Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival
Abstract
:1. Introduction
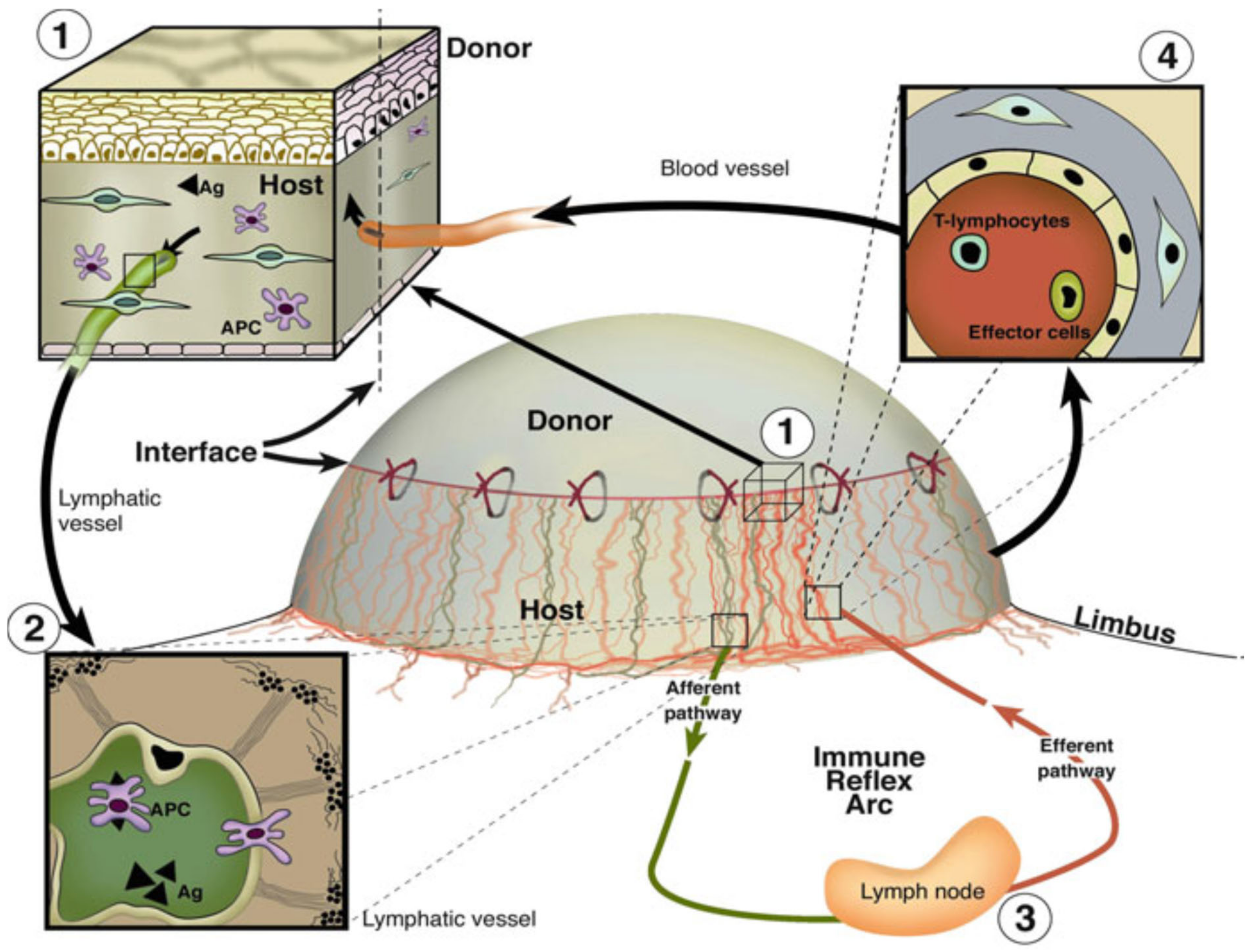
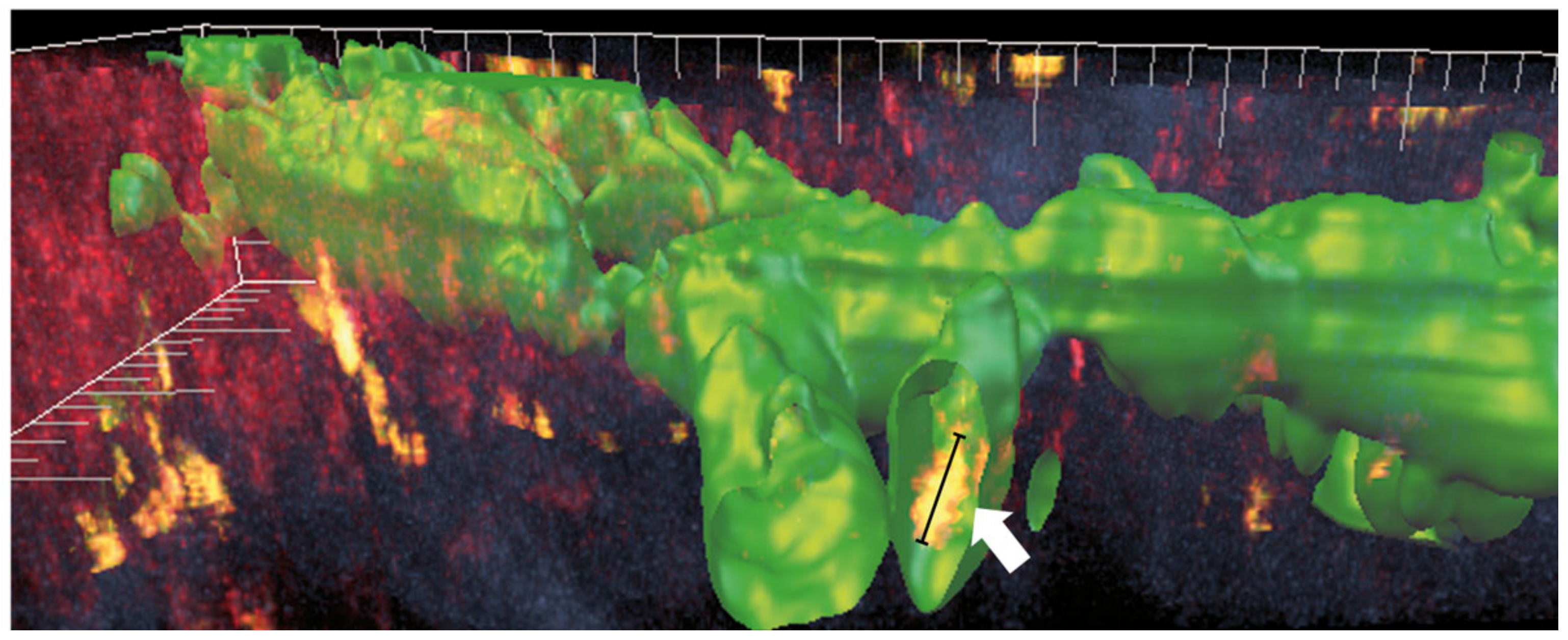
2. Lymphatic Endothelial and Immune Cell Interaction
3. Role of Lymphatic Vessels in Allograft Rejection after Solid Organ Transplantation
4. Targeting Active Corneal (Lymph)angiogenesis to Promote Corneal Transplant Survival—Concept and Current State of Art
5. Experimental Modulation of Lymphatic Trafficking via UVA-Light Crosslinking (CXL) and Fine-Needle Diathermy (FND) to Regress Pathologic Corneal Lymphatics to Promote Corneal Transplant Survival: The Eye as a Model of Organ Transplant Immunology (“Lymphangioregressive Preconditioning”)
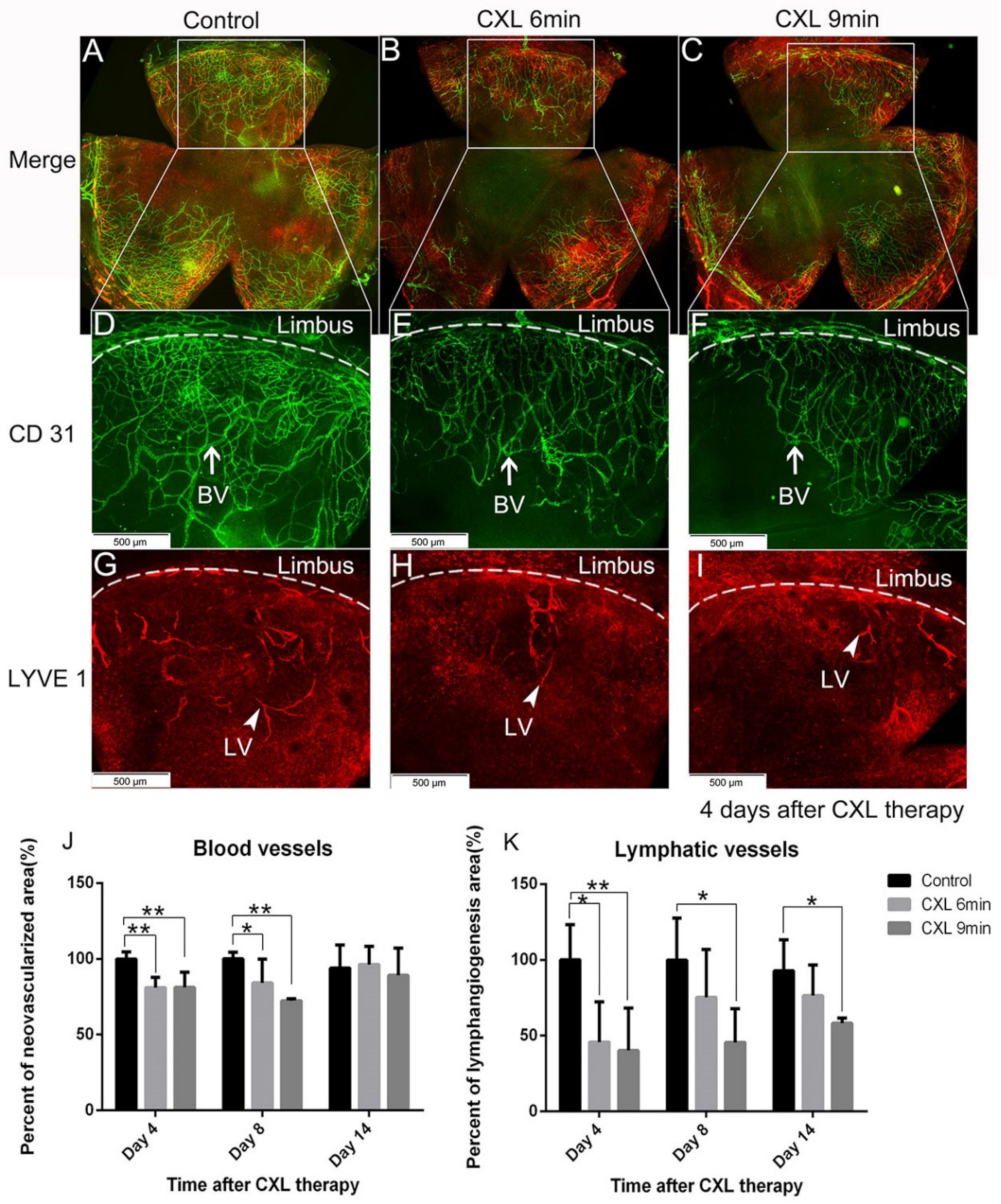
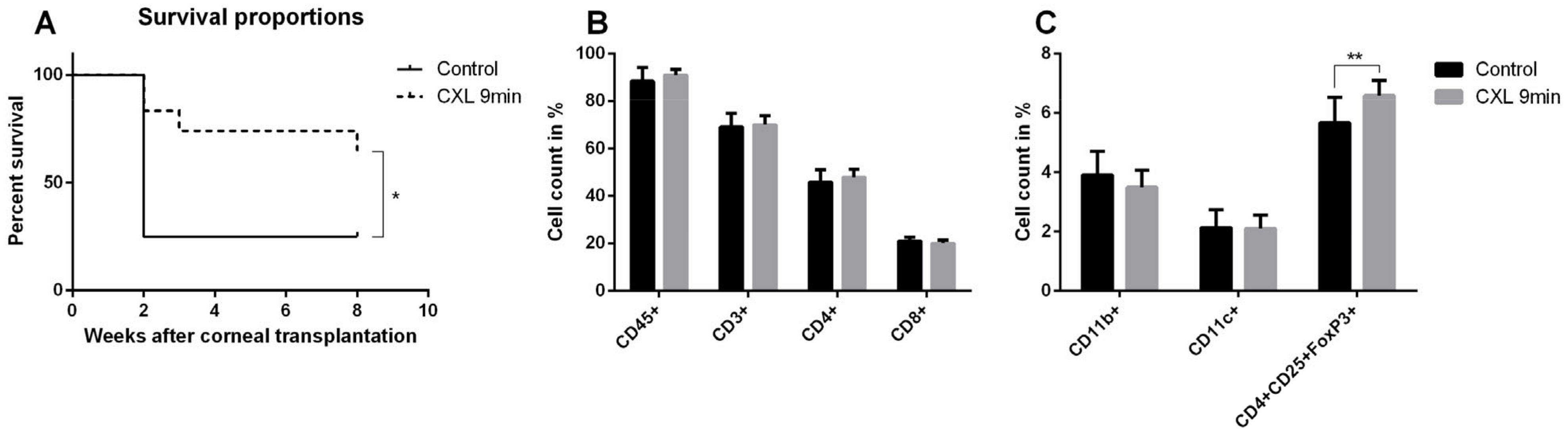
5.1. Preoperative UV-A-Light Based CXL Regresses Corneal Lymphatic Vessels in Prevascularized High-Risk Eyes and Significantly Improves Subsequent Graft Survival in Experimental Settings
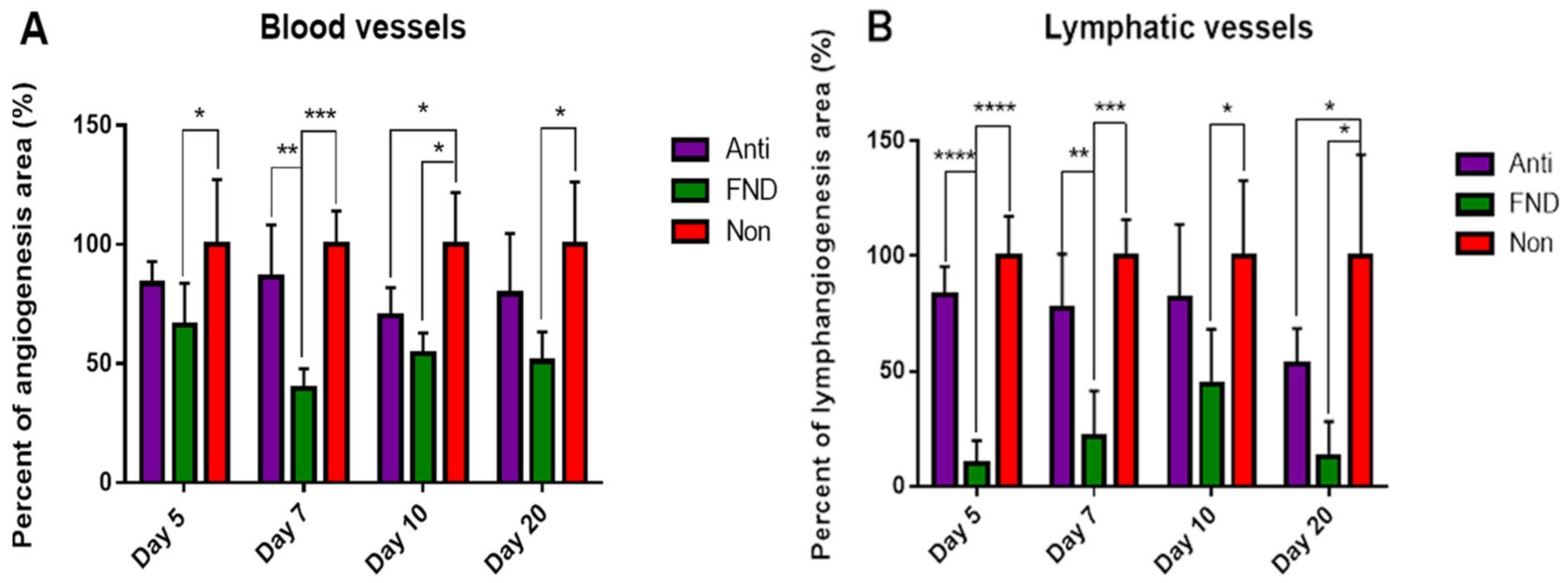
5.2. FND in Combination with Anti-VEGF Antibody (Bevacizumab) Application Improves Corneal Graft Survival via Lymphatic Trafficking Modulation in Mice
6. Translation of Temporary Pretransplant (Lymph)angioregressive Therapy at the Recipient Site via UVA-Based CXL and FND Approaches into Clinical Practice
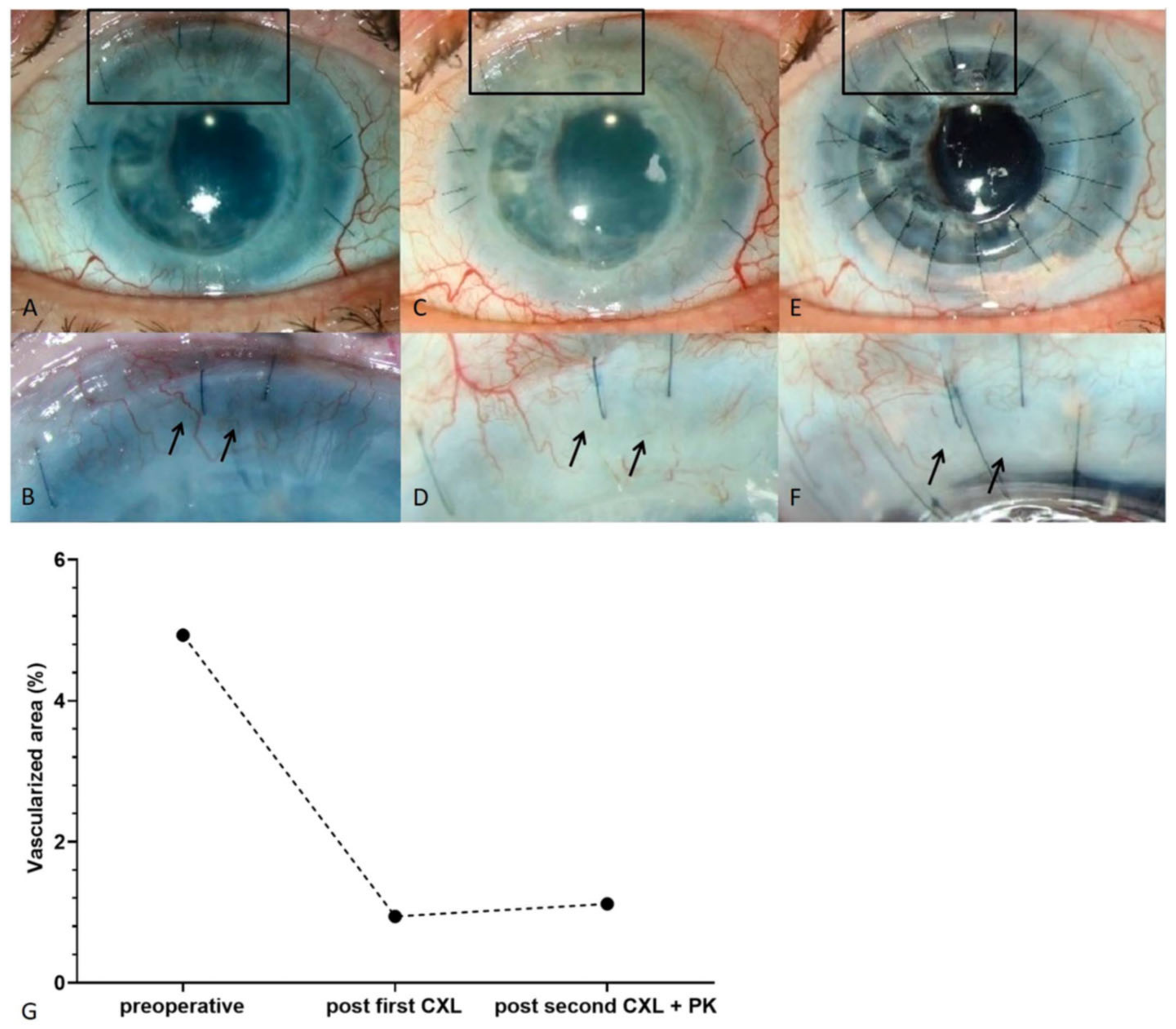
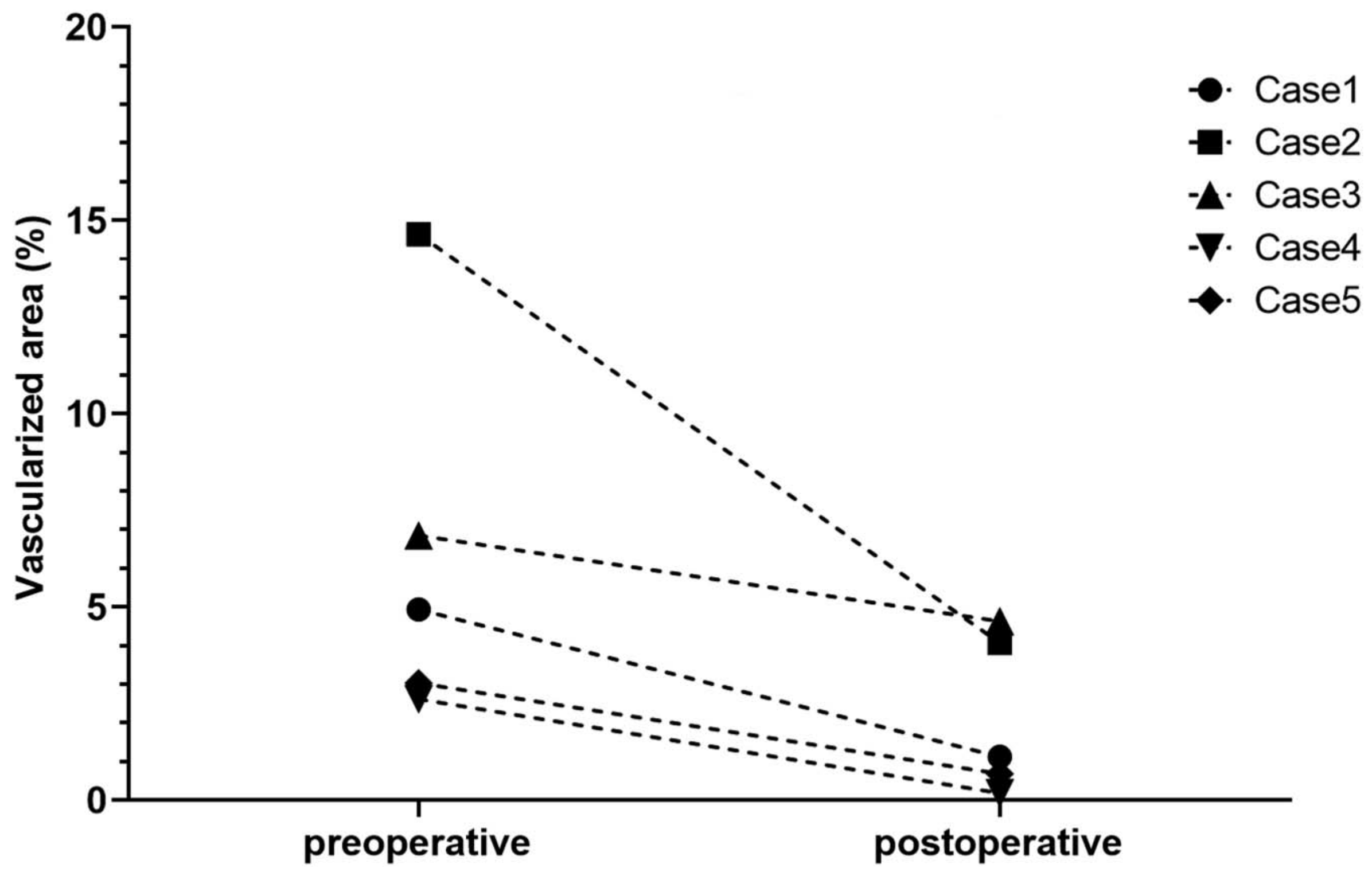
6.1. Clinical Pilot Study of Peripheral UV-A-Light Based CXL Shows the Possibility of Enhancing Transplant Survival in High-Risk Eyes
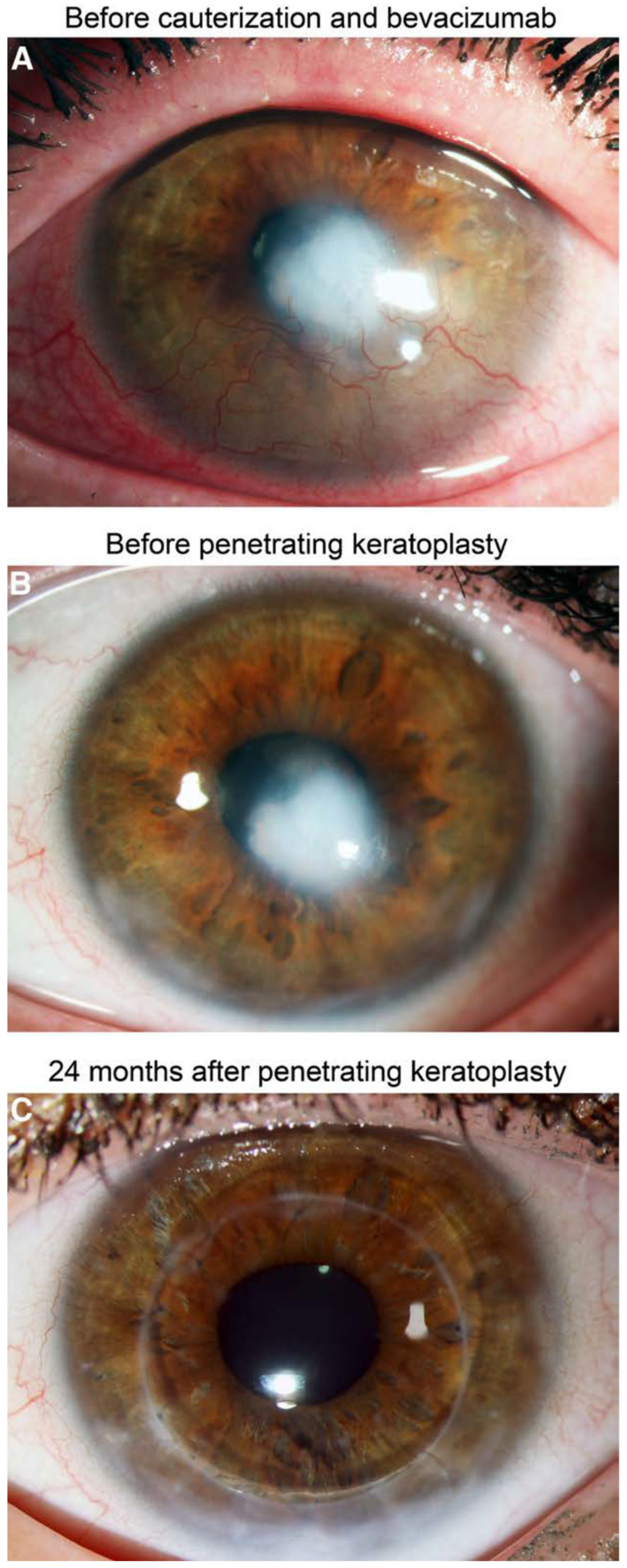
6.2. Interventional Clinical Study of FND Combined with Bevacizumab Indicates Increase of Rejection-Free Corneal Graft Survival Rates in Lymphangioregressively Pretreated Recipient Eyes
7. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 2007, 92, 50–57. [Google Scholar] [CrossRef]
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis : What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef]
- Cursiefen, C.; Rummelt, C.; Jünemann, A.; Vorwerk, C.; Neuhuber, W.; Kruse, F.E.; Schroedl, F. Absence of blood and lymphatic vessels in the developing human cornea. Cornea 2006, 25, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Hussein, H.; Abd El-Baky, N. Molecular underpinnings of corneal angiogenesis: Advances over the past decade. Int. J. Ophthalmol. 2016, 9, 768–779. [Google Scholar] [CrossRef]
- Al-Debasi, T.; Al-Bekairy, A.; Al-Katheri, A.; Al Harbi, S.; Mansour, M. Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization. Saudi J. Ophthalmol. 2017, 31, 99–105. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiculescu, O.B.; Voinea, L.M.; Alexandrescu, C. Corneal neovascularization and biological therapy. J. Med. Life 2015, 8, 444–448. [Google Scholar]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35 Suppl, S224–S243. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.-C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Invest. Ophthalmol. Vis. Sci. 2016, 57, 6554–6560. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, R.J.C.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Jackson, D.G.; Streilein, J.W.; Kruse, F.E. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 2006, 25, 443–447. [Google Scholar] [CrossRef]
