ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Placental Explant Culture
2.2. Immunohistochemistry
2.3. Image Analysis and Quantification
2.4. Immunofluorescence Staining
2.5. Quantitative Real Time PCR (qPCR)
2.6. Immunoblotting
2.7. Immune Cell Isolation
2.8. Flow Cytometry
2.9. Statistical Analysis
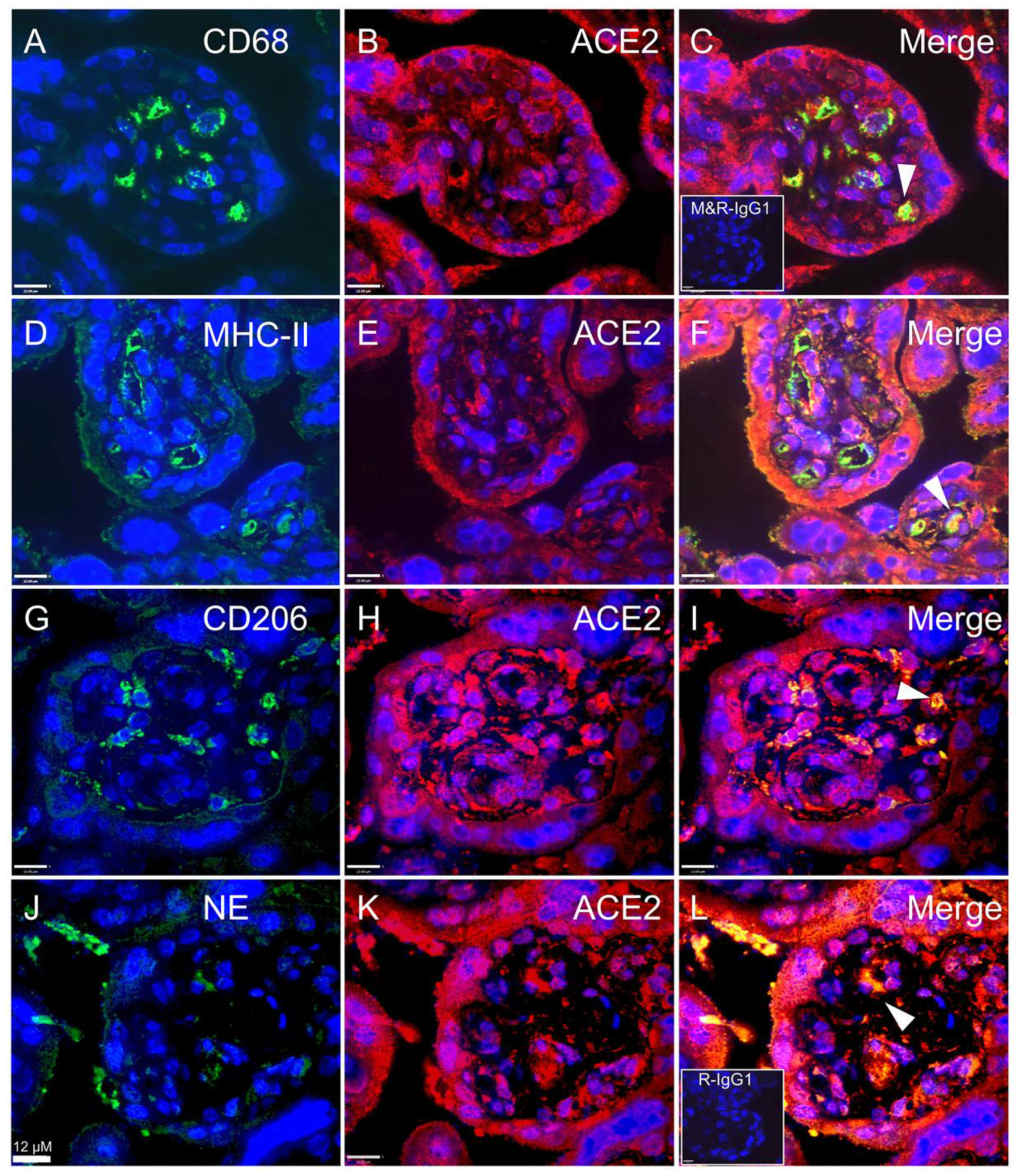
3. Results
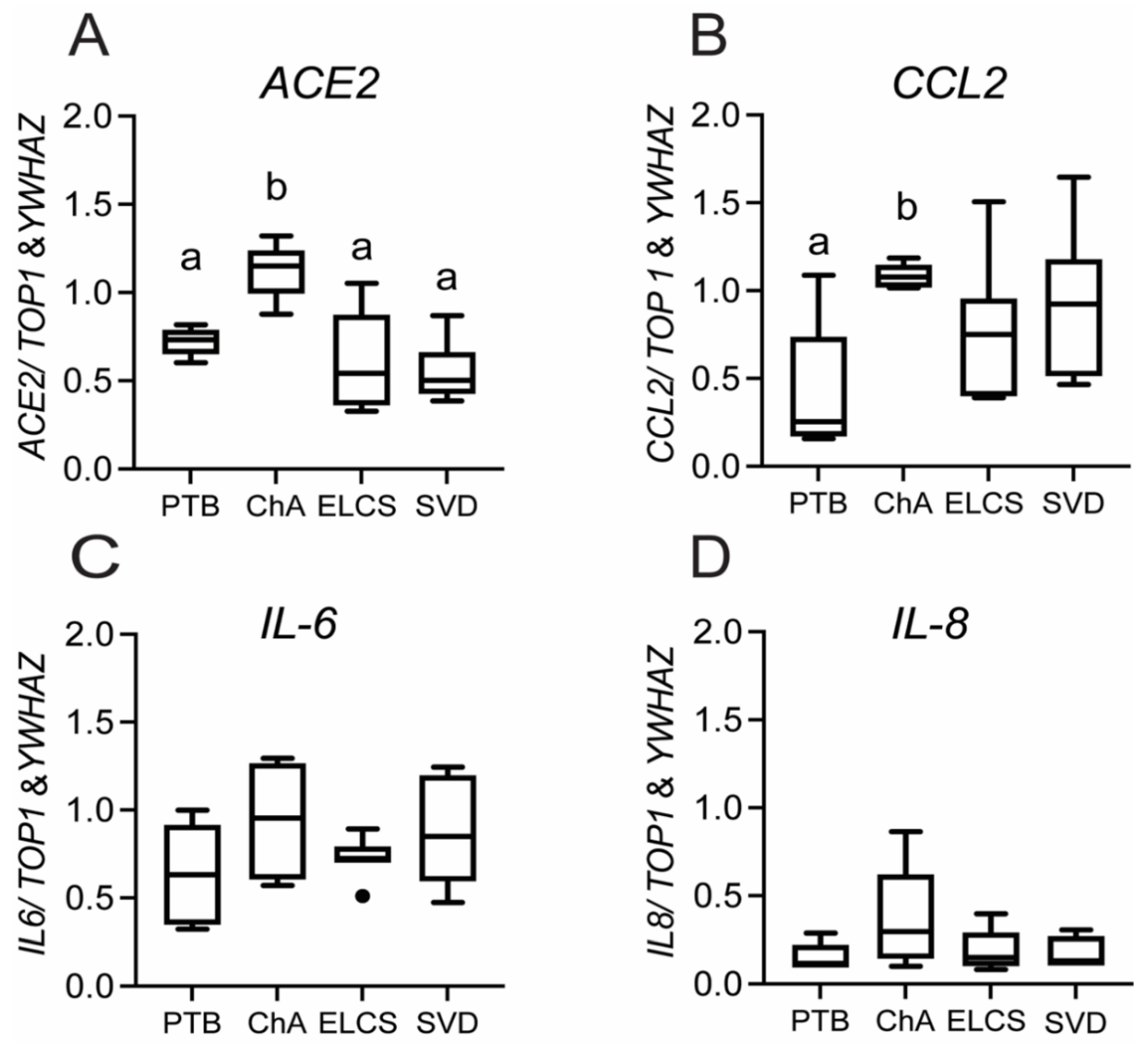
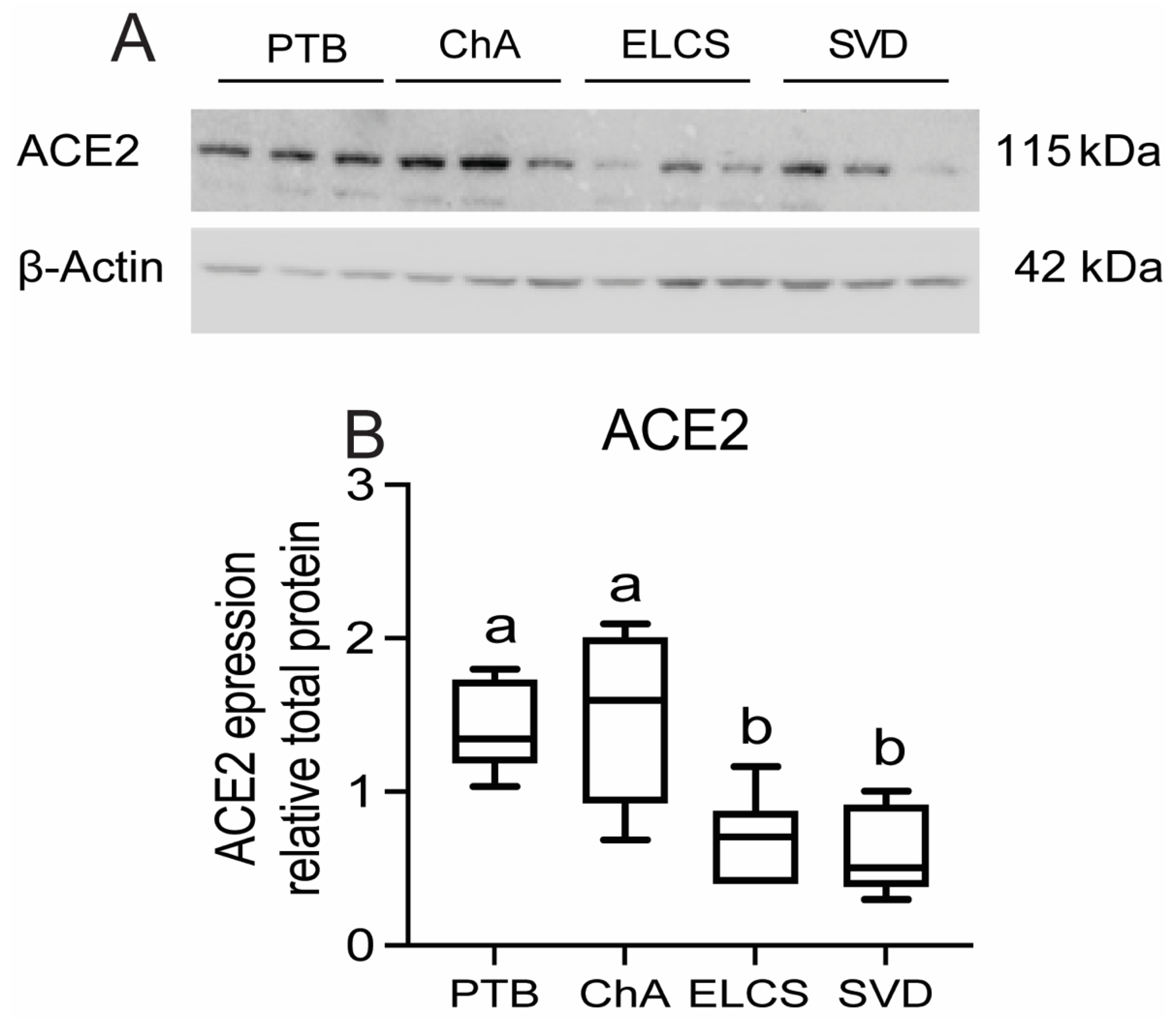
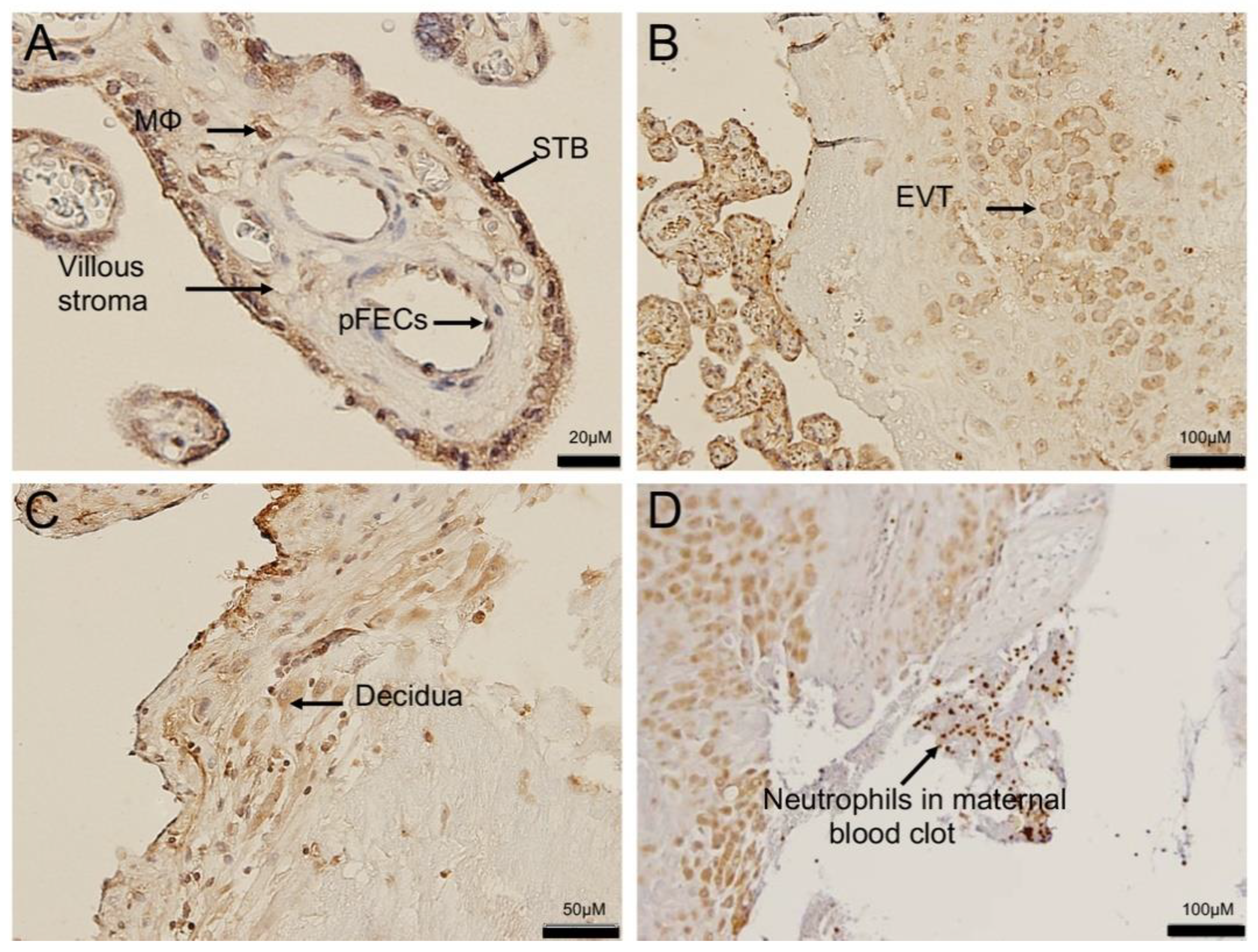
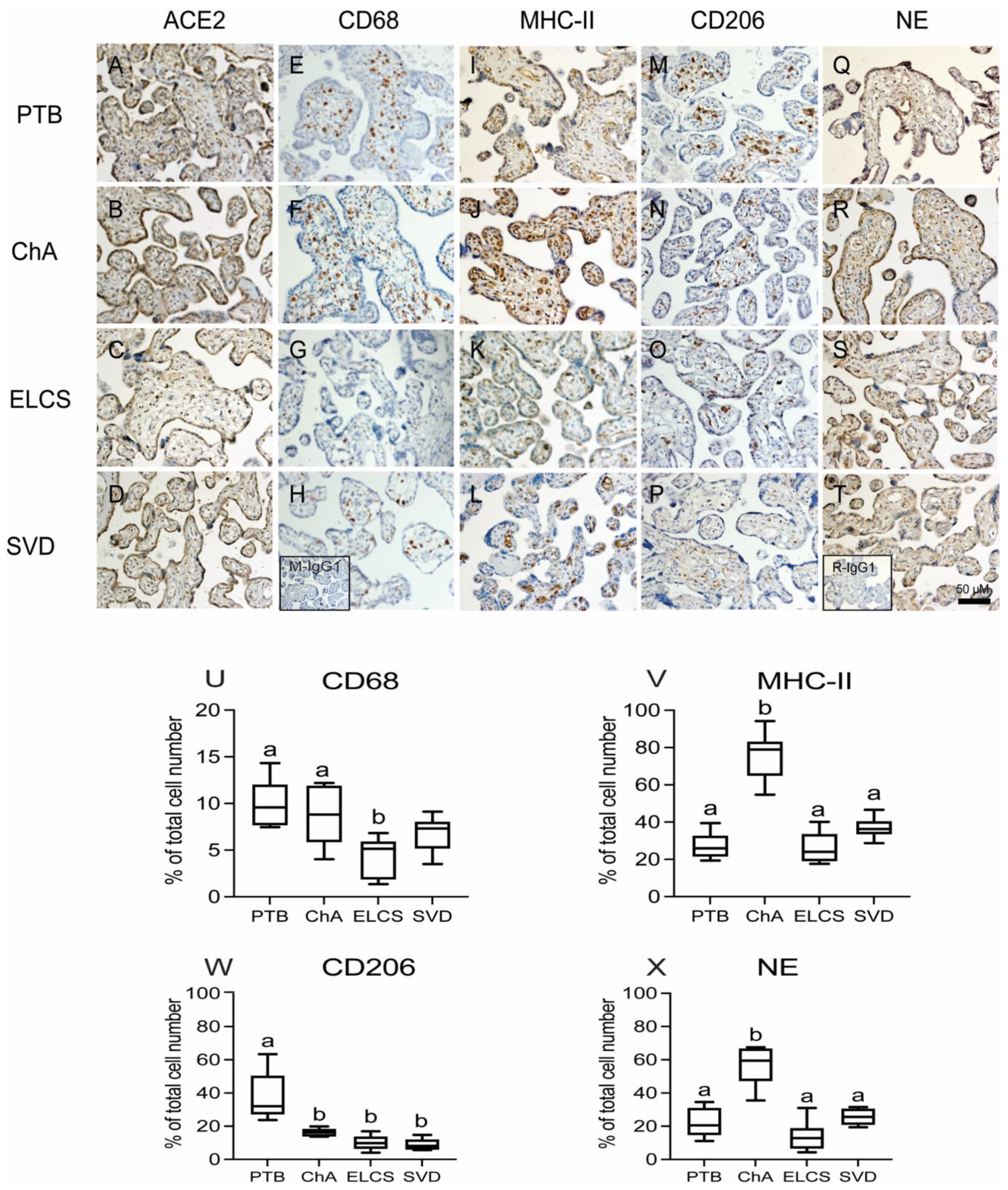
3.1. Chorioamnionitis and LPS Exposure Are Associated with Increased Placental ACE2 Expression and an Inflammatory Cytokine/Chemokine Response
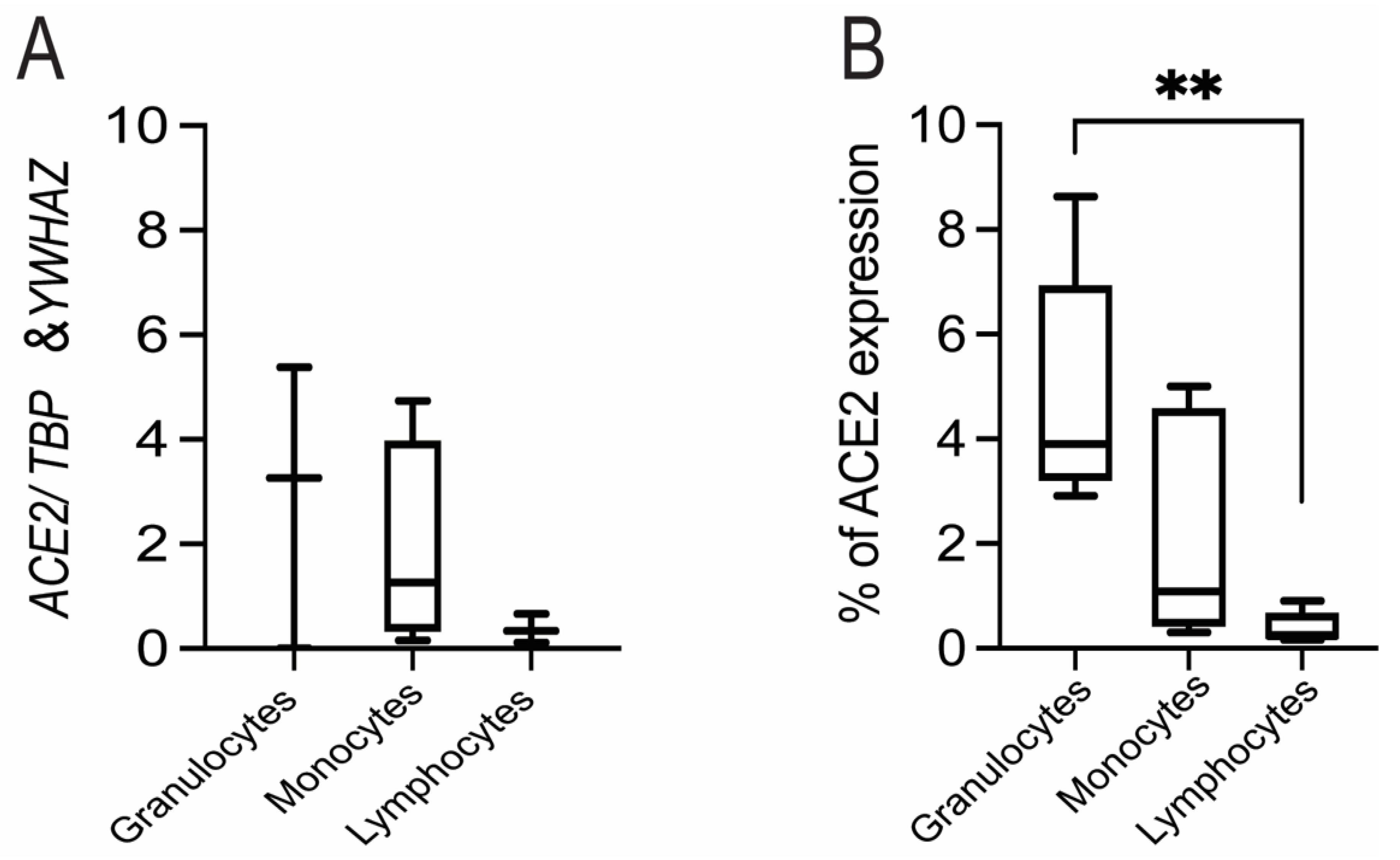
3.2. Expression of ACE2 in Circulating Maternal Immune Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, D.A. An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef]
- Bloise, E.; Zhang, J.; Nakpu, J.; Hamada, H.; Dunk, C.E.; Li, S.; Imperio, G.E.; Nadeem, L.; Kibschull, M.; Lye, P.; et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2021, 224, 298.e1–298.e8. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.-D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, e58716. [Google Scholar] [CrossRef]
- Cui, D.; Liu, Y.; Jiang, X.; Ding, C.; Poon, L.C.; Wang, H.; Yang, H. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 2021, 57, 248–256. [Google Scholar] [CrossRef]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bagalkot, T.; Fitzgerald, W.; Sadovsky, E.; Chu, T.; Martínez-Marchal, A.; Brieño-Enríquez, M.; Su, E.J.; Margolis, L.; Sorkin, A.; et al. Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin. mSphere 2021, 6, e00250-21. [Google Scholar] [CrossRef]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, G.; Saderi, L.; Aliberti, S.; Mondoni, M.; Piana, A.; Dessole, F.; Dessole, M.; Cherchi, P.L.; Dessole, S.; Sotgiu, G. COVID-19 in pregnant women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 543–558. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Yang, R.; Mei, H.; Zheng, T.; Fu, Q.; Zhang, Y.; Buka, S.; Yao, X.; Tang, Z.; Zhang, X.; Qiu, L.; et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: A population-based cohort study in Wuhan, China. BMC Med. 2020, 18, 330. [Google Scholar] [CrossRef]
- Smith, V.; Seo, D.; Warty, R.; Payne, O.; Salih, M.; Chin, K.L.; Ofori-Asenso, R.; Krishnan, S.; Costa, F.D.S.; Vollenhoven, B.; et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE 2020, 15, e0234187. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Cavalcante, C.T.D.M.B.; Sarno, M.; Barini, R.; Kwak-Kim, J. Maternal immune responses and obstetrical outcomes of pregnant women with COVID-19 and possible health risks of offspring. J. Reprod. Immunol. 2021, 143, 103250. [Google Scholar] [CrossRef]
- Moreno, S.C.; To, J.; Chun, H.; Ngai, I.M. Vertical Transmission of COVID-19 to the Neonate. Infect. Dis. Obstet. Gynecol. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Bellos, I.; Pandita, A.; Panza, R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 194–204. [Google Scholar] [CrossRef]
- Richtmann, R.; Torloni, M.R.; Otani, A.R.O.; Levi, J.E.; Tobara, M.C.; Silva, C.D.A.; Dias, L.; Miglioli-Galvão, L.; Silva, P.M.; Kondo, M.M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep. Womens Health 2020, 27, e00243. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 2020, 59, 102951. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Baergen, R.N.; Heller, D.S. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef]
- Gulersen, M.; Prasannan, L.; Tam, H.T.; Metz, C.N.; Rochelson, B.; Meirowitz, N.; Shan, W.; Edelman, M.; Millington, K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM 2020, 2, 100211. [Google Scholar] [CrossRef]
