Participation of L-Lactate and Its Receptor HCAR1/GPR81 in Neurovisual Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Animals
2.2. Genotypic Screening
2.3. Reagents
2.4. Tissue Preparation for Immunohistochemistry
2.5. Immunohistochemistry
2.6. L-Lactate Solution
2.7. Retinal Explant Culture
2.8. Growth Cone Behavior Assay
2.9. Primary Neuron Culture
2.10. Immunocytochemistry
2.11. Western Blot Analysis
2.12. Eye Specific Segregation
2.13. Statistical Analysis
3. Results
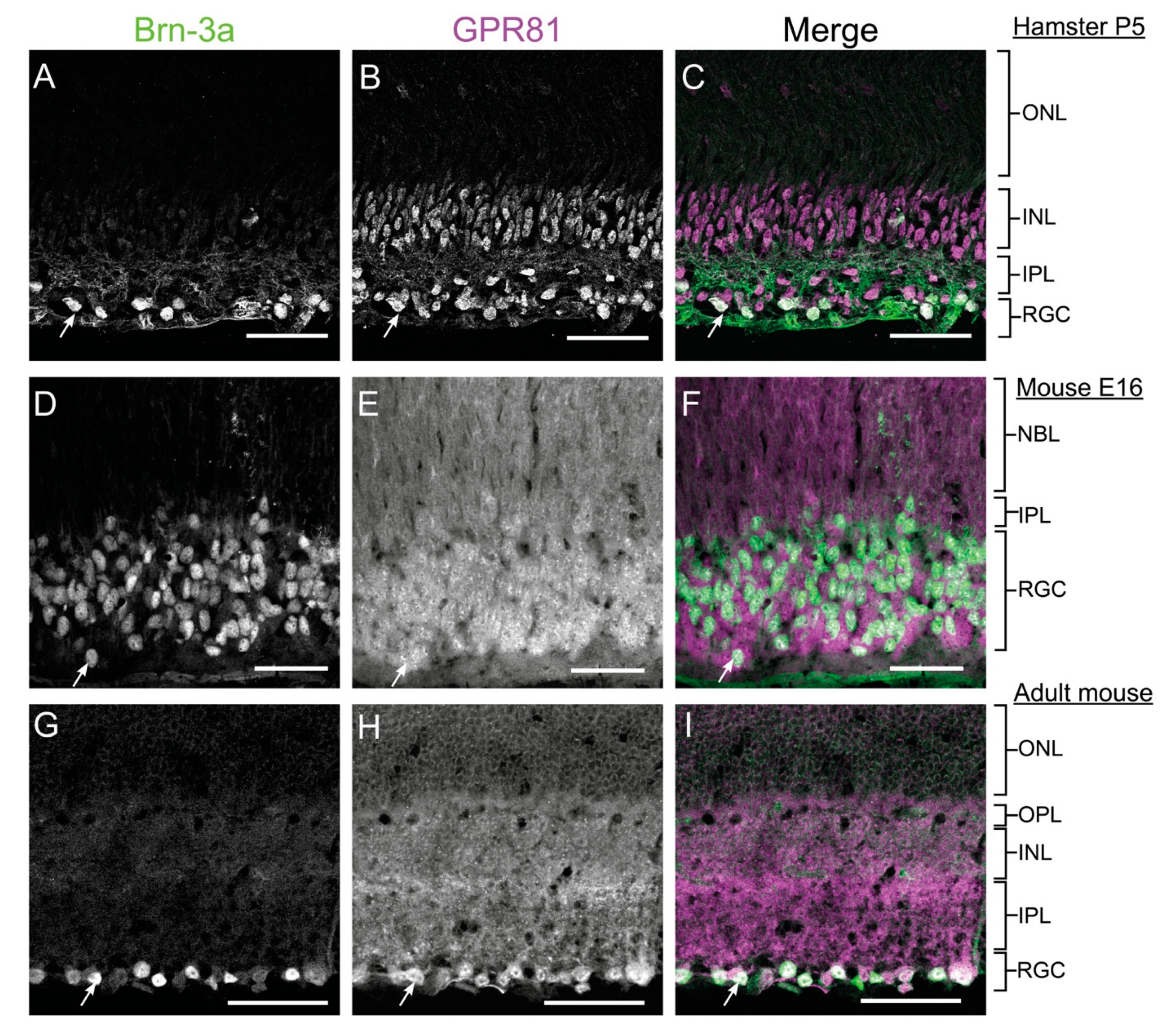
3.1. GPR81 Is Expressed in the Retina
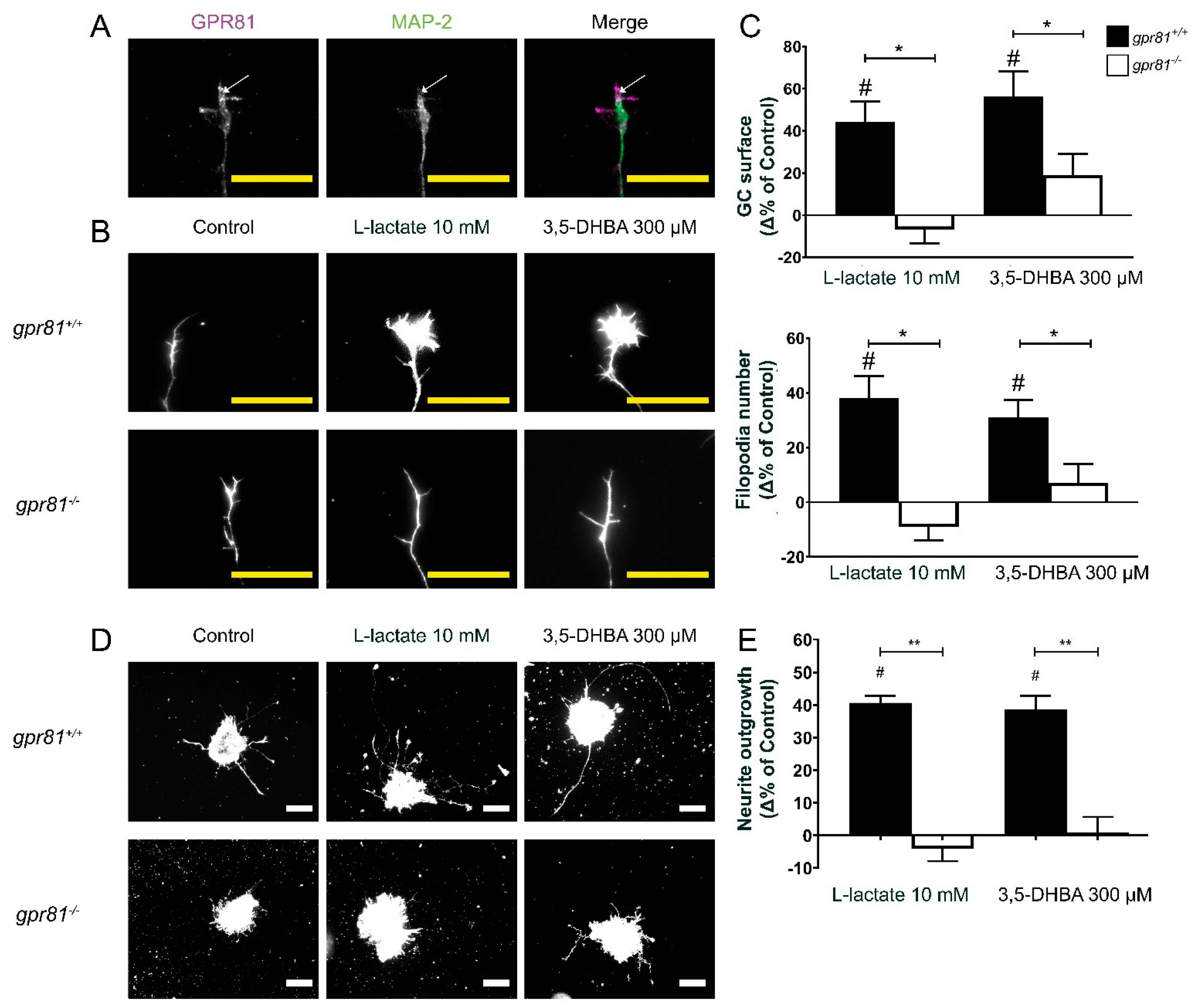
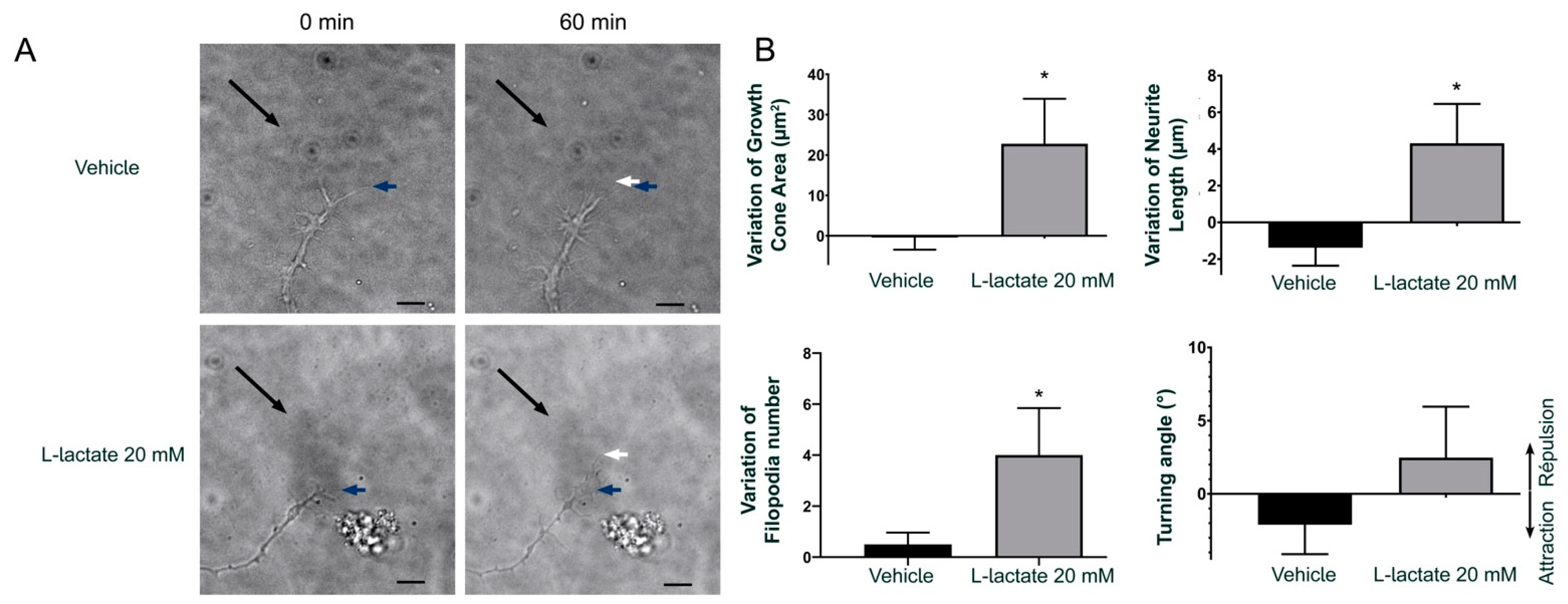
3.2. GPR81 Influences GC Morphology and Axon Growth
3.3. Lactate Increases PKC and PKA Phosphorylation
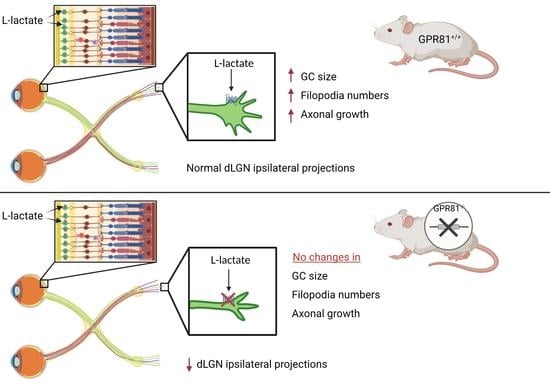
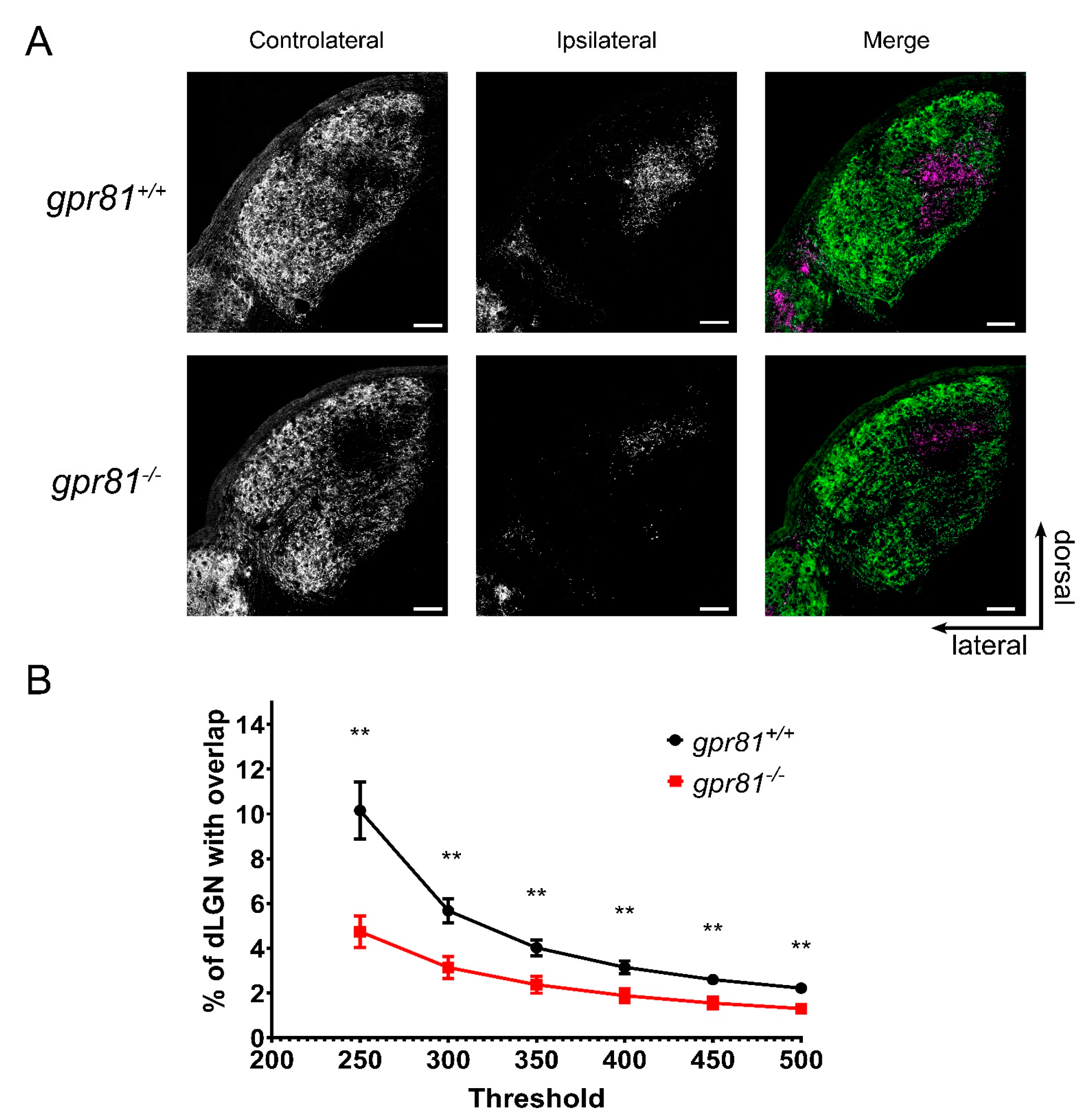
3.4. GPR81 Affects Retinothalamic Projections In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef]
- Liu, C.; Kuei, C.; Zhu, J.; Yu, J.; Zhang, L.; Shih, A.; Mirzadegan, T.; Shelton, J.; Sutton, S.; Connelly, M.A.; et al. 3,5-Dihydroxybenzoic Acid, a Specific Agonist for Hydroxycarboxylic Acid 1, Inhibits Lipolysis in Adipocytes. J. Pharmacol. Exp. Ther. 2012, 341, 794. [Google Scholar] [CrossRef] [Green Version]
- Madaan, A.; Chaudhari, P.; Nadeau-Vallee, M.; Hamel, D.; Zhu, T.; Mitchell, G.; Samuels, M.; Pundir, S.; Dabouz, R.; Howe Cheng, C.W.; et al. Muller Cell-Localized G-Protein-Coupled Receptor 81 (Hydroxycarboxylic Acid Receptor 1) Regulates Inner Retinal Vasculature via Norrin/Wnt Pathways. (1525-2191 (Electronic)). Am. J. Pathol. 2019, 189, 1878–1896. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar] [CrossRef] [Green Version]
- Wong-Riley, M.T.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef] [Green Version]
- Plump, A.S.; Erskine, L.; Sabatier, C.; Brose, K.; Epstein, C.J.; Goodman, C.S.; Mason, C.A.; Tessier-Lavigne, M. Slit1 and Slit2 Cooperate to Prevent Premature Midline Crossing of Retinal Axons in the Mouse Visual System. Neuron 2002, 33, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Brennan, C.; Johnson, K.G.; Shewan, D.; Harris, W.A.; Holt, C.E. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron 2000, 25, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Reese, B.E. Development of the retina and optic pathway. Vis. Res. 2011, 51, 613–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Bittar, P.G.; Charnay, Y.; Pellerin, L.; Bouras, C.; Magistretti, P.J. Selective Distribution of Lactate Dehydrogenase Isoenzymes in Neurons and Astrocytes of Human Brain. J. Cereb. Blood Flow Metab. 1996, 16, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Friede, R.L.; Fleming, L.M. A mapping of the distribution of lactic dehydrogenase in the brain of the rhesus monkey. Am. J. Anat. 1963, 113, 215–234. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.; Frith, K.; Sjödin, B.; Gollnick, P.D.; Saltin, B. Distribution of LDH Isozymes in Human Skeletal Muscle. Scand. J. Clin. Lab. Investig. 1974, 33, 307–312. [Google Scholar] [CrossRef]
- Sjödin, B.; Thorstensson, A.; Frith, K.; Karlsson, J. Effect of Physical Training on LDH Activity and LDH Isozyme Pattern in Human Skeletal Muscle. Acta Physiol. Scand. 1976, 97, 150–157. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Tseng, M.T.; Rigor, B.M. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001, 895, 268–272. [Google Scholar] [CrossRef]