- Schönberg, A.; Hamdorf, M.; Bock, F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1280. [Google Scholar] [CrossRef]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.C.; Horstmann, J.; et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305.e7. [Google Scholar] [CrossRef]
- Meduri, A.; Grenga, P.L.; Scorolli, L.; Ceruti, P.; Ferreri, G. Role of cysteine in corneal wound healing after photorefractive keratectomy. Ophthalmic Res. 2009, 41, 76–82. [Google Scholar] [CrossRef]
- Coppini, L.P.; Visniauskas, B.; Costa, E.F.; Filho, M.N.; Rodrigues, E.B.; Chagas, J.R.; Farah, M.E.; Barros, N.M.T.; Carmona, A.K. Corneal angiogenesis modulation by cysteine cathepsins: In vitro and in vivo studies. Exp. Eye Res. 2015, 134, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bao, L.; Zhao, M.; Cao, J.; Zheng, H. Progress in Research on the Role of FGF in the Formation and Treatment of Corneal Neovascularization. Front. Pharmacol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Scalinci, S.Z.; Scorolli, L.; Meduri, A.; Grenga, P.L.; Corradetti, G.; Metrangolo, C. Effect of basic fibroblast growth factor and cytochrome c peroxidase combination in transgenic mice corneal epithelial healing process after excimer laser photoablation. Clin. Ophthalmol. 2011, 5, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.L.X.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 2016, 7, 10824. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef] [Green Version]
- Salabarria, A.-C.; Koch, M.; Schönberg, A.; Zinser, E.; Hos, D.; Hamdorf, M.; Imhof, T.; Braun, G.; Cursiefen, C.; Bock, F. Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Le, V.N.H.; Clahsen, T.; Schneider, A.C.; Bock, F.; Cursiefen, C. Photodynamic therapy leads to time-dependent regression of pathologic corneal (lymph) angiogenesis and promotes high-risk corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5862–5869. [Google Scholar] [CrossRef] [Green Version]
- Salabarria, A.-C.C.; Braun, G.; Heykants, M.; Koch, M.; Reuten, R.; Mahabir, E.; Cursiefen, C.; Bock, F. Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am. J. Transplant. 2019, 19, 2446–2456. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Tóth, G.; Siebelmann, S.; Horstmann, J.; Gabriel, T.; Bock, F.; Cursiefen, C. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am. J. Transplant. 2018, 18, 2873–2884. [Google Scholar] [CrossRef] [PubMed]
- Le, V.N.H.; Schneider, A.C.; Scholz, R.; Bock, F.; Cursiefen, C. Fine Needle-Diathermy Regresses Pathological Corneal (Lymph) Angiogenesis and Promotes High-Risk Corneal Transplant Survival. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting Edge: Lymphatic Vessels, Not Blood Vessels, Primarily Mediate Immune Rejections After Transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2666–2673. [Google Scholar] [CrossRef] [Green Version]
- Koenig, Y.; Bock, F.; Kruse, F.E.; Stock, K.; Cursiefen, C. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: Fine-needle vessel coagulation combined with anti-VEGFs. Cornea 2012, 31, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating Stress Induces T Cell-Mediated Sjögren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Goyal, S.; Chauhan, S.K.; Dana, R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch. Ophthalmol. 2012, 130, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Schaumburg, C.S.; Siemasko, K.F.; De Paiva, C.S.; Wheeler, L.A.; Niederkorn, J.Y.; Pflugfelder, S.C.; Stern, M.E. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 2011, 187, 3653–3662. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D., 3rd; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Schlereth, S.; Lee, H.S.; Khandelwal, P.; Saban, D.R. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am. J. Pathol. 2012, 180, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Zgraggen, S.; Ochsenbein, A.M.; Detmar, M. An important role of blood and lymphatic vessels in inflammation and allergy. J. Allergy 2013, 2013, 672381. [Google Scholar] [CrossRef] [Green Version]
- Saban, D.R. The chemokine receptor CCR7 expressed by dendritic cells: A key player in corneal and ocular surface inflammation. Ocul. Surf. 2014, 12, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Wuest, T.R.; Carr, D.J.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Matthaei, M.; Sandhaeger, H.; Hermel, M.; Adler, W.; Jun, A.S.; Cursiefen, C.; Heindl, L.M. Changing Indications in Penetrating Keratoplasty: A Systematic Review of 34 Years of Global Reporting. Transplantation 2017, 101, 1387–1399. [Google Scholar] [CrossRef]
- Cursiefen, C.; Hos, D. Cutting Edge: Novel Treatment Options Targeting Corneal Neovascularization to Improve High-Risk Corneal Graft Survival. Cornea 2021. [Google Scholar] [CrossRef]
- Hayashi, T.; Zhang, W.; Hos, D.; Schrittenlocher, S.; Nhat Hung Le, V.; Siebelmann, S.; Matthaei, M.; Bock, F.; Bachmann, B.; Cursiefen, C. Descemet Membrane Endothelial Keratoplasty in Vascularized Eyes: Outcome and Effect on Corneal Neovascularization. Cornea 2021, 40, 685–689. [Google Scholar] [CrossRef]
- Coster, D.J.; Williams, K.A. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am. J. Ophthalmol. 2005, 140, 1112–1122. [Google Scholar] [CrossRef] [Green Version]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.W. Ocular Immune Privilege and Transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Hos, D.; Le, V.N.H.; Hellmich, M.; Siebelmann, S.; Roters, S.; Bachmann, B.O.; Cursiefen, C. Risk of Corneal Graft Rejection after High-risk Keratoplasty Following Fine-needle Vessel Coagulation of Corneal Neovascularization Combined with Bevacizumab: A Pilot Study. Transplant. Direct 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Sessa, R.; Kang, G.J.; Shi, M.; Ge, S.; Gong, A.J.; Wen, Y.; Chintharlapalli, S.; Chen, L. Angiopoietin-2 blockade promotes survival of corneal transplants. Investig. Ophthalmol. Vis. Sci. 2017, 58, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hamrah, P.; Zhang, Q.; Taylor, A.W.; Dana, M.R. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J. Exp. Med. 2002, 195, 259–268. [Google Scholar] [CrossRef]
- Kaplan, H.J.; Streilein, J.W.; Stevens, T.R. Transplantation immunology of the anterior chamber of the eye. II. Immune response to allogeneic cells. J. Immunol. 1975, 115, 805–810. [Google Scholar]