- Prabhu, M.; Cagino, K.; Matthews, K.C.; Friedlander, R.L.; Glynn, S.M.; Kubiak, J.M.; Yang, Y.J.; Zhao, Z.; Baergen, R.N.; DiPace, J.I.; et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hösli, I.; Bruder, E. Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion. Pathobiology 2021, 88, 69–77. [Google Scholar] [CrossRef]
- Pulinx, B.; Kieffer, D.; Michiels, I.; Petermans, S.; Strybol, D.; Delvaux, S.; Baldewijns, M.; Raymaekers, M.; Cartuyvels, R.; Maurissen, W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2441–2445. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D. Placental Pathology of COVID-19 with and without Fetal and Neonatal Infection: Trophoblast Necrosis and Chronic Histiocytic Intervillositis as Risk Factors for Transplacental Transmission of SARS-CoV-2. Viruses 2020, 12, 1308. [Google Scholar] [CrossRef]
- Silasi, M.; Cardenas, I.; Kwon, J.-Y.; Racicot, K.; Aldo, P.; Mor, G. Viral Infections During Pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef]
- Francis, F.; Bhat, V.; Mondal, N.; Adhisivam, B.; Jacob, S.; Dorairajan, G.; Harish, B.N. Fetal inflammatory response syndrome (FIRS) and outcome of preterm neonates—A prospective analytical study. J. Matern. Neonatal Med. 2017, 32, 488–492. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Romero, R.; Mor, G. New Insights into the Relationship between Viral Infection and Pregnancy Complications. Am. J. Reprod. Immunol. 2014, 71, 387–390. [Google Scholar] [CrossRef]
- Cardenas, I.; Mor, G.; Aldo, P.; Lang, S.M.; Stabach, P.; Sharp, A.; Romero, R.; Mazaki-Tovi, S.; Gervasi, M.; Means, R.E. Placental Viral Infection Sensitizes to Endotoxin-Induced Pre-Term Labor: A Double Hit Hypothesis. Am. J. Reprod. Immunol. 2010, 65, 110–117. [Google Scholar] [CrossRef]
- Hazan, A.D.; Smith, S.D.; Jones, R.L.; Whittle, W.; Lye, S.J.; Dunk, C.E. Vascular-Leukocyte Interactions: Mechanisms of Human Decidual Spiral Artery Remodeling in Vitro. Am. J. Pathol. 2010, 177, 1017–1030. [Google Scholar] [CrossRef]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages Infiltrate the Human and Rat Decidua During Term and Preterm Labor: Evidence That Decidual Inflammation Precedes Labor1. Biol. Reprod. 2012, 86, 39. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Zhang, J.; Dunk, C.; Lye, S. Myometrial activation: Novel concepts underlying labor. Placenta 2020, 92, 28–36. [Google Scholar] [CrossRef]
- Cappelletti, M.; Presicce, P.; Kallapur, S.G. Immunobiology of Acute Chorioamnionitis. Front. Immunol. 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z. Mineralocorticoid Receptor Blocker Increases Angiotensin-Converting Enzyme 2 Activity in Congestive Heart Failure Patients. Circ. Res. 2005, 97, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, X.; Zhang, L.; Ran, Q.; Wang, J.; Xiong, A.; Wu, D.; Chen, F.; Sun, J.; Chang, C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020, 112, 102463. [Google Scholar] [CrossRef]
- Império, G.E.D.; Bloise, E.; Javam, M.; Lye, P.; Constantinof, A.; Dunk, C.; Dos Reisf, F.M.; Lye, S.J.; Gibb, W.; Ortiga-Carvalho, T.M.; et al. Chorioamnionitis Induces a Specific Signature of Placental ABC Transporters Associated with an Increase of miR-331-5p in the Human Preterm Placenta. Cell. Physiol. Biochem. 2018, 45, 591–604. [Google Scholar] [CrossRef]
- Torricelli, M.; Novembri, R.; Bloise, E.; De Bonis, M.; Challis, J.R.; Petraglia, F. Changes in Placental CRH, Urocortins, and CRH-Receptor mRNA Expression Associated with Preterm Delivery and Chorioamnionitis. J. Clin. Endocrinol. Metab. 2011, 96, 534–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lye, P.; Bloise, E.; Javam, M.; Gibb, W.; Lye, S.J.; Matthews, S.G. Impact of Bacterial and Viral Challenge on Multidrug Resistance in First- and Third-Trimester Human Placenta. Am. J. Pathol. 2015, 185, 1666–1675. [Google Scholar] [CrossRef]