- Shen, Z.; Jiang, L.; Yuan, Y.; Deng, T.; Zheng, Y.-R.; Zhao, Y.-Y.; Li, W.-L.; Wu, J.-Y.; Gao, J.-Q.; Hu, W.-W.; et al. Inhibition of G Protein-Coupled Receptor 81 (GPR81) Protects Against Ischemic Brain Injury. CNS Neurosci. Ther. 2015, 21, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell Surface Lactate Receptor GPR81 Is Crucial for Cancer Cell Survival. Cancer Res. 2014, 74, 5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate Reduces Liver and Pancreatic Injury in Toll-Like Receptor– and Inflammasome-Mediated Inflammation via GPR81-Mediated Suppression of Innate Immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Madaan, A.; Nadeau-Vallée, M.; Rivera, J.C.; Obari, D.; Hou, X.; Sierra, E.M.; Girard, S.; Olson, D.M.; Chemtob, S. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am. J. Obstet. Gynecol. 2017, 216, 60.e1–60.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolko, M.; Vosborg, F.; Henriksen, U.L.; Hasan-Olive, M.M.; Diget, E.H.; Vohra, R.; Gurubaran, I.R.S.; Gjedde, A.; Mariga, S.T.; Skytt, D.M.; et al. Erratum to: Lactate Transport and Receptor Actions in Retina: Potential Roles in Retinal Function and Disease. Neurochem. Res. 2016, 41, 1237. [Google Scholar] [CrossRef] [Green Version]
- Cherif, H.; Duhamel, F.; Cécyre, B.; Bouchard, A.; Quintal, A.; Chemtob, S.; Bouchard, J.-F. Receptors of intermediates of carbohydrate metabolism, GPR91 and GPR99, mediate axon growth. PLoS Biol. 2018, 16, e2003619. [Google Scholar] [CrossRef] [Green Version]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.S.; Cho, J.H.; Honoré, J.C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. (1546-170X (Electronic)). Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef]
- Argaw, A.; Duff, G.; Zabouri, N.; Cécyre, B.; Chainé, N.; Cherif, H.; Tea, N.; Lutz, B.; Ptito, M.; Bouchard, J.-F. Concerted Action of CB1 Cannabinoid Receptor and Deleted in Colorectal Cancer in Axon Guidance. J. Neurosci. 2011, 31, 1489. [Google Scholar] [CrossRef] [Green Version]
- Cherif, H.; Argaw, A.; Cécyre, B.; Bouchard, A.; Gagnon, J.; Javadi, P.; Desgent, S.; Mackie, K.; Bouchard, J.-F. Role of GPR55 during Axon Growth and Target Innervation. eNeuro 2015, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duff, G.; Argaw, A.; Cecyre, B.; Cherif, H.; Tea, N.; Zabouri, N.; Casanova, C.; Ptito, M.; Bouchard, J.-F. Cannabinoid Receptor CB2 Modulates Axon Guidance. PLoS ONE 2013, 8, e70849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.C.; Posch, A. The Design of a Quantitative Western Blot Experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
- Torborg, C.L.; Feller, M.B. Unbiased analysis of bulk axonal segregation patterns. J. Neurosci. Methods 2004, 135, 17–26. [Google Scholar] [CrossRef]
- Muir-Robinson, G.; Hwang, B.J.; Feller, M.B. Retinogeniculate Axons Undergo Eye-Specific Segregation in the Absence of Eye-Specific Layers. J. Neurosci. 2002, 22, 5259. [Google Scholar] [CrossRef] [PubMed]
- Jassen, A.K.; Yang, H.; Miller, G.M.; Calder, E.; Madras, B.K. Receptor Regulation of Gene Expression of Axon Guidance Molecules: Implications for Adaptation. Mol. Pharmacol. 2006, 70, 71. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, Y.; Zhang, Z.; Cui, K.; Wang, S.; Yuan, X.-B.; Wu, C.-P.; Poo, M.-M.; Duan, S. Nerve growth cone guidance mediated by G protein–coupled receptors. Nat. Neurosci. 2002, 5, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.-Q.; Ren, N.; Jin, L.; Cheng, K.; Kash, S.; Chen, R.; Wright, S.D.; Taggart, A.K.P.; Waters, M.G. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 2008, 377, 987–991. [Google Scholar] [CrossRef]
- Shanks, J.A.; Ito, S.; Schaevitz, L.; Yamada, J.; Chen, B.; Litke, A.; Feldheim, D.A. Corticothalamic Axons Are Essential for Retinal Ganglion Cell Axon Targeting to the Mouse Dorsal Lateral Geniculate Nucleus. J. Neurosci. 2016, 36, 5252–5263. [Google Scholar] [CrossRef] [Green Version]
- Chih, C.-P.; Roberts, E.L. Energy Substrates for Neurons during Neural Activity: A Critical Review of the Astrocyte-Neuron Lactate Shuttle Hypothesis. J. Cereb. Blood Flow Metab. 2003, 23, 1263–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; TÖRnquist, P.; Bill, A. Glucose metabolism of the inner retina in pigs in darkness and light. Acta Physiol. Scand. 1997, 160, 71–74. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, J.-F.; Horn, K.E.; Stroh, T.; Kennedy, T.E. Depolarization recruits DCC to the plasma membrane of embryonic cortical neurons and enhances axon extension in response to netrin-1. J. Neurochem. 2008, 107, 398–417. [Google Scholar] [CrossRef]
- Sanes, D.H.; Reh, T.A.; Harris, W.A. 4-Determination and differentiation. In Development of the Nervous System, 3rd ed.; Sanes, D.H., Reh, T.A., Harris, W.A., Eds.; Academic Press: London, UK, 2012; pp. 77–104. [Google Scholar] [CrossRef]
- Hsu, L.; Jeng, A.Y.; Chen, K.Y. Induction of neurite outgrowth from chick embryonic ganglia explants by activators of protein kinase C. Neurosci. Lett. 1989, 99, 257–262. [Google Scholar] [CrossRef]
- Mot, A.I.; Liddell, J.R.; White, A.R.; Crouch, P.J. Circumventing the Crabtree Effect: A method to induce lactate consumption and increase oxidative phosphorylation in cell culture. Int. J. Biochem. Cell Biol. 2016, 79, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kolkova, K.; Novitskaya, V.; Pedersen, N.; Berezin, V.; Bock, E. Neural Cell Adhesion Molecule-Stimulated Neurite Outgrowth Depends on Activation of Protein Kinase C and the Ras–Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2000, 20, 2238. [Google Scholar] [CrossRef]
- Rosdahl, J.A.; Mourton, T.L.; Brady-Kalnay, S.M. Protein Kinase C δ (PKCδ) Is Required for Protein Tyrosine Phosphatase μ (PTPμ)-Dependent Neurite Outgrowth. Mol. Cell. Neurosci. 2002, 19, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Heacock, A.M.; Agranoff, B.W. Protein Kinase Inhibitors Block Neurite Outgrowth from Explants of Goldfish Retina. Neurochem. Res. 1997, 22, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, V.; Yebra, M. Netrins: Beyond the brain. Nat. Rev. Mol. Cell Biol. 2007, 8, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.-F.; Moore, S.W.; Tritsch, N.X.; Roux, P.P.; Shekarabi, M.; Barker, P.A.; Kennedy, T.E. Protein Kinase A Activation Promotes Plasma Membrane Insertion of DCC from an Intracellular Pool: A Novel Mechanism Regulating Commissural Axon Extension. J. Neurosci. 2004, 24, 3040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barallobre, M.J.; Pascual, M.; Del Río, J.A.; Soriano, E. The Netrin family of guidance factors: Emphasis on Netrin-1 signalling. Brain Res. Rev. 2005, 49, 22–47. [Google Scholar] [CrossRef]
- Argaw, A.; Duff, G.; Boire, D.; Ptito, M.; Bouchard, J.-F. Protein kinase A modulates retinal ganglion cell growth during development. Exp. Neurol. 2008, 211, 494–502. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625. [Google Scholar] [CrossRef] [Green Version]
- Berthet, C.; Lei, H.; Thevenet, J.; Gruetter, R.; Magistretti, P.J.; Hirt, L. Neuroprotective Role of Lactate after Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 1780–1789. [Google Scholar] [CrossRef]
- Berthet, C.; Castillo, X.; Magistretti, P.J.; Hirt, L. New Evidence of Neuroprotection by Lactate after Transient Focal Cerebral Ischaemia: Extended Benefit after Intracerebroventricular Injection and Efficacy of Intravenous Administration. Cerebrovasc. Dis. 2012, 34, 329–335. [Google Scholar] [CrossRef]
- Zhai, X.; Li, J.; Li, L.; Sun, Y.; Zhang, X.; Xue, Y.; Gao, Y.; Li, S.; Yan, W.; Yin, S.; et al. L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res. 2020, 1746, 146945. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laroche, S.; Stil, A.; Germain, P.; Cherif, H.; Chemtob, S.; Bouchard, J.-F. Participation of L-Lactate and Its Receptor HCAR1/GPR81 in Neurovisual Development. Cells 2021, 10, 1640. https://doi.org/10.3390/cells10071640
Laroche S, Stil A, Germain P, Cherif H, Chemtob S, Bouchard J-F. Participation of L-Lactate and Its Receptor HCAR1/GPR81 in Neurovisual Development. Cells. 2021; 10(7):1640. https://doi.org/10.3390/cells10071640
Chicago/Turabian StyleLaroche, Samuel, Aurélie Stil, Philippe Germain, Hosni Cherif, Sylvain Chemtob, and Jean-François Bouchard. 2021. "Participation of L-Lactate and Its Receptor HCAR1/GPR81 in Neurovisual Development" Cells 10, no. 7: 1640. https://doi.org/10.3390/cells10071640
APA StyleLaroche, S., Stil, A., Germain, P., Cherif, H., Chemtob, S., & Bouchard, J.-F. (2021). Participation of L-Lactate and Its Receptor HCAR1/GPR81 in Neurovisual Development. Cells, 10(7), 1640. https://doi.org/10.3390/cells10071640