- Maddula, S.; Davis, D.K.; Maddula, S.; Burrow, M.K.; Ambati, B.K. Horizons in therapy for corneal angiogenesis. Ophthalmology 2011, 118, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hamrah, P.; Cursiefen, C.; Zhang, Q.; Pytowski, B.; Streilein, J.W.; Dana, M.R. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat. Med. 2004, 10, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W. New thoughts on the immunology of corneal transplantation. Eye 2003, 17, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef] [Green Version]
- Podgrabinska, S.; Kamalu, O.; Mayer, L.; Shimaoka, M.; Snoeck, H.; Randolph, G.J.; Skobe, M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 2009, 183, 1767–1779. [Google Scholar] [CrossRef]
- Hos, D.; Dörrie, J.; Schaft, N.; Bock, F.; Notara, M.; Kruse, F.E.; Krautwald, S.; Cursiefen, C.; Bachmann, B.O. Blockade of CCR7 leads to decreased dendritic cell migration to draining lymph nodes and promotes graft survival in low-risk corneal transplantation. Exp. Eye Res. 2016, 146, 1–6. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Bock, F.; Wiegand, S.J.; Maruyama, K.; Dana, M.R.; Kruse, F.E.; Luetjen-Drecoll, E.; Cursiefen, C. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch. Ophthalmol. 2008, 126, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Bucher, F.; Bi, Y.; Gehlsen, U.; Hos, D.; Cursiefen, C.; Bock, F. Regression of mature lymphatic vessels in the cornea by photodynamic therapy. Br. J. Ophthalmol. 2014, 98, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Dana, M.R.; Tsuru, T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea 2002, 21, 405–409. [Google Scholar] [CrossRef]
- Sallusto, F.; Schaerli, P.; Loetscher, P.; Schaniel, C.; Lenig, D.; Mackay, C.R.; Qin, S.; Lanzavecchia, A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998, 28, 2760–2769. [Google Scholar] [CrossRef]
- Saeki, H.; Moore, A.M.; Brown, M.J.; Hwang, S.T. Cutting edge: Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999, 162, 2472–2475. [Google Scholar]
- Jin, Y.; Shen, L.; Chong, E.-M.; Hamrah, P.; Zhang, Q.; Chen, L.; Dana, M.R. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol. Vis. 2007, 13, 626–634. [Google Scholar] [PubMed]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Soriano, S.F.; Hons, M.; Schumann, K.; Kumar, V.; Dennier, T.J.; Lyck, R.; Sixt, M.; Stein, J. V In vivo analysis of uropod function during physiological T cell trafficking. J. Immunol. 2011, 187, 2356–2364. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Banerji, S.; Lawrance, W.; Gileadi, U.; Prota, G.; Holder, K.A.; Roshorm, Y.M.; Hanke, T.; Cerundolo, V.; Gale, N.W.; et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 2017, 18, 762–770. [Google Scholar] [CrossRef]
- Nitschké, M.; Aebischer, D.; Abadier, M.; Haener, S.; Lucic, M.; Vigl, B.; Luche, H.; Fehling, H.J.; Biehlmaier, O.; Lyck, R.; et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood 2012, 120, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Steven, P.; Bock, F.; Hüttmann, G.; Cursiefen, C. Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS ONE 2011, 6, e26253. [Google Scholar] [CrossRef]
- Torzicky, M.; Viznerova, P.; Richter, S.; Strobl, H.; Scheinecker, C.; Foedinger, D.; Riedl, E. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and CD99 are critical in lymphatic transmigration of human dendritic cells. J. Invest. Dermatol. 2012, 132, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777. [Google Scholar] [CrossRef] [Green Version]
- Maddaluno, L.; Verbrugge, S.E.; Martinoli, C.; Matteoli, G.; Chiavelli, A.; Zeng, Y.; Williams, E.D.; Rescigno, M.; Cavallaro, U. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J. Exp. Med. 2009, 206, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Teijeira, Á.; Palazón, A.; Garasa, S.; Marré, D.; Aubá, C.; Rogel, A.; Murillo, O.; Martínez-Forero, I.; Lang, F.; Melero, I.; et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J. 2012, 26, 3380–3392. [Google Scholar] [CrossRef]
- Buhusi, M.; Demyanenko, G.P.; Jannie, K.M.; Dalal, J.; Darnell, E.P.B.; Weiner, J.A.; Maness, P.F. ALCAM regulates mediolateral retinotopic mapping in the superior colliculus. J. Neurosci. 2009, 29, 15630–15641. [Google Scholar] [CrossRef] [Green Version]
- Willrodt, A.-H.H.; Salabarria, A.-C.C.; Schineis, P.; Ignatova, D.; Hunter, M.C.; Vranova, M.; Golding-Ochsenbein, A.M.; Sigmund, E.; Romagna, A.; Strassberger, V.; et al. ALCAM Mediates DC Migration Through Afferent Lymphatics and Promotes Allospecific Immune Reactions. Front. Immunol. 2019, 10, 759. [Google Scholar] [CrossRef] [Green Version]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Dashkevich, A.; Heilmann, C.; Kayser, G.; Germann, M.; Beyersdorf, F.; Passlick, B.; Geissler, H.J. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann. Thorac. Surg. 2010, 90, 406–411. [Google Scholar] [CrossRef]
- Nykänen, A.I.; Sandelin, H.; Krebs, R.; Keränen, M.A.I.; Tuuminen, R.; Kärpänen, T.; Wu, Y.; Pytowski, B.; Koskinen, P.K.; Ylä-Herttuala, S.; et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation 2010, 121, 1413–1422. [Google Scholar] [CrossRef]
- Stuht, S.; Gwinner, W.; Franz, I.; Schwarz, A.; Jonigk, D.; Kreipe, H.; Kerjaschki, D.; Haller, H.; Mengel, M. Lymphatic neoangiogenesis in human renal allografts: Results from sequential protocol biopsies. Am. J. Transplant. 2007, 7, 377–384. [Google Scholar] [CrossRef]
- Geissler, H.J.; Dashkevich, A.; Fischer, U.M.; Fries, J.W.U.; Kuhn-Régnier, F.; Addicks, K.; Mehlhorn, U.; Bloch, W. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur. J. Cardio Thoracic Surg. 2006, 29, 767–771. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, K.; Monzon-Medina, M.E.; Padera, R.F.; Wang, H.; George, G.; Toprak, D.; Abdelnour, E.; D’Agostino, E.; Goldberg, H.J.; et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J. Clin. Investig. 2015, 125, 4255–4268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hos, D.; Cursiefen, C. Lymphatic vessels in the development of tissue and organ rejection. Adv. Anat. Embryol. Cell Biol. 2014, 214, 119–141. [Google Scholar] [CrossRef]
- Lipp, M.; Bucher, F.; Parthasarathy, A.; Hos, D.; Onderka, J.; Cursiefen, C.; Bock, F. Blockade of the VEGF isoforms in inflammatory corneal hemangiogenesis and lymphangiogenesis. Graefe Arch. Clin. Exp. Ophthalmol. 2014, 252, 943–949. [Google Scholar] [CrossRef]
- Büttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase Is a Novel Endogenous Regulator of Developmental and Inflammatory Lymphangiogenesis. Am. J. Pathol. 2019, 189, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Le, V.N.H.; Hos, D.; Hou, Y.; Witt, M.; Barkovskiy, M.; Bock, F.; Cursiefen, C. VEGF trapR1R2 suspended in the semifluorinated alkane f6h8 inhibits inflammatory corneal hem-and lymphangiogenesis. Transl. Vis. Sci. Technol. 2020, 9, 1–2. [Google Scholar] [CrossRef]
- Cursiefen, C.; Bock, F.; Horn, F.K.; Kruse, F.E.; Seitz, B.; Borderie, V.; Früh, B.; Thiel, M.A.; Wilhelm, F.; Geudelin, B.; et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: Interim results of a randomized phase II trial. Ophthalmology 2009, 116, 1630–1637. [Google Scholar] [CrossRef]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5778–5785. [Google Scholar] [CrossRef] [Green Version]
- Bucher, F.; Parthasarathy, A.; Bergua, A.; Onderka, J.; Regenfuss, B.; Cursiefen, C.; Bock, F. Topical Ranibizumab inhibits inflammatory corneal hem- and lymphangiogenesis. Acta Ophthalmol. 2014, 92, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch. Ophthalmol. 2011, 129, 445–452. [Google Scholar] [CrossRef]
- Schaub, F.; Hou, Y.; Zhang, W.; Bock, F.; Hos, D.; Cursiefen, C. Corneal Crosslinking to Regress Pathologic Corneal Neovascularization Before High-Risk Keratoplasty. Cornea 2021, 40, 147–155. [Google Scholar] [CrossRef]
- Le, V.N.H.; Hou, Y.; Bock, F.; Cursiefen, C. Supplemental Anti Vegf A-Therapy Prevents Rebound Neovascularisation after Fine Needle Diathermy Treatment to Regress Pathological Corneal (LYMPH)Angiogenesis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöllhorn, L.; Bock, F.; Cursiefen, C. Thrombospondin-1 as a Regulator of Corneal Inflammation and Lymphangiogenesis: Effects on Dry Eye Disease and Corneal Graft Immunology. J. Ocul. Pharmacol. Ther. 2015, 31, 376–385. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefe Arch. Clin. Exp. Ophthalmol. 2009, 247, 1375–1382. [Google Scholar] [CrossRef]
- Bock, F.; König, Y.; Kruse, F.; Baier, M.; Cursiefen, C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefe Arch. Clin. Exp. Ophthalmol. 2008, 246, 281–284. [Google Scholar] [CrossRef]
- Ferrari, G.; Dastjerdi, M.H.; Okanobo, A.; Cheng, S.-F.; Amparo, F.; Nallasamy, N.; Dana, R. Topical ranibizumab as a treatment of corneal neovascularization. Cornea 2013, 32, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Detry, B.; Blacher, S.; Erpicum, C.; Paupert, J.; Maertens, L.; Maillard, C.; Munaut, C.; Sounni, N.E.; Lambert, V.; Foidart, J.-M.; et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3082–3093. [Google Scholar] [CrossRef] [Green Version]
- Platania, C.B.M.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [Green Version]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefe Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.-M.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1244–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2007, 48, 2545–2552. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Saban, D.R.; Okanobo, A.; Nallasamy, N.; Sadrai, Z.; Chauhan, S.K.; Hajrasouliha, A.R.; Dana, R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Bignami, F.; Lorusso, A.; Rama, P.; Ferrari, G. Growth inhibition of formed corneal neovascularization following Fosaprepitant treatment. Acta Ophthalmol. 2017, 95, e641–e648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Dietrich, T.; Onderka, J.; Bock, F.; Kruse, F.E.; Vossmeyer, D.; Stragies, R.; Zahn, G.; Cursiefen, C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am. J. Pathol. 2007, 171, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Garmy-Susini, B.; Varner, J.A. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat. Res. Biol. 2008, 6, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.J.; Truong, T.; Huang, E.; Su, V.; Ge, S.; Chen, L. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5935–5939. [Google Scholar] [CrossRef] [Green Version]
- Reuer, T.; Schneider, A.C.; Cakir, B.; Bühler, A.D.; Walz, J.M.; Lapp, T.; Lange, C.; Agostini, H.; Schlunck, G.; Cursiefen, C.; et al. Semaphorin 3F modulates corneal lymphangiogenesis and promotes corneal graft survival. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5277–5284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, Y.; Maruyama, K.; Kato, Y.; Kajiya, K.; Moritoh, S.; Yamamoto, K.; Matsumoto, Y.; Sawane, M.; Kerjaschki, D.; Nakazawa, T.; et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4813–4822. [Google Scholar] [CrossRef] [Green Version]
- Du, H.-T.T.; Du, L.-L.L.; Tang, X.-L.L.; Ge, H.-Y.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe Arch. Clin. Exp. Ophthalmol. 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Cursiefen, C.; Colin, J.; Dana, R.; Diaz-Llopis, M.; Faraj, L.A.; Garcia-Delpech, S.; Geerling, G.; Price, F.W.; Remeijer, L.; Rouse, B.T.; et al. Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: Outcome of an expert roundtable. Br. J. Ophthalmol. 2012, 96, 3–9. [Google Scholar] [CrossRef]
- Cursiefen, C.; Cordeiro, F.; Cunha-Vaz, J.; Wheeler-Schilling, T.; Scholl, H.P.N. Unmet Needs in Ophthalmology: A European Vision Institute-Consensus Roadmap 2019–2025. Ophthalmic Res. 2019, 62, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, M.; Andrieu-Soler, C.; Doat, M.; Courtois, Y.; BenEzra, D.; Behar-Cohen, F. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest. Ophthalmol. Vis. Sci. 2005, 46, 4072–4078. [Google Scholar] [CrossRef] [Green Version]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecová, P.; Lévy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The I-CAN study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiskup, F.; Spoerl, E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul. Surf. 2013, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Reber, F.; Seiler, T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye 2004, 18, 718–722. [Google Scholar] [CrossRef]
- Mencucci, R.; Marini, M.; Paladini, I.; Sarchielli, E.; Sgambati, E.; Menchini, U.; Vannelli, G.B. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clin. Experiment. Ophthalmol. 2010, 38, 49–56. [Google Scholar] [CrossRef]
- Spoerl, E.; Mrochen, M.; Sliney, D.; Trokel, S.; Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007, 26, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.B.; Park, S.Y.; Ko, J.H.; Park, J.W.; Yoon, C.H.; Kim, D.H.; Kim, J.H.; Kim, M.K.; Lee, R.H.; Prockop, D.J.; et al. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol. Ther. 2018, 26, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, K.J.; Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003, 3, 199–210. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Cunnusamy, K.; Niederkorn, J.Y. IFN-γ blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. Am. J. Transplant. 2013, 13, 3076–3084. [Google Scholar] [CrossRef] [Green Version]
- Reyes, N.J.; Chen, P.W.; Niederkorn, J.Y. Allergic conjunctivitis renders CD4(+) T cells resistant to t regulatory cells and exacerbates corneal allograft rejection. Am. J. Transplant. 2013, 13, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.K.; Saban, D.R.; Lee, H.K.; Dana, R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009, 182, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Cunnusamy, K.; Chen, P.W.; Niederkorn, J.Y. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J. Immunol. 2011, 186, 6737–6745. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.T.; Dua, H.S.; Hossain, P. Fine needle diathermy occlusion of corneal vessels. Invest. Ophthalmol. Vis. Sci. 2000, 41, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Faraj, L.A.; Elalfy, M.S.; Said, D.G.; Dua, H.S. Fine needle diathermy occlusion of corneal vessels. Br. J. Ophthalmol. 2014, 98, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Thatte, S. Fine needle diathermy—A choice for managing corneal vascularization. Nepal. J. Ophthalmol. 2011, 3, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Spiteri, N.; Romano, V.; Zheng, Y.; Yadav, S.; Dwivedi, R.; Chen, J.; Ahmad, S.; Willoughby, C.E.; Kaye, S.B. Corneal angiography for guiding and evaluating fine-needle diathermy treatment of corneal neovascularization. Ophthalmology 2015, 122, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, U.; Mireskandari, K.; Shen, C.; Ali, A. Corneal fine needle diathermy with adjuvant bevacizumab to treat corneal neovascularization in children. Cornea 2015, 34, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Savant, V. Fine-Needle Diathermy with Simultaneous Subconjunctival Bevacizumab. Semin. Ophthalmol. 2017, 32, 550–552. [Google Scholar] [CrossRef]
- Trikha, S.; Parikh, S.; Osmond, C.; Anderson, D.F.; Hossain, P.N. Long-term outcomes of Fine Needle Diathermy for established corneal neovascularisation. Br. J. Ophthalmol. 2014, 98, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.A.; Keane, M.C.; Galettis, R.A.; Jones, V.J.; Mills, R.A.; Coster, D.J. The Australian Corneal Graft Registry 2015 Report. Available online: http//hdl.handle.net/2328/35402 (accessed on 18 February 2019).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Bock, F.; Hos, D.; Cursiefen, C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells 2021, 10, 1661. https://doi.org/10.3390/cells10071661
Hou Y, Bock F, Hos D, Cursiefen C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells. 2021; 10(7):1661. https://doi.org/10.3390/cells10071661
Chicago/Turabian StyleHou, Yanhong, Felix Bock, Deniz Hos, and Claus Cursiefen. 2021. "Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival" Cells 10, no. 7: 1661. https://doi.org/10.3390/cells10071661