- Lye, P.; Bloise, E.; Nadeem, L.; Peng, C.; Gibb, W.; Ortiga-Carvalho, T.M.; Lye, S.J.; Matthews, S.G. Breast Cancer Resistance Protein (BCRP/ABCG2) Inhibits Extra Villous Trophoblast Migration: The Impact of Bacterial and Viral Infection. Cells 2019, 8, 1150. [Google Scholar] [CrossRef]
- Dunk, C.; Kwan, M.; Hazan, A.; Walker, S.; Wright, J.K.; Harris, L.; Jones, R.L.; Keating, S.; Kingdom, J.C.P.; Whittle, W.; et al. Failure of Decidualization and Maternal Immune Tolerance Underlies Uterovascular Resistance in Intra Uterine Growth Restriction. Front. Endocrinol. 2019, 10, 160. [Google Scholar] [CrossRef]
- Salio, C.; Lossi, L.; Ferrini, F.; Merighi, A. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur. J. Neurosci. 2005, 22, 1951–1966. [Google Scholar] [CrossRef]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Harris, L.K.; Aplin, J.; Jones, R.L. Decidual leucocytes infiltrating human spiral arterioles are rich source of matrix metalloproteinases and degrade extracellular matrix in vitro and in situ. Am. J. Reprod. Immunol. 2018, 81, e13054. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Drewlo, S.; Levytska, K.; Kingdom, J. Revisiting the housekeeping genes of human placental development and insufficiency syndromes. Placenta 2012, 33, 952–954. [Google Scholar] [CrossRef]
- Paquette, A.G.; Shynlova, O.; Kibschull, M.; Price, N.; Lye, S.J. Comparative analysis of gene expression in maternal peripheral blood and monocytes during spontaneous preterm labor. Am. J. Obstet. Gynecol. 2018, 218, 345.e1–345.e30. [Google Scholar] [CrossRef]
- Amsalem, H.; Kwan, M.; Hazan, A.; Zhang, J.; Jones, R.; Whittle, W.; Kingdom, J.C.P.; Croy, B.A.; Lye, S.J.; Dunk, C.E. Identification of a Novel Neutrophil Population: Proangiogenic Granulocytes in Second-Trimester Human Decidua. J. Immunol. 2014, 193, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Wei, M.; Cheng, B.H.; Zhou, X.C.; Li, J.; Tian, J.H.; Dong, L.; Hu, R.H. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020, 55, E009. [Google Scholar]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef]
- Shah, P.S.; Diambomba, Y.; Acharya, G.; Morris, S.K.; Bitnun, A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020, 99, 565–568. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Ebadi, A.; Mir, S.A.; Rahmani, Z.; Haghshenas, M.; Azizi, S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 2020, 40, 1759–1761. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Cao, J.D.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS–CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.A.; Roman, A. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM 2020, 2, 100133. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef]
- Moore, K.M.; Suthar, M.S. Comprehensive analysis of COVID-19 during pregnancy. Biochem. Biophys. Res. Commun. 2021, 538, 180–186. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef]
- Pringle, K.; Tadros, M.; Callister, R.; Lumbers, E. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef]
- Valdés, G.; Neves, L.; Anton, L.; Corthorn, J.; Chacón, C.; Germain, A.; Merrill, D.; Ferrario, C.; Sarao, R.; Penninger, J.; et al. Distribution of Angiotensin-(1-7) and ACE2 in Human Placentas of Normal and Pathological Pregnancies. Placenta 2006, 27, 200–207. [Google Scholar] [CrossRef]
- Delforce, S.; Lumbers, E.R.; Ellery, S.J.; Murthi, P.; Pringle, K.G. Dysregulation of the placental renin–angiotensin system in human fetal growth restriction. Reproduction 2019, 158, 237–245. [Google Scholar] [CrossRef]
- Tamanna, S.; Clifton, V.L.; Rae, K.; Van Helden, D.F.; Lumbers, E.R.; Pringle, K.G. Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front. Physiol. 2020, 11, 590787. [Google Scholar] [CrossRef]
- Verma, S.; Joshi, C.S.; Silverstein, R.B.; He, M.; Carter, E.B.; Mysorekar, I.U. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med 2021, 2, 575–590.e5. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Leng, Y.; Garcia-Flores, V.; Miller, D.; Jacques, S.M.; Hassan, S.S.; Faro, J.; Alsamsam, A.; et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am. J. Obstet. Gynecol. 2017, 217, 693.e1–693.e16. [Google Scholar] [CrossRef]
- Abassi, Z.; Knaney, Y.; Karram, T.; Heyman, S.N. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020, 11, 1312. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Glauser, M.; Zanetti, G.; Baumgartner, J.-D.; Cohen, J. Septic shock: Pathogenesis. Lancet 1991, 338, 732–736. [Google Scholar] [CrossRef]
- Boumaza, A.; Gay, L.; Mezouar, S.; Bestion, E.; Diallo, A.B.; Michel, M.; Desnues, B.; Raoult, D.; La Scola, B.; Halfon, P.; et al. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef]
- Duval, C.; Brien, M.-E.; Gaudreault, V.; Boufaied, I.; Baker, B.; Jones, R.; Girard, S. Differential effect of LPS and IL-1β in term placental explants. Placenta 2019, 75, 9–15. [Google Scholar] [CrossRef]
- Bhoopat, L.; Khunamornpong, S.; Sirivatanapa, P.; Rithaporn, T.; Lerdsrimongkol, P.; Thorner, P.S.; Bhoopat, T. Chorioamnionitis is associated with placental transmission of human immunodeficiency virus-1 subtype E in the early gestational period. Mod. Pathol. 2005, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.H.; Mudenda, V.; Levy, J.; Sinkala, M.; Goldenberg, R.L.; Stringer, J.S. Acute and chronic chorioamnionitis and the risk of perinatal human immunodeficiency virus-1 transmission. Am. J. Obstet. Gynecol. 2006, 194, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jering, K.S.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Vardeny, O.; Greene, M.F.; Solomon, S.D. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth with and Without COVID-19. JAMA Intern. Med. 2021, 181, 714–717. [Google Scholar] [CrossRef]

| Pregnancies | PTB | ChA | ELCS | SVD | p Value | Flow Cytometry |
|---|---|---|---|---|---|---|
| Number of patients | (n = 12) | (n = 13) | (n = 13) | (n = 13) | (n = 6) | |
| Maternal Characteristics | ||||||
| Maternal Age (years) | 29.6 ± 1.83 a (21–43) | 28.5 ± 2.25 a (17–40) | 35.8 ± 0.85 b (34–42) | 32.4 ± 1.06 a (27–43) | <0.05 | 35.0 ± 3.52 (29–39) |
| Labor (Y/N/U) | 10:2:0 | 12:1:0 | 0:13:0 | 13:0:0 | - | 0:6:0 |
| BMI | 22.4 ± 1.18 a (18.26–29.75) | 23.8 ± 1.12 a (18.30–29.75) | 22.6 ± 1.77 a (15.24–32.44) | 23.2 ± 1.34 a (19.08–32.91) | NS | 23.4 ± 2.91 (19.9 ± 7.3) |
| Fetal Characteristics | ||||||
| Gestational age (weeks) | 31.7 ± 0.93 a (25.3–36.0) | 29.3 ± 1.01 a (26–33) | 38.6 ± 0.30 b (37–41) | 39.0 ± 0.32 b (37–40.4) | <0.05 | 39.2 ± 0.08 (39.1–39.3) |
| Blood draw: Gestational age (weeks) | - | - | - | - | - | 14.4 ± 3.36 (12.3–20.9) |
| Neonatal sex (Male/Female/U) | 9:3:0 | 9:4:0 | 8:5:0 | 7:6:0 | - | 3:0:3 |
| Pathology Characteristics | ||||||
| Chorioamnionitis (G1,G2,G3,U) | No | 1:10:1:U | No | No | - | No |
| Glucocorticoid treatment | Yes | Yes | No | No | - | No |
| Primer Name | Sequence | Reference |
|---|---|---|
| ACE2 | Forward: 5′-GGAGTGATAGTGGTTGGCATTGTC-3′ | * |
| Reverse: 5′-GCTAATATCGATGGAGGCATAAGGA-3′ | ||
| IL-6 | Forward: 5′-TGCAGAAAAAGGCAAAGAAT-3′ | [43] |
| Reverse: 5′-CTGACCAGAