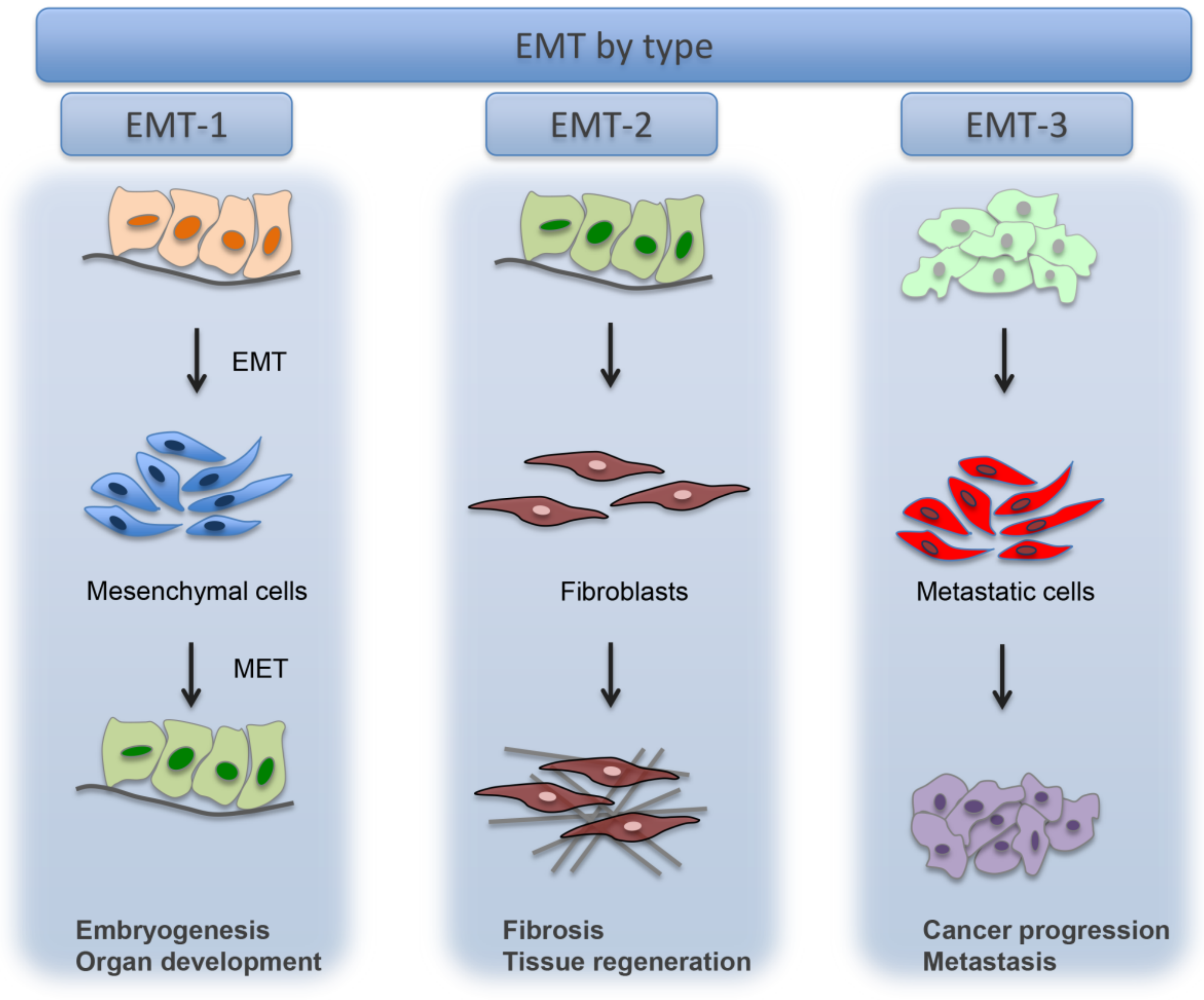
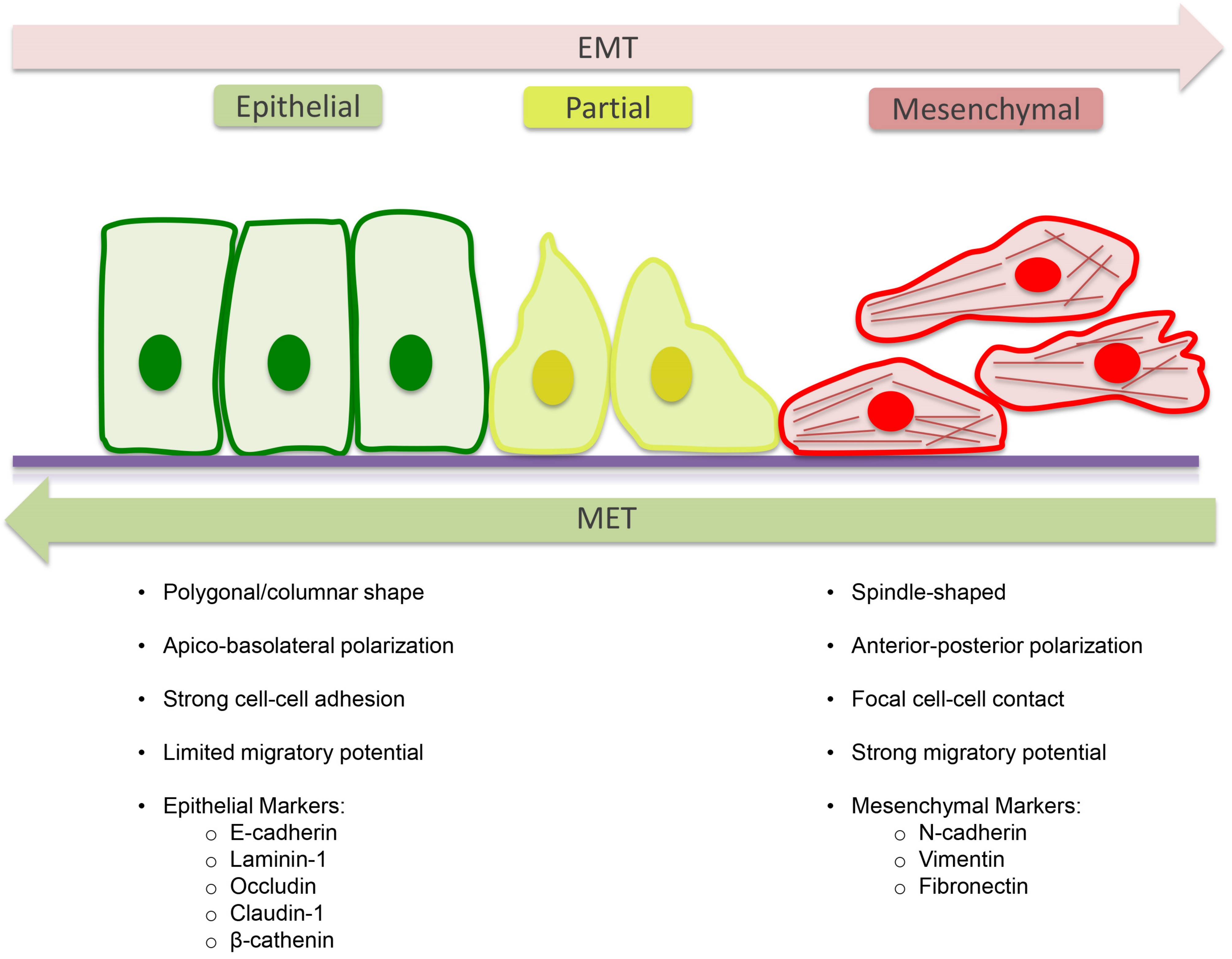
4. Epithelial to Mesenchymal Transition (EMT)
5. Molecular Mechanism of EMT
6. Tissue Regeneration and EMT
7. Modulation of EMT by Biomaterials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Barriere, G.; Fici, P.; Gallerani, G.; Fabbri, F.; Rigaud, M. Epithelial Mesenchymal Transition: A double-edged sword. Clin. Transl. Med. 2015, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Lin, Y.W.; Dong, C.F.; Zhou, B.H.P. Epigenetic Regulation of EMT: The Snail Story. Curr. Pharm. Des. 2014, 20, 1698–1705. [Google Scholar] [CrossRef]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Li, Z.D.; Jimenez, S.A. Role of Endothelial-Mesenchymal Transition (EndoMT) in the Pathogenesis of Fibrotic Disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef]
- Li, M.R.; Luan, F.X.; Zhao, Y.L.; Hao, H.J.; Zhou, Y.; Han, W.D.; Fu, X.B. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp. Biol. Med. 2016, 241, 1–13. [Google Scholar] [CrossRef]
- Skovierova, H.; Okajcekova, T.; Strnadel, J.; Vidomanova, E.; Halasova, E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis. Int. J. Mol. Med. 2018, 41, 1187–1200. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef]
- Kim, D.H.; Xing, T.S.; Yang, Z.B.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2018, 7, 1. [Google Scholar] [CrossRef]
- Willis, B.C.; duBois, R.M.; Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 2006, 3, 377–382. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.Q.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1054. [Google Scholar] [CrossRef]
- Mack, M.; Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef]
- Kreuter, M.; Bonella, F.; Wijsenbeek, M.; Maher, T.M.; Spagnolo, P. Pharmacological Treatment of Idiopathic Pulmonary Fibrosis: Current Approaches, Unsolved Issues, and Future Perspectives. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Yuan, F.L.; Sun, Z.L.; Feng, Y.; Liu, S.Y.; Du, Y.; Yu, S.; Yang, M.L.; Lv, G.Z. Epithelial-mesenchymal transition in the formation of hypertrophic scars and keloids. J. Cell. Physiol. 2019, 234, 21662–21669. [Google Scholar] [CrossRef]
- Yan, C.; Grimm, W.A.; Garner, W.L.; Qin, L.; Travis, T.; Tan, N.; Han, Y.P. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am. J. Pathol. 2010, 176, 2247–2258. [Google Scholar] [CrossRef]
- Haensel, D.; Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev. Dyn. 2018, 247, 473–480. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Hantash, B.M.; Zhao, L.M.; Knowles, J.A.; Lorenz, H.P. Adult and fetal wound healing. Front. Biosci. 2008, 13, 51–61. [Google Scholar] [CrossRef]
- Ke, T.; Yang, M.; Mao, D.; Zhu, M.; Che, Y.; Kong, D.; Li, C. Co-Transplantation of Skin-Derived Precursors and Collagen Sponge Facilitates Diabetic Wound Healing by Promoting Local Vascular Regeneration. Cell. Physiol. Biochem. 2015, 37, 1725–1737. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Abdelaziz, T.T.; Abdel Razk, A.A.K.; Ashour, M.M.M.; Abdelrahman, A.S. Interreader reproducibility of the Neck Imaging Reporting and Data system (NI-RADS) lexicon for the detection of residual/recurrent disease in treated head and neck squamous cell carcinoma (HNSCC). Cancer Imaging 2020, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.P.; Sharan, A.; Singh, R.; Prasad, H.K.; Rao, P. Effects of limited access dressing in chronic wounds: A biochemical and histological study. Indian J. Plast. Surg. 2015, 48, 22–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Diomede, F.; Merciaro, I.; Caputi, S.; Tartaro, A.; Guarnieri, S.; Trubiani, O. Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease. J. Cell. Physiol. 2018, 233, 6734–6747. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Dai, H.P. Type 2 epithelial mesenchymal transition in vivo: Truth or pitfalls? Chin. Med. J. 2012, 125, 3312–3317. [Google Scholar]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Karahan, A.; AAbbasoğlu, A.; Isik, S.A.; Cevik, B.; Saltan, C.; Elbas, N.O.; Yalili, A. Factors Affecting Wound Healing in Individuals with Pressure Ulcers: A Retrospective Study. Ostomy Wound Manag. 2018, 64, 32–39. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmouliere, A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011, 19, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Darby, I.A.; Gabbiani, G. Normal and pathologic soft tissue remodeling: Role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab. Investig. 2003, 83, 1689–1707. [Google Scholar] [CrossRef]
- Virag, J.I.; Murry, C.E. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am. J. Pathol. 2003, 163, 2433–2440. [Google Scholar] [CrossRef]
- Phan, S.H. The myofibroblast in pulmonary fibrosis. Chest 2002, 122, 286s–289s. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P., 3rd; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr. Opin. Nephrol. Hypertens. 2003, 12, 25–29. [Google Scholar] [CrossRef]
- Seidel, H.; Richter, J.; Kurerov, N.N.; Schajpak, E.J.; Bluthner, R.; Erdmann, U.; Hinz, B. Psychophysical assessment of sinusoidal whole-body vibration in z-axis between 0.6 and 5 Hz combined with different noise levels. Int. Arch. Occup. Environ. Health 1989, 61, 413–422. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmouliere, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell. Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast: A key cell for wound healing and fibrocontractive diseases. Prog. Clin. Biol. Res. 1981, 54, 183–194. [Google Scholar]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Powell, D.W.; Mifflin, R.C.; Valentich, J.D.; Crowe, S.E.; Saada, J.I.; West, A.B. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. 1999, 277, C1–C19. [Google Scholar] [CrossRef]
- Eyden, B. The myofibroblast: A study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. Part 2—Tumours and tumour-like lesions. J. Submicrosc. Cytol. Pathol. 2005, 37, 231–296. [Google Scholar]
- Kohan, M.; Muro, A.F.; White, E.S.; Berkman, N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010, 24, 4503–4512. [Google Scholar] [CrossRef]
- Van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral Health 2015, 7, 75–80. [Google Scholar]
- Ko, U.H.; Choi, J.; Choung, J.; Moon, S.; Shin, J.H. Physicochemically Tuned Myofibroblasts for Wound Healing Strategy. Sci. Rep. 2019, 9, 16070. [Google Scholar] [CrossRef]
- Lian, N.; Li, T. Growth factor pathways in hypertrophic scars: Molecular pathogenesis and therapeutic implications. Biomed. Pharmacother. 2016, 84, 42–50. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Du, C.; Shan, S.; Zhang, Y.; Du, Z.; Han, D. Honokiol Alleviates Hypertrophic Scar by Targeting Transforming Growth Factor-beta/Smad2/3 Signaling Pathway. Front. Pharmacol. 2017, 8, 206. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; Bayat, A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 141–152. [Google Scholar] [CrossRef]
- Moulin, V.; Castilloux, G.; Auger, F.A.; Garrel, D.; O’Connor-McCourt, M.D.; Germain, L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp. Cell Res. 1998, 238, 283–293. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Fibrosis: Recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol. Rep. 2010, 2, 78. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef]
- Hu, M.S.; Maan, Z.N.; Wu, J.C.; Rennert, R.C.; Hong, W.X.; Lai, T.S.; Cheung, A.T.; Walmsley, G.G.; Chung, M.T.; McArdle, A.; et al. Tissue engineering and regenerative repair in wound healing. Ann. Biomed. Eng. 2014, 42, 1494–1507. [Google Scholar] [CrossRef]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Twigg, S.; Chen, X.; Polhill, T.S.; Poronnik, P.; Gilbert, R.E.; Pollock, C.A. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am. J. Physiol. Ren. Physiol. 2005, 288, F800–F809. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.; Mamalis, A.; Lev-Tov, H.; Jagdeo, J. The role of microRNAs in skin fibrosis. Arch. Dermatol. Res. 2013, 305, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Peng, A.P.; Huang, L.T.; Wang, Y.T.; Lan, C.W.; Yang, N.S. The Phytochemical Shikonin Stimulates Epithelial-Mesenchymal Transition (EMT) in Skin Wound Healing. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Fintha, A.; Gasparics, A.; Rosivall, L.; Sebe, A. Therapeutic Targeting of Fibrotic Epithelial-Mesenchymal Transition-An Outstanding Challenge. Front. Pharmacol. 2019, 10, 388. [Google Scholar] [CrossRef]
- Huang, R.Y.; Guilford, P.; Thiery, J.P. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J. Cell Sci. 2012, 125, 4417–4422. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Xu, R.; Won, J.Y.; Kim, C.H.; Kim, D.E.; Yim, H. Roles of the Phosphorylation of Transcriptional Factors in Epithelial-Mesenchymal Transition. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Fuxe, J.; Vincent, T.; Garcia de Herreros, A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef]
- Bongiovanni, L.; D’Andrea, A.; Romanucci, M.; Malatesta, D.; Candolini, M.; Salda, L.D.; Mechelli, L.; Sforna, M.; Brachelente, C. Epithelial-to-mesenchymal transition: Immunohistochemical investigation of related molecules in canine cutaneous epithelial tumours. Vet. Dermatol. 2013, 24, 195–203. [Google Scholar] [CrossRef]
- Iwano, M. EMT and TGF-beta in renal fibrosis. Front. Biosci. 2010, 2, 229–238. [Google Scholar] [CrossRef]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized Scaffold in Bone Tissue Repair. Int. J. Mol. Sci. 2018, 19, 1022. [Google Scholar] [CrossRef]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Expression of Alzheimer’s Disease-Related Genes in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Ettorre, V.; Bramanti, A.; Piattelli, A.; Gatta, V.; Mazzon, E.; Fontana, A.; et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018, 13, 3805–3825. [Google Scholar] [CrossRef]
- Trubiani, O.; Toniato, E.; Di Iorio, D.; Diomede, F.; Merciaro, I.; D’Arcangelo, C.; Caputi, S.; Oriana, T. Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. Int. J. Immunopathol. Pharmacol. 2012, 25, 637–643. [Google Scholar] [CrossRef]
- Vibert, L.; Daulny, A.; Jarriault, S. Wound healing, cellular regeneration and plasticity: The elegans way. Int. J. Dev. Biol. 2018, 62, 491–505. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Bando, T.; Yokoyama, H.; Nakamura, H. Wound repair, remodeling, and regeneration. Dev. Growth Differ. 2018, 60, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Akita, S. Wound Repair and Regeneration: Mechanisms, Signaling. Int. J. Mol. Sci. 2019, 20, 6328. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Abnave, P.; Aboukhatwa, E.; Kosaka, N.; Thompson, J.; Hill, M.A.; Aboobaker, A.A. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 2017, 144, 3440–3453. [Google Scholar] [CrossRef]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Diomede, F.; Gugliandolo, A.; Chiricosta, L.; Bramanti, P.; Merciaro, I.; Orsini, T.; Mazzon, E.; Trubiani, O. 3D Printing PLA/Gingival Stem Cells/EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment. Int. J. Mol. Sci. 2019, 20, 3256. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Pierdomenico, S.D.; Piattelli, A.; Varvara, G.; Fonticoli, L.; Trubiani, O.; Diomede, F. 3D Human Periodontal Stem Cells and Endothelial Cells Promote Bone Development in Bovine Pericardium-Based Tissue Biomaterial. Materials 2019, 12, 2157. [Google Scholar] [CrossRef]
- Featherston, T.; Brasch, H.D.; Siljee, S.D.; van Schaijik, B.; Patel, J.; de Jongh, J.; Marsh, R.W.; Itinteang, T.; Tan, S.T. Cancer Stem Cells in Head and Neck Cutaneous Squamous Cell Carcinoma Express Cathepsins. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3042. [Google Scholar] [CrossRef]
- You, S.; Avidan, O.; Tariq, A.; Ahluwalia, I.; Stark, P.C.; Kublin, C.L.; Zoukhri, D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 126–135. [Google Scholar] [CrossRef]
- Forte, E.; Chimenti, I.; Rosa, P.; Angelini, F.; Pagano, F.; Calogero, A.; Giacomello, A.; Messina, E. EMT/MET at the Crossroad of Stemness, Regeneration and Oncogenesis: The Ying-Yang Equilibrium Recapitulated in Cell Spheroids. Cancers 2017, 9, 98. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sanchez-Tomero, J.A.; Selgas, R.; Lopez-Cabrera, M.; Aguilera, A.; Mateo, G.G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Razali, R.A.; Lokanathan, Y.; Yazid, M.D.; Ansari, A.S.; Bin Saim, A.; Idrus, R.B.H. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. Int. J. Mol. Sci. 2019, 20, 3492. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Burlando, B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: Comparison among different honeys. Wound Repair Regen. 2012, 20, 778–785. [Google Scholar] [CrossRef]
- Zhang, L.M.; Su, L.X.; Hu, J.Z.; Wang, M.; Ju, H.Y.; Li, X.; Han, Y.F.; Xia, W.Y.; Guo, W.; Ren, G.X.; et al. Epigenetic regulation of VENTXP1 suppresses tumor proliferation via miR-205-5p/ANKRD2/NF-kB signaling in head and neck squamous cell carcinoma. Cell Death Dis. 2020, 11, 838. [Google Scholar] [CrossRef]
- Morgan, E.L.; Chen, Z.; Van Waes, C. Regulation of NFkappaB Signalling by Ubiquitination: A Potential Therapeutic Target in Head and Neck Squamous Cell Carcinoma? Cancers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Mihalko, E.P.; Brown, A.C. Material Strategies for Modulating Epithelial to Mesenchymal Transitions. ACS Biomater. Sci. Eng. 2018, 4, 1149–1161. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Ozdemir, T.; Bah, M.; Baskerville, K.A.; Shah, S.I.; Rajasekaran, A.K.; Jia, X.Q. Regulation of Epithelial-to-Mesenchymal Transition Using Biomimetic Fibrous Scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 17915–17926. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Wang, W.Y.; Huang, X.M.; Sun, Y.Y.; Tian, S.; Cai, P. Reduced graphene oxide triggered epithelial-mesenchymal transition in A549 cells. Sci Rep. 2018, 8. [Google Scholar] [CrossRef]
- Horejs, C.M.; St-Pierre, J.P.; Ojala, J.R.M.; Steele, J.A.M.; da Silva, P.B.; Rynne-Vidal, A.; Maynard, S.A.; Hansel, C.S.; Rodriguez-Fernandez, C.; Mazo, M.M.; et al. Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. https://doi.org/10.3390/cells10071587
Marconi GD, Fonticoli L, Rajan TS, Pierdomenico SD, Trubiani O, Pizzicannella J, Diomede F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells. 2021; 10(7):1587. https://doi.org/10.3390/cells10071587
Chicago/Turabian StyleMarconi, Guya D., Luigia Fonticoli, Thangavelu Soundara Rajan, Sante D. Pierdomenico, Oriana Trubiani, Jacopo Pizzicannella, and Francesca Diomede. 2021. "Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis" Cells 10, no. 7: 1587. https://doi.org/10.3390/cells10071587
APA StyleMarconi, G. D., Fonticoli, L., Rajan, T. S., Pierdomenico, S. D., Trubiani, O., Pizzicannella, J., & Diomede, F. (2021). Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells, 10(7), 1587. https://doi.org/10.3390/cells10071587






