Mast Cell-Specific Deletion of Group III Secreted Phospholipase A2 Impairs Mast Cell Maturation and Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Maturation and Activation of BMMCs
2.3. Flow Cytometry
2.4. Quantitative RT-PCR
2.5. Anaphylaxis
2.6. Dermatitis
2.7. MC Reconstitution
2.8. Histology
2.9. Statistical Analysis
3. Results
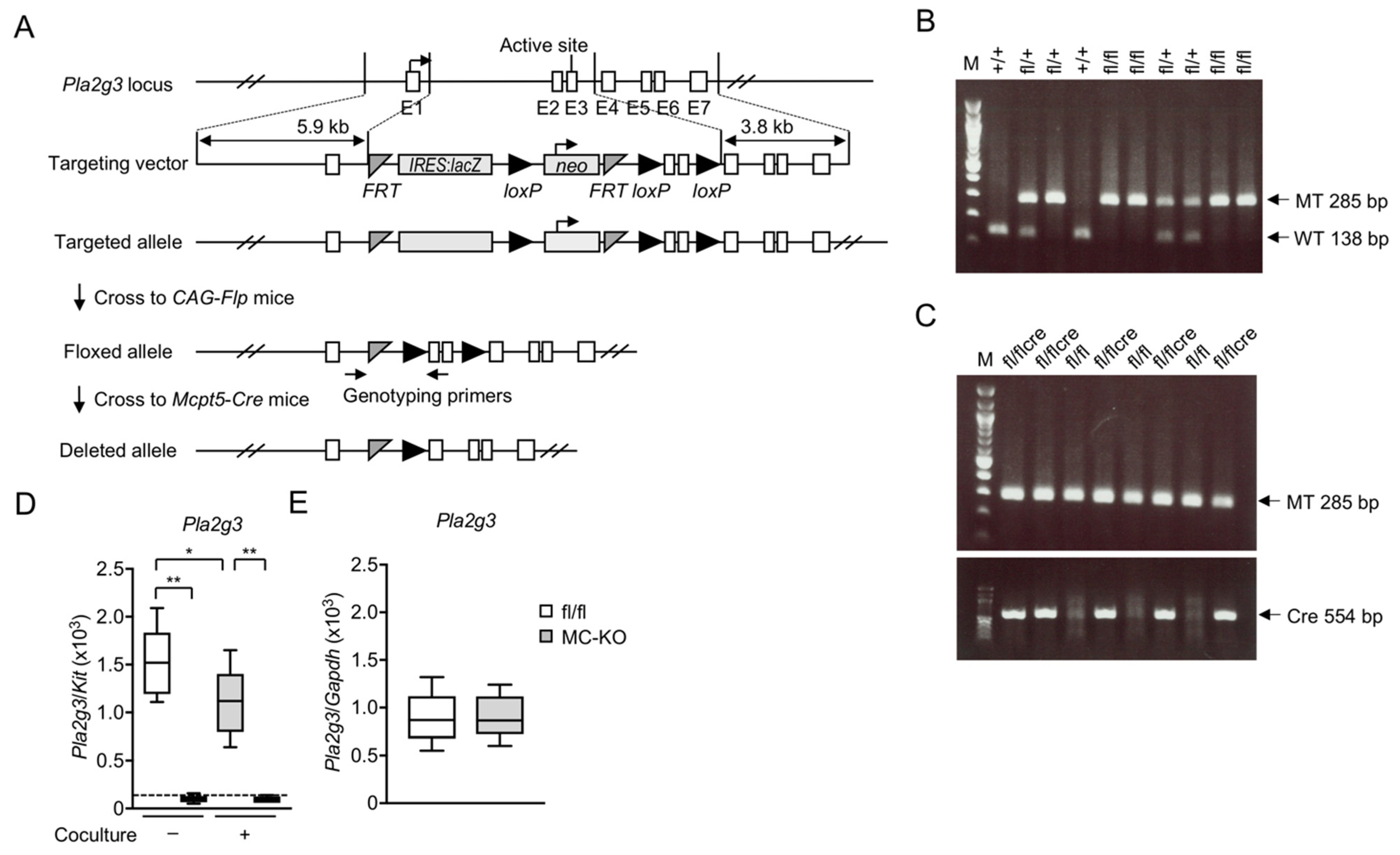
3.1. Generation of MC-Specific Pla2g3-Deficient Mice
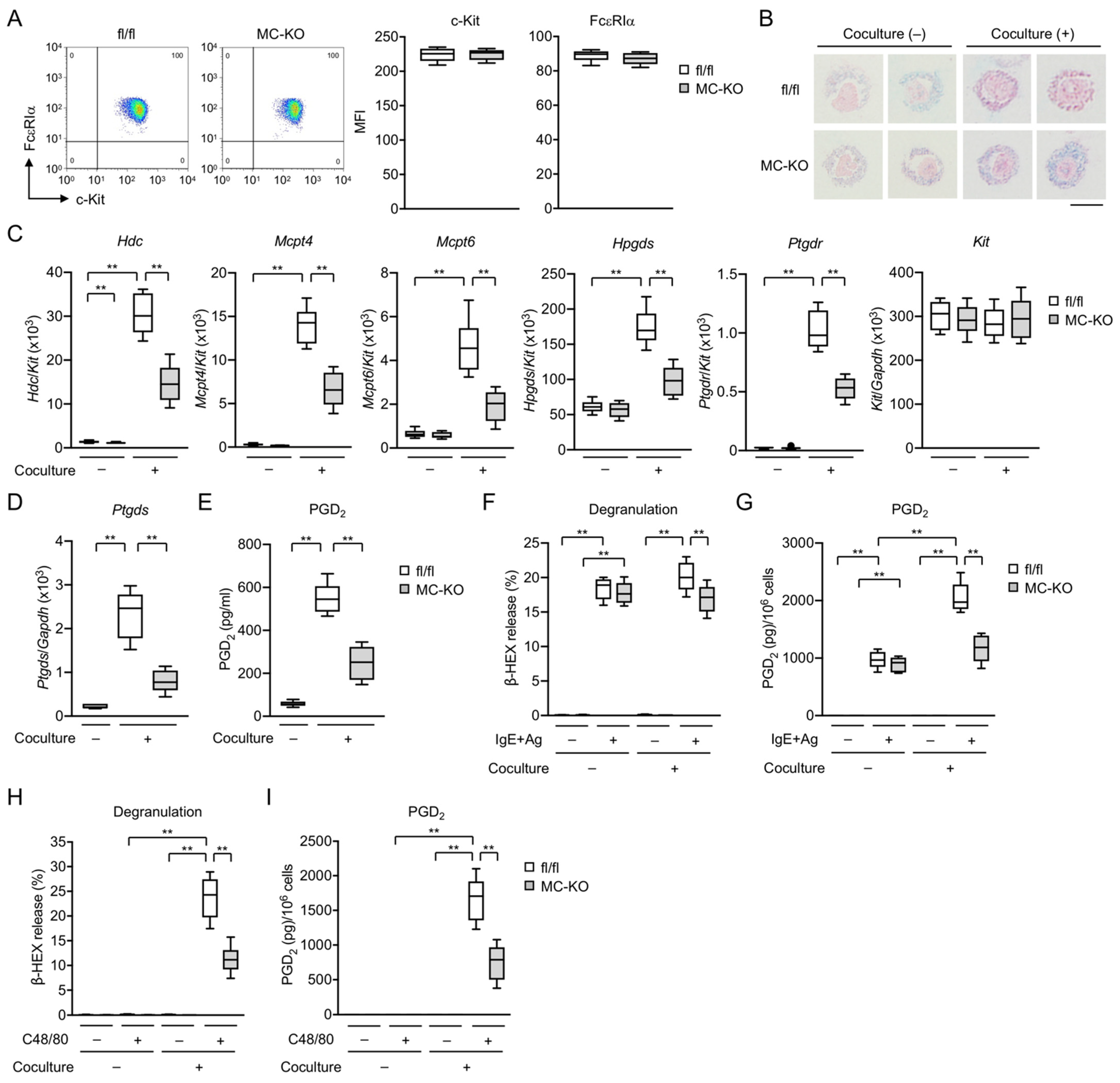
3.2. MC-Specific Pla2g3 Ablation Impairs MC Maturation
3.3. MC-Specific Pla2g3 Ablation Ameliorates MC-Associated Anaphylaxis and Irritant Dermatitis
3.4. MC-Specific Pla2g3 Ablation Excacerbates CHS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast cells in inflammation and disease: Recent progress and ongoing concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Hallgren, J.; Gurish, M.F. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 2011, 716, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu. Rev. Immunol. 1989, 7, 59–76. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajenoff, M. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult connective tissue-resident mast cells originate from late erythro-myeloid progenitors. Immunity 2018, 49, 640–653.e5. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Oboki, K.; Ito, A. Development of mast cells. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Hallgren, J.; Gurish, M.F. Pathways of murine mast cell development and trafficking: Tracking the roots and routes of the mast cell. Immunol. Rev. 2007, 217, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, Y.; Ueno, N.; Kojima, T.; Sato, H.; Murase, R.; Yamamoto, K.; Tanaka, S.; Sakanaka, M.; Nakamura, M.; Nishito, Y.; et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013, 14, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M. Lipoquality control by phospholipase A2 enzymes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 677–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent progress in phospholipase A2 research: From cells to animals to humans. Prog. Lipid Res. 2011, 50, 152–192. [Google Scholar] [CrossRef]
- Fujishima, H.; Sanchez Mejia, R.O.; Bingham, C.O., 3rd; Lam, B.K.; Sapirstein, A.; Bonventre, J.V.; Austen, K.F.; Arm, J.P. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc. Natl. Acad. Sci. USA 1999, 96, 4803–4807. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, N.; Uozumi, N.; Kume, K.; Murakami, M.; Kudo, I.; Shimizu, T. Role of cytosolic phospholipase A2 in the production of lipid mediators and histamine release in mouse bone-marrow-derived mast cells. Biochem. J. 2000, 352, 311–317. [Google Scholar] [CrossRef]
- Murakami, M.; Taketomi, Y. Secreted phospholipase A2 and mast cells. Allergol. Int. 2015, 64, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, T.; Hirata, M.; Tanaka, H.; Takahashi, Y.; Murata, T.; Kabashima, K.; Sugimoto, Y.; Kobayashi, T.; Ushikubi, F.; Aze, Y.; et al. Prostaglandin D2 as a mediator of allergic asthma. Science 2000, 287, 2013–2017. [Google Scholar] [CrossRef] [Green Version]
- Spik, I.; Brenuchon, C.; Angeli, V.; Staumont, D.; Fleury, S.; Capron, M.; Trottein, F.; Dombrowicz, D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J. Immunol. 2005, 174, 3703–3708. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.G.; Newson, J.; Rajakariar, R.; Jacques, T.S.; Hannon, R.; Kanaoka, Y.; Eguchi, N.; Colville-Nash, P.; Gilroy, D.W. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc. Natl. Acad. Sci. USA 2006, 103, 5179–5184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad, H.; Kool, M.; Soullie, T.; Narumiya, S.; Trottein, F.; Hoogsteden, H.C.; Lambrecht, B.N. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 2007, 204, 357–367. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Otani, S.; Hirai, H.; Nagata, K.; Aritake, K.; Urade, Y.; Narumiya, S.; Yokozeki, H.; Nakamura, M.; Satoh, T. Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2. Am. J. Pathol. 2011, 179, 302–314. [Google Scholar] [CrossRef]
- Nakamura, T.; Maeda, S.; Horiguchi, K.; Maehara, T.; Aritake, K.; Choi, B.I.; Iwakura, Y.; Urade, Y.; Murata, T. PGD2 deficiency exacerbates food antigen-induced mast cell hyperplasia. Nat. Commun. 2015, 6, 7514. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Gelb, M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008, 77, 495–520. [Google Scholar] [CrossRef] [Green Version]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 2000, 275, 7492–7496. [Google Scholar] [CrossRef] [Green Version]
- Grimbaldeston, M.A.; Chen, C.C.; Piliponsky, A.M.; Tsai, M.; Tam, S.Y.; Galli, S.J. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005, 167, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Nigrovic, P.A.; Gray, D.H.; Jones, T.; Hallgren, J.; Kuo, F.C.; Chaletzky, B.; Gurish, M.; Mathis, D.; Benoist, C.; Lee, D.M. Genetic inversion in mast cell-deficient Wsh mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am. J. Pathol. 2008, 173, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.L.; Marichal, T.; Galli, S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Gaudenzio, N.; Marichal, T.; Galli, S.J.; Reber, L.L. Genetic and imaging approaches reveal pro-inflammatory and immunoregulatory roles of mast cells in contact hypersensitivity. Front. Immunol. 2018, 9, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, J.; Ghouse, S.M.; Lehmann, C.H.; Hoppe, A.; Schubert, N.; Nedospasov, S.A.; Dudziak, D.; Dudeck, A. Mast-cell-derived TNF amplifies CD8+ dendritic cell functionality and CD8+ T cell priming. Cell Rep. 2015, 13, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reber, L.L.; Sibilano, R.; Starkl, P.; Roers, A.; Grimbaldeston, M.A.; Tsai, M.; Gaudenzio, N.; Galli, S.J. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, B.; Harmacek, L.; Long, Z.; Liang, J.; Lukin, K.; Leach, S.M.; O’Connor, B.; Gerber, A.N.; Hagman, J.; et al. The transcription factors GATA2 and microphthalmia-associated transcription factor regulate Hdc gene expression in mast cells and are required for IgE/mast cell-mediated anaphylaxis. J. Allergy Clin. Immunol. 2018, 142, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Mishina, M.; Sakimura, K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci. Res. 2007, 58, 105–112. [Google Scholar] [CrossRef]
- Scholten, J.; Hartmann, K.; Gerbaulet, A.; Krieg, T.; Muller, W.; Testa, G.; Roers, A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008, 17, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Taketomi, Y.; Isogai, Y.; Miki, Y.; Yamamoto, K.; Masuda, S.; Hosono, T.; Arata, S.; Ishikawa, Y.; Ishii, T.; et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 2010, 120, 1400–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohri, I.; Taniike, M.; Taniguchi, H.; Kanekiyo, T.; Aritake, K.; Inui, T.; Fukumoto, N.; Eguchi, N.; Kushi, A.; Sasai, H.; et al. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J. Neurosci. 2006, 26, 4383–4393. [Google Scholar] [CrossRef]
- Murakami, M.; Matsumoto, R.; Austen, K.F.; Arm, J.P. Prostaglandin endoperoxide synthase-1 and -2 couple to different transmembrane stimuli to generate prostaglandin D2 in mouse bone marrow-derived mast cells. J. Biol. Chem. 1994, 269, 22269–22275. [Google Scholar] [CrossRef]
- Ogasawara, T.; Murakami, M.; Suzuki-Nishimura, T.; Uchida, M.K.; Kudo, I. Mouse bone marrow-derived mast cells undergo exocytosis, prostanoid generation, and cytokine expression in response to G protein-activating polybasic compounds after coculture with fibroblasts in the presence of c-kit ligand. J. Immunol. 1997, 158, 393–404. [Google Scholar] [PubMed]
- Taketomi, Y.; Sugiki, T.; Saito, T.; Ishii, S.; Hisada, M.; Suzuki-Nishimura, T.; Uchida, M.K.; Moon, T.C.; Chang, H.W.; Natori, Y.; et al. Identification of NDRG1 as an early inducible gene during in vitro maturation of cultured mast cells. Biochem. Biophys. Res. Commun. 2003, 306, 339–346. [Google Scholar] [CrossRef]
- Taketomi, Y.; Sunaga, K.; Tanaka, S.; Nakamura, M.; Arata, S.; Okuda, T.; Moon, T.C.; Chang, H.W.; Sugimoto, Y.; Kokame, K.; et al. Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1-deficient mice. J. Immunol. 2007, 178, 7042–7053. [Google Scholar] [CrossRef] [Green Version]
- Miki, Y.; Yamamoto, K.; Taketomi, Y.; Sato, H.; Shimo, K.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; Nakanishi, H.; Ikeda, K.; et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013, 210, 1217–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Miki, Y.; Sato, M.; Taketomi, Y.; Nishito, Y.; Taya, C.; Muramatsu, K.; Ikeda, K.; Nakanishi, H.; Taguchi, R.; et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 2015, 212, 1901–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Edwards, T.N.; Chaudhri, V.K.; Wu, J.; Cohen, J.A.; Hirai, T.; Rittenhouse, N.; Schmitz, E.G.; Zhou, P.Y.; McNeil, B.D.; et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 2021, 184, 2151–2166.e16. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askenase, P.W.; Van Loveren, H.; Kraeuter-Kops, S.; Ron, Y.; Meade, R.; Theoharides, T.C.; Nordlund, J.J.; Scovern, H.; Gerhson, M.D.; Ptak, W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J. Immunol. 1983, 131, 2687–2694. [Google Scholar] [PubMed]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hultner, L.; Rocken, M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000, 192, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Bryce, P.J.; Miller, M.L.; Miyajima, I.; Tsai, M.; Galli, S.J.; Oettgen, H.C. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity 2004, 20, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Kohler, A.; Peschke, K.; Vohringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, A.; Kubo, M.; Honda, T.; Egawa, G.; Nakajima, S.; Tanizaki, H.; Kim, B.; Matsuoka, S.; Watanabe, T.; Nakae, S.; et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 2011, 6, e25538. [Google Scholar] [CrossRef] [Green Version]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsai, M.; Galli, S.J. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef]
- Hershko, A.Y.; Suzuki, R.; Charles, N.; Alvarez-Errico, D.; Sargent, J.L.; Laurence, A.; Rivera, J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 2011, 35, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Rivera, V.A.; Siebenhaar, F.; Zimmermann, C.; Siiskonen, H.; Metz, M.; Maurer, M. Mast cells limit the exacerbation of chronic allergic contact dermatitis in response to repeated allergen exposure. J. Immunol. 2016, 197, 4240–4246. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Hammel, I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science 1984, 226, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Mekori, Y.A.; Galli, S.J. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J. Immunol. 1985, 135, 879–885. [Google Scholar]
- Mekori, Y.A.; Chang, J.C.; Wershil, B.K.; Galli, S.J. Studies of the role of mast cells in contact sensitivity responses. Passive transfer of the reaction into mast cell-deficient mice locally reconstituted with cultured mast cells: Effect of reserpine on transfer of the reaction with DNP-specific cloned T cells. Cell. Immunol. 1987, 109, 39–52. [Google Scholar] [CrossRef]
- Xu, H.; DiIulio, N.A.; Fairchild, R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996, 183, 1001–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Wei, Y.; Chhiba, K.D.; Zhang, F.; Ye, X.; Wang, L.; Zhang, L.; Robida, P.A.; Moreno-Vinasco, L.; Schnaar, R.L.; Roers, A.; et al. Mast cell-specific expression of human Siglec-8 in conditional knock-in mice. Int. J. Mol. Sci. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilla, J.N.; Chen, C.C.; Mukai, K.; BenBarak, M.J.; Franco, C.B.; Kalesnikoff, J.; Yu, M.; Tsai, M.; Piliponsky, A.M.; Galli, S.J. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 2011, 118, 6930–6938. [Google Scholar] [CrossRef] [Green Version]
- Suto, H.; Nakae, S.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 2006, 176, 4102–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Yamamoto, K.; Miki, Y.; Murase, R.; Sato, H.; Taketomi, Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016, 132, 91–134. [Google Scholar] [CrossRef]
- Murakami, M.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Yamamoto, K. Group IID, IIE, IIF and III secreted phospholipase A2s. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 803–818. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taketomi, Y.; Endo, Y.; Higashi, T.; Murase, R.; Ono, T.; Taya, C.; Kobayashi, T.; Murakami, M. Mast Cell-Specific Deletion of Group III Secreted Phospholipase A2 Impairs Mast Cell Maturation and Functions. Cells 2021, 10, 1691. https://doi.org/10.3390/cells10071691
Taketomi Y, Endo Y, Higashi T, Murase R, Ono T, Taya C, Kobayashi T, Murakami M. Mast Cell-Specific Deletion of Group III Secreted Phospholipase A2 Impairs Mast Cell Maturation and Functions. Cells. 2021; 10(7):1691. https://doi.org/10.3390/cells10071691
Chicago/Turabian StyleTaketomi, Yoshitaka, Yuki Endo, Takayoshi Higashi, Remi Murase, Tomio Ono, Choji Taya, Tetsuyuki Kobayashi, and Makoto Murakami. 2021. "Mast Cell-Specific Deletion of Group III Secreted Phospholipase A2 Impairs Mast Cell Maturation and Functions" Cells 10, no. 7: 1691. https://doi.org/10.3390/cells10071691
APA StyleTaketomi, Y., Endo, Y., Higashi, T., Murase, R., Ono, T., Taya, C., Kobayashi, T., & Murakami, M. (2021). Mast Cell-Specific Deletion of Group III Secreted Phospholipase A2 Impairs Mast Cell Maturation and Functions. Cells, 10(7), 1691. https://doi.org/10.3390/cells10071691





