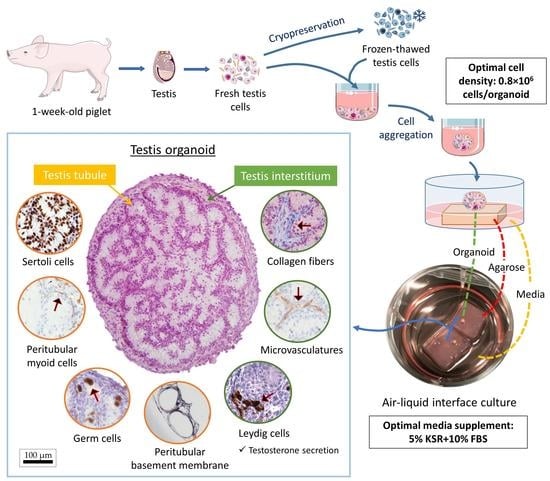
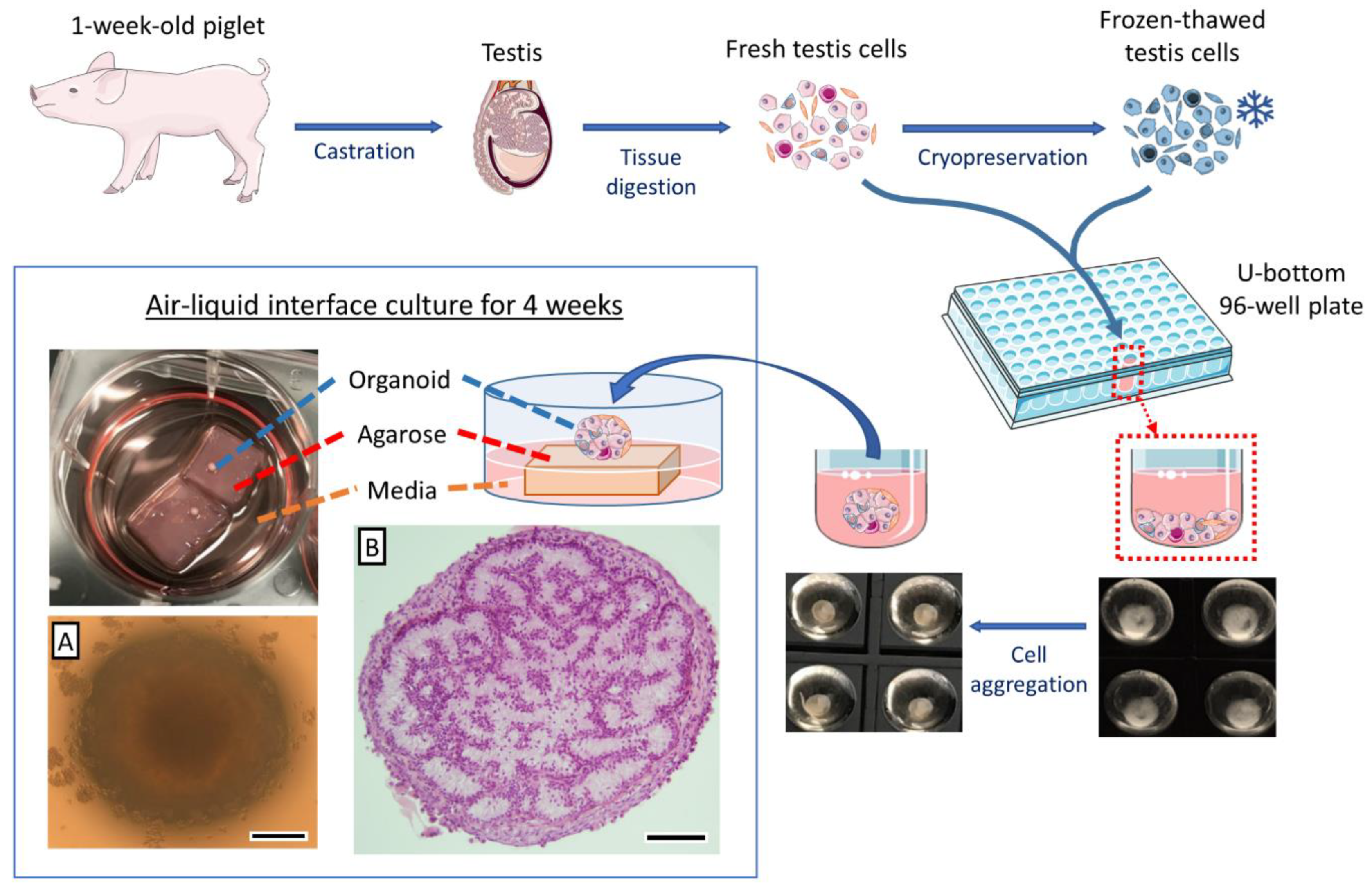
Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Testis Collection and Preparation
2.3. Testis Cell Isolation
2.4. Testis Cell Cryopreservation
2.5. Testis Organoid Culture System
2.6. Histological Analysis
2.7. Immunohistochemistry (IHC) and Tissue-Specific Staining
2.8. Transmission Electron Microscopy (TEM)
2.9. Luteinizing Hormone (LH) Induction and Testosterone Measurements
2.10. Statistical Analyses
3. Results
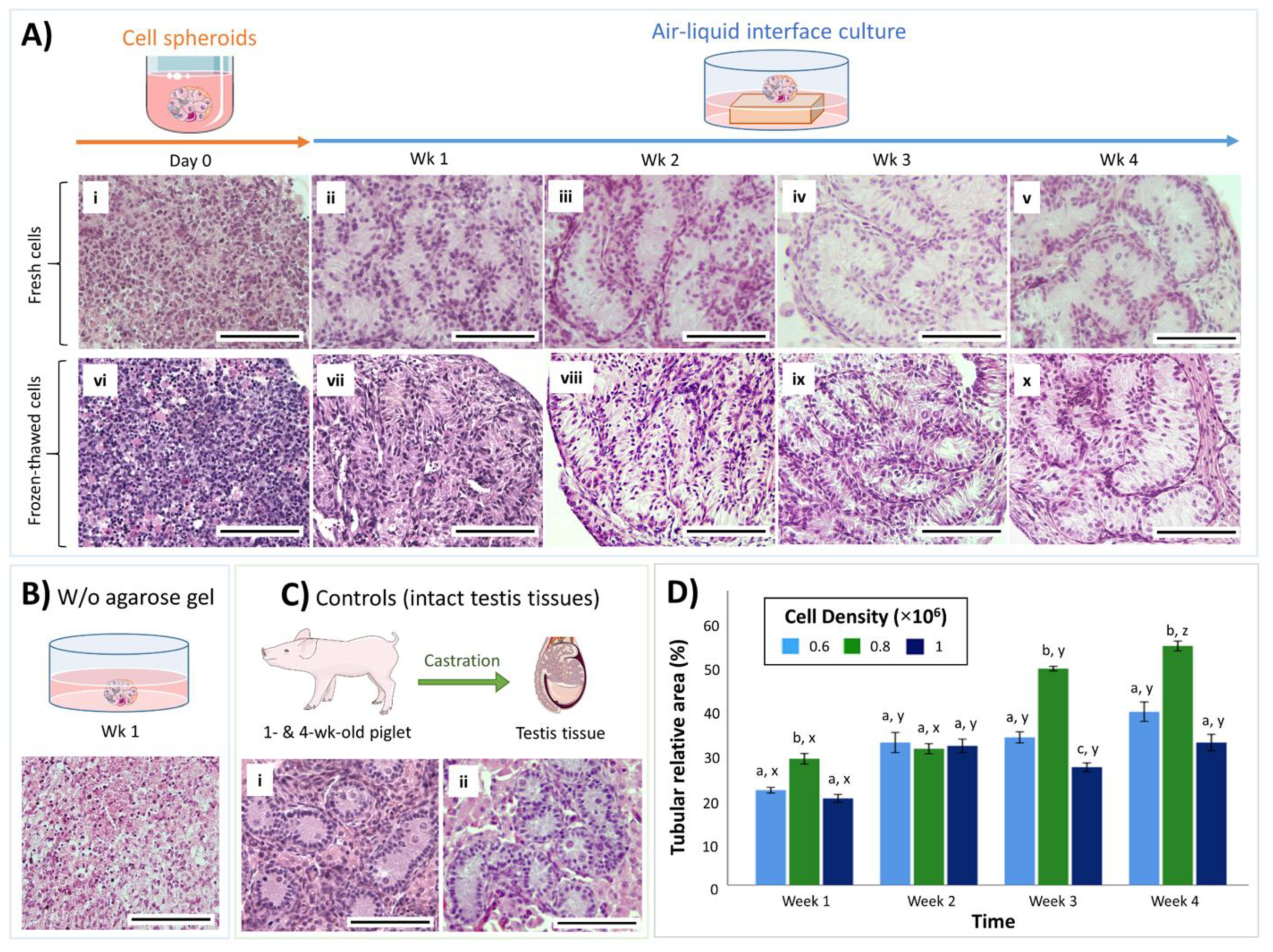
3.1. Both Fresh and Cryopreserved Testis Cells can Form Organoids
3.2. Cell Density Affects In Vitro Tubulogenesis of Organoids
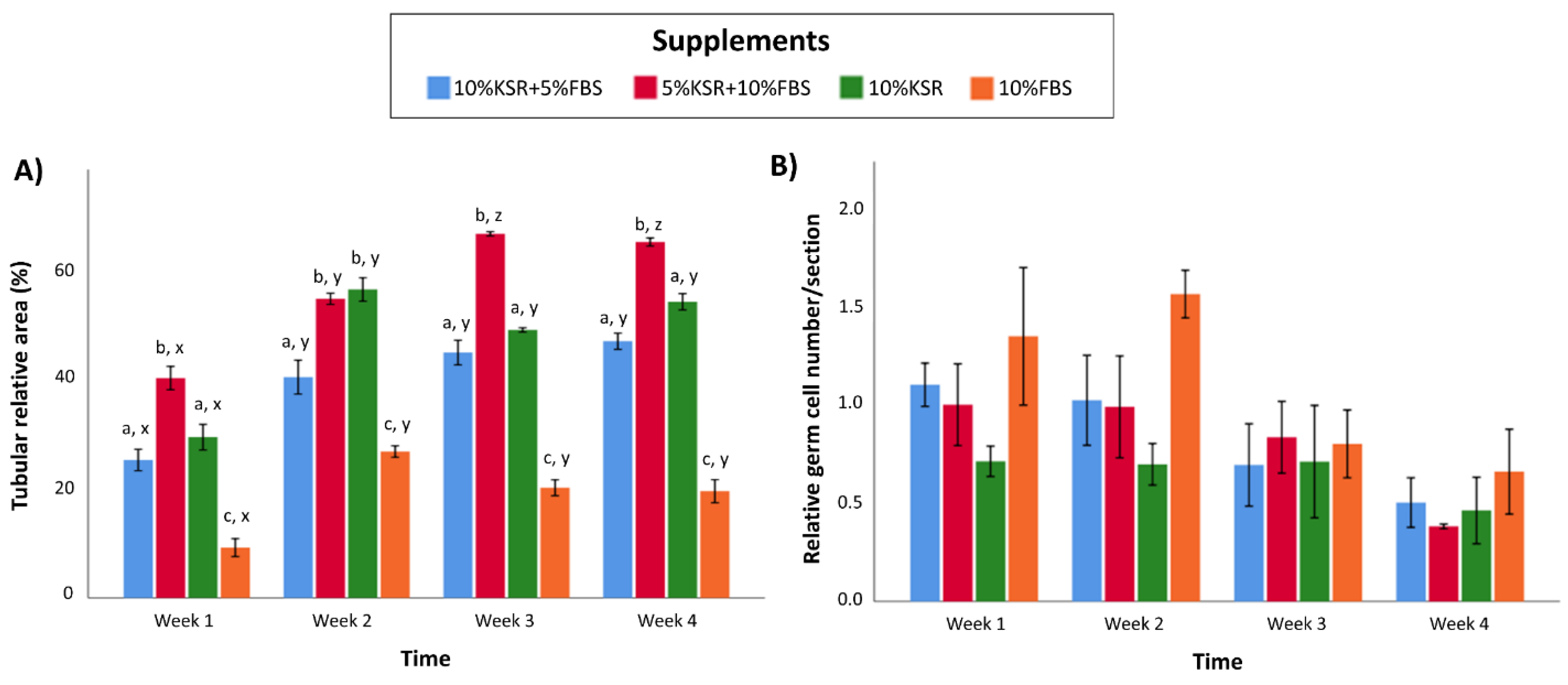
3.3. Media Supplementation Affects In Vitro Tubulogenesis and Germ Cell Ratios in Organoids
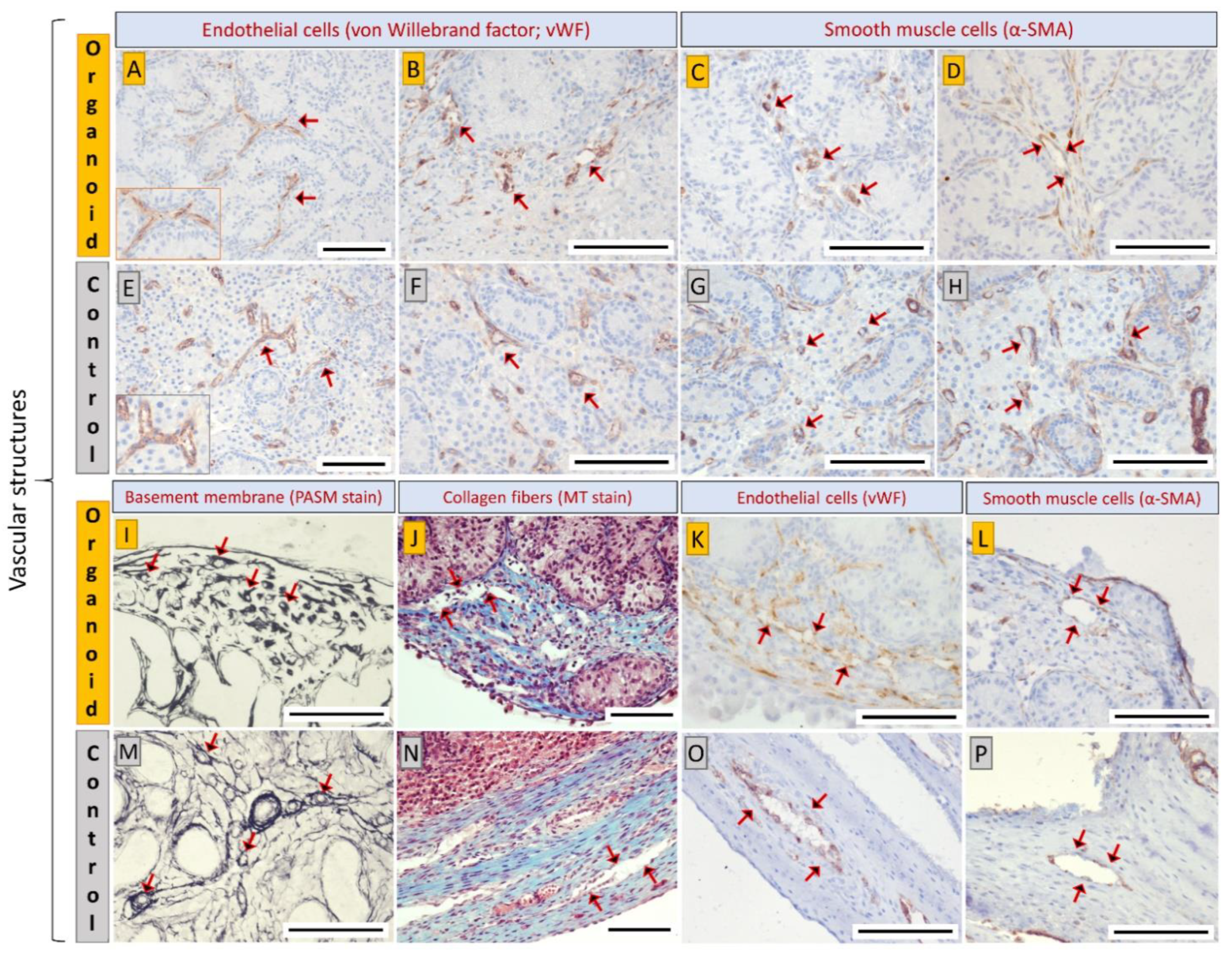
3.4. Cell Types and Structural Components of Organoids Correspond with Intact Tissue
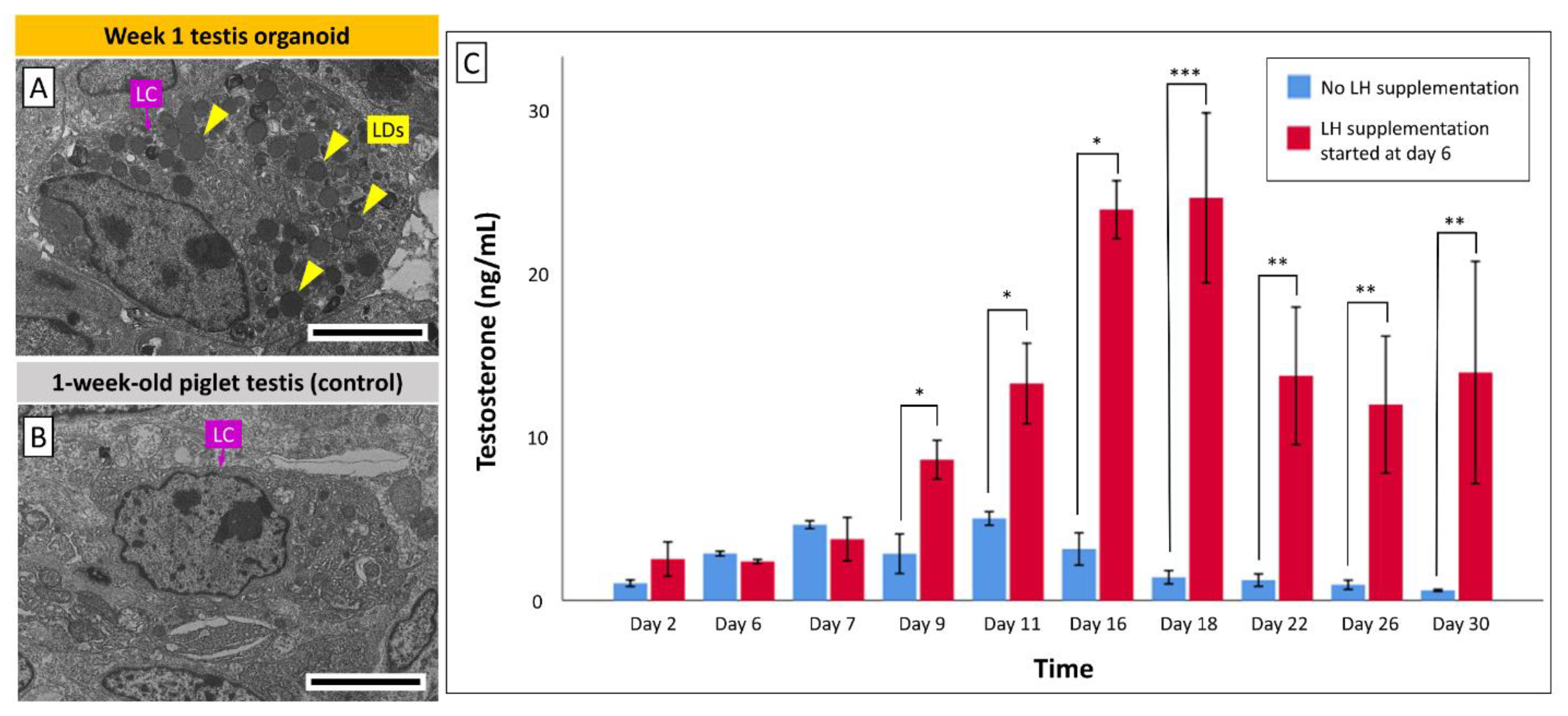
3.5. Leydig Cells in Organoids Secrete Testosterone in Response to LH Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Richer, G.; Baert, Y.; Goossens, E. In-vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 2019, 8, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Cham, T.-C.; Chen, X.; Honaramooz, A. Current progress, challenges, and future prospects of testis organoids†. Biol. Reprod. 2021, 104, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Saksena, S.; Sadri-Ardekani, H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis†. Biol. Reprod. 2020, 103, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Maleki, B.H.; Tartibian, B. COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reprod. 2021, 161, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Koopman, P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar] [CrossRef]
- Chemes, H.E. Infancy is not a quiescent period of testicular development. Int. J. Androl. 2001, 24, 2–7. [Google Scholar] [CrossRef]
- Rey, R.A.; Musse, M.; Venara, M.; Chemes, H.E. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: Its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 2009, 72, 787–795. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Colon, E.; Söder, O. Ontogenesis of Testis Development and Function in Humans. Sex. Dev. 2010, 4, 199–212. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Tung, P.S.; Fritz, I.B. Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: Inhibition of the morphogenetic cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev. Biol. 1987, 120, 139–153. [Google Scholar] [CrossRef]
- Tung, P.S.; Skinner, M.K.; Fritz, I.B. Cooperativity between Sertoli Cells and Peritubular Myoid Cells in the Formation of the Basal Lamina in the Seminiferous Tubule. Ann. N. Y. Acad. Sci. 1984, 438, 435–446. [Google Scholar] [CrossRef]
- Hughes, I.A. Disorders of sex development: a new definition and classification. Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 119–134. [Google Scholar] [CrossRef]
- Skakkebæk, N.; Meyts, E.R.-D.; Main, K.M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Edmonds, M.E.; Woodruff, T.K. Testicular organoid formation is a property of immature somatic cells, which self-assemble and exhibit long-term hormone-responsive endocrine function. Biofabrication 2020, 12, 045002. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, J.P.; Stukenborg, J.-B. Testicular organoids: a new model to study the testicular microenvironment in vitro? Hum. Reprod. Update 2017, 24, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; De Kock, J.; Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B.; Goossens, E. Primary Human Testicular Cells Self-Organize into Organoids with Testicular Properties. Stem Cell Rep. 2017, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kim, K.-S.; Yang, Y.H.; Oh, H.S.; Lee, S.H.; Chung, T.G.; Cho, J.H.; Kim, H.J.; Yoon, T.K.; Cha, K.Y. Isolation of male germ stem cell-like cells from testicular tissue of non-obstructive azoospermic patients and differentiation into haploid male germ cells in vitro. Hum. Reprod. 2005, 21, 471–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.-H.; Gye, M.C.; Choi, K.W.; Hong, J.Y.; Lee, Y.B.; Park, D.-W.; Lee, S.J.; Min, C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007, 87, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Uchida, A.; Valenzuela-Leon, P.; Yu, Y.; Valli-Pulaski, H.; Orwig, K.; Ungrin, M.; Dobrinski, I. Formation of organotypic testicular organoids in microwell culture†. Biol. Reprod. 2019, 100, 1648–1660. [Google Scholar] [CrossRef]
- Vermeulen, M.; Del Vento, F.; Kanbar, M.; Ruys, S.P.D.; Vertommen, D.; Poels, J.; Wyns, C. Generation of Organized Porcine Testicular Organoids in Solubilized Hydrogels from Decellularized Extracellular Matrix. Int. J. Mol. Sci. 2019, 20, 5476. [Google Scholar] [CrossRef]
- Johnson, L.; Varner, D.; Roberts, M.; Smith, T.; Keillor, G.; Scrutchfield, W. Efficiency of spermatogenesis: a comparative approach. Anim. Reprod. Sci. 2000, 60-61, 471–480. [Google Scholar] [CrossRef]
- Ibtisham, F.; Honaramooz, A. Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef]
- Ibtisham, F.; Awang-Junaidi, A.H.; Honaramooz, A. The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res. 2020, 380, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Goodyear, S.M.; Abramowitz, L.K.; Bartolomei, M.S.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum. Reprod. 2012, 27, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Picton, H.M.; Wyns, C.; Anderson, R.A.; Goossens, E.; Jahnukainen, K.; Kliesch, S.; Mitchell, R.; Pennings, G.; Rives, N.; Tournaye, H.; et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum. Reprod. 2015, 30, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pichel, J.G.; Mayeli, T.; Sariola, H.; Lu, B.; Westphal, H. Gdnf Haploinsufficiency Causes Hirschsprung-Like Intestinal Obstruction and Early-Onset Lethality in Mice. Am. J. Hum. Genet. 2002, 70, 435–447. [Google Scholar] [CrossRef][Green Version]
- Strange, D.P.; Zarandi, N.P.; Trivedi, G.; Atala, A.; Bishop, C.E.; Sadri-Ardekani, H.; Verma, S. Human testicular organoid system as a novel tool to study Zika virus pathogenesis. Emerg. Microbes Infect. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Yang, Y.; Honaramooz, A. Efficient purification of neonatal porcine gonocytes with Nycodenz and differential plating. Reprod. Fertil. Dev. 2011, 23, 496–505. [Google Scholar] [CrossRef]
- Junaidi, A.H.A.; Honaramooz, A. Optimization of culture conditions for short-term maintenance, proliferation, and colony formation of porcine gonocytes. J. Anim. Sci. Biotechnol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Honaramooz, A.; Snedaker, A.K.; Boiani, M.; Schöler, H.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nat. Cell Biol. 2002, 418, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, M.A.; Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Validation of ultrasound biomicroscopy for the assessment of xenogeneic testis tissue grafts and cell implants in recipient mice. Andrology 2020, 8, 1332–1346. [Google Scholar] [CrossRef] [PubMed]
- Awang-Junaidi, A.H.; Fayaz, M.A.; Kawamura, E.; Sobchishin, L.; Macphee, D.J.; Honaramooz, A. Live-cell imaging and ultrastructural analysis reveal remarkable features of cultured porcine gonocytes. Cell Tissue Res. 2020, 381, 361–377. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Honaramooz, A.; Megee, S.O.; Rathi, R.; Dobrinski, I. Building a Testis: Formation of Functional Testis Tissue after Transplantation of Isolated Porcine (Sus scrofa) Testis Cells1. Biol. Reprod. 2007, 76, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Junaidi, A.H.A.; Singh, J.; Honaramooz, A. Regeneration of testis tissue after ectopic implantation of porcine testis cell aggregates in mice: improved consistency of outcomes and in situ monitoring. Reprod. Fertil. Dev. 2020, 32, 594. [Google Scholar] [CrossRef]
- Yokonishi, T.; Sato, T.; Katagiri, K.; Komeya, M.; Kubota, Y.; Ogawa, T. In Vitro Reconstruction of Mouse Seminiferous Tubules Supporting Germ Cell Differentiation1. Biol. Reprod. 2013, 89, 15. [Google Scholar] [CrossRef]
- Gholami, K.; Vermeulen, M.; Del Vento, F.; De Michele, F.; Giudice, M.G.; Wyns, C. The air-liquid interface culture of the mechanically isolated seminiferous tubules embedded in agarose or alginate improves in vitro spermatogenesis at the expense of attenuating their integrity. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 261–270. [Google Scholar] [CrossRef]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef]
- Nakamura, N.; Merry, G.E.; Inselman, A.; Sloper, D.T.; Del Valle, P.L.; Sato, T.; Ogawa, T.; Hansen, D.K. Evaluation of Culture Time and Media in anIn VitroTestis Organ Culture System. Birth Defects Res. 2017, 109, 465–474. [Google Scholar] [CrossRef]
- Sakib, S.; Voigt, A.; Goldsmith, T.; Dobrinski, I. Three-dimensional testicular organoids as novel in vitro models of testicular biology and toxicology. Environ. Epigenetics 2019, 5, dvz011. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Goldsmith, T.; Voigt, A.; Dobrinski, I. Testicular organoids to study cell–cell interactions in the mammalian testis. Andrology 2020, 8, 835–841. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Van der Wee, K.; Hofmann, M.-C. An in Vitro Tubule Assay Identifies HGF as a Morphogen for the Formation of Seminiferous Tubules in the Postnatal Mouse Testis. Exp. Cell Res. 1999, 252, 175–185. [Google Scholar] [CrossRef]
- Schlatt, S.; De Kretser, D.M.; Loveland, K.L. Discriminative Analysis of Rat Sertoli and Peritubular Cells and their Proliferation in Vitro: Evidence for Follicle-Stimulating Hormone-Mediated Contact Inhibition of Sertoli Cell Mitosis1. Biol. Reprod. 1996, 55, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gassei, K.; Schlatt, S.; Ehmcke, J. De Novo Morphogenesis of Seminiferous Tubules From Dissociated Immature Rat Testicular Cells in Xenografts. J. Androl. 2006, 27, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hatakeyama, J.; Eto, K.; Abe, S.-I. Reconstruction of a seminiferous tubule-like structure in a 3 dimensional culture system of re-aggregated mouse neonatal testicular cells within a collagen matrix. Gen. Comp. Endocrinol. 2014, 205, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gharenaz, N.M.; Movahedin, M.; Mazaheri, Z. Three-Dimensional Culture of Mouse Spermatogonial Stem Cells Using A Decellularised Testicular Scaffold. Cell J. Yakhteh 2019, 21, 410–418. [Google Scholar]
- Zhang, X.; Wang, L.; Zhang, X.; Ren, L.; Shi, W.; Tian, Y.; Zhu, J.; Zhang, T. The use of KnockOut serum replacement (KSR) in three dimensional rat testicular cells co-culture model: An improved male reproductive toxicity testing system. Food Chem. Toxicol. 2017, 106, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Topraggaleh, T.R.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomater. Sci. 2019, 7, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Komrskova, K.; Margaryan, H.; Goossens, E. Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication 2019, 11, 035011. [Google Scholar] [CrossRef]
- Youssefi, R.; Tajik, P.; Movahedin, M.; Akbarinejad, V. Enhancement in colonization of bovine spermatogonial stem cells following addition of knock-out serum replacement to culture medium. Vet. Res. Forum Int. Q. J. 2016, 7, 275–280. [Google Scholar]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells†. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, Y.; Chen, H.; Jesus, T.; Tang, E.; Li, N.; Lian, Q.; Ge, R.-S.; Cheng, C.Y. Cell polarity, cell adhesion, and spermatogenesis: role of cytoskeletons. F1000Research 2017, 6, 1565. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, J.H.; Kim, M.R.; Min, C.K. Evaluation of in vitro spermatogenesis using poly(D,L-lactic-co-glycolic acid) (PLGA)-based macroporous biodegradable scaffolds. J. Tissue Eng. Regen. Med. 2011, 5, 130–137. [Google Scholar] [CrossRef]
- Yu, X.; Sidhu, J.S.; Hong, S.; Faustman, E.M. Essential Role of Extracellular Matrix (ECM) Overlay in Establishing the Functional Integrity of Primary Neonatal Rat Sertoli Cell/Gonocyte Co-cultures: An Improved In Vitro Model for Assessment of Male Reproductive Toxicity. Toxicol. Sci. 2005, 84, 378–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, X.; Hong, S.; Moreira, E.; Faustman, E.M. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol. Appl. Pharmacol. 2009, 239, 325–336. [Google Scholar] [CrossRef]
- França, L.R.; Silva, V.A.; Chiarini-Garcia, H.; Garcia, S.K.; Debeljuk, L. Cell Proliferation and Hormonal Changes During Postnatal Development of the Testis in the Pig1. Biol. Reprod. 2000, 63, 1629–1636. [Google Scholar] [CrossRef]
- Kita, K.; Watanabe, T.; Ohsaka, K.; Hayashi, H.; Kubota, Y.; Nagashima, Y.; Aoki, I.; Taniguchi, H.; Noce, T.; Inoue, K.; et al. Production of Functional Spermatids from Mouse Germline Stem Cells in Ectopically Reconstituted Seminiferous Tubules1. Biol. Reprod. 2007, 76, 211–217. [Google Scholar] [CrossRef]
- Fayaz, M.A.; Awang-Junaidi, A.H.; Singh, J.; Honaramooz, A. Long-Term Monitoring of Donor Xenogeneic Testis Tissue Grafts and Cell Implants in Recipient Mice Using Ultrasound Biomicroscopy. Ultrasound Med. Biol. 2020, 46, 3088–3103. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Garland, K.K.; Russell, L.D. Desert hedgehog (Dhh) Gene Is Required in the Mouse Testis for Formation of Adult-Type Leydig Cells and Normal Development of Peritubular Cells and Seminiferous Tubules. Biol. Reprod. 2000, 63, 1825–1838. [Google Scholar] [CrossRef]
- Setchell, B.P. Blood-Testis Barrier, Junctional and Transport Proteins and Spermatogenesis. In Chemistry and Biology of Pteridines and Folates; Springer Science and Business Media LLC: Berlin, Germany, 2009; Volume 636, pp. 212–233. [Google Scholar]
- Bhang, D.H.; Kim, B.-J.; Kim, B.G.; Schadler, K.; Baek, K.-H.; Kim, Y.H.; Hsiao, W.; Ding, B.-S.; Rafii, S.; Weiss, M.; et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 17, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.H.-C.; Aardema, J.; Holthusen, K. Sexually Dimorphic Regulation of Inhibin Beta B in Establishing Gonadal Vasculature in Mice1. Biol. Reprod. 2006, 74, 978–983. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Cheng, Q.; Diao, F.; Zeng, Q.; Yang, X.; Wu, Y.; Zhang, H.; Huang, M.; Chen, J.; et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 2020, 30, 244–255. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. hCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Kerr, J.B.; Sharpe, R.M. Effects and interactions of LH and LHRH agonist on testicular morphology and function in hypophysectomized rats. Reproduction 1986, 76, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.-B.; Schlatt, S.; Simoni, M.; Yeung, C.-H.; Abu Elhija, M.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009, 15, 521–529. [Google Scholar] [CrossRef]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Preston, B.T.; Stevenson, I.R.; Lincoln, G.A.; Monfort, S.L.; Pilkington, J.G.; Wilson, K. Testes size, testosterone production and reproductive behaviour in a natural mammalian mating system. J. Anim. Ecol. 2011, 81, 296–305. [Google Scholar] [CrossRef]
- Sofikitis, N.; Giotitsas, N.; Tsounapi, P.; Baltogiannis, D.; Giannakis, D.; Pardalidis, N. Hormonal regulation of spermatogenesis and spermiogenesis. J. Steroid Biochem. Mol. Biol. 2008, 109, 323–330. [Google Scholar] [CrossRef]
- Hadley, M.A.; Byers, S.W.; Suárez-Quian, C.A.; Kleinman, H.K.; Dym, M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985, 101, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.A.; Weeks, B.S.; Kleinman, H.K.; Dym, M. Laminin promotes formation of cord-like structures by sertoli cells in vitro. Dev. Biol. 1990, 140, 318–327. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Kim, H.; Lee, S.J.; Gye, M.C. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006, 27, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.; Froment, P.; Desmots, S.; LeComte, A.; Habert, R.; Lemazurier, E. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials 2010, 31, 4492–4505. [Google Scholar] [CrossRef]
- Auchus, R.J. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 2004, 15, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Holdcraft, R.W.; Braun, R.E. Hormonal regulation of spermatogenesis. Int. J. Androl. 2004, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wilbert, D.M.; Griffin, J.E.; Wilson, J.D. Characterization of the Cytosol Androgen Receptor of the Human Prostate*. J. Clin. Endocrinol. Metab. 1983, 56, 113–120. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cham, T.-C.; Ibtisham, F.; Fayaz, M.A.; Honaramooz, A. Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue. Cells 2021, 10, 1696. https://doi.org/10.3390/cells10071696
Cham T-C, Ibtisham F, Fayaz MA, Honaramooz A. Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue. Cells. 2021; 10(7):1696. https://doi.org/10.3390/cells10071696
Chicago/Turabian StyleCham, Tat-Chuan, Fahar Ibtisham, Mohammad Amin Fayaz, and Ali Honaramooz. 2021. "Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue" Cells 10, no. 7: 1696. https://doi.org/10.3390/cells10071696
APA StyleCham, T.-C., Ibtisham, F., Fayaz, M. A., & Honaramooz, A. (2021). Generation of a Highly Biomimetic Organoid, Including Vasculature, Resembling the Native Immature Testis Tissue. Cells, 10(7), 1696. https://doi.org/10.3390/cells10071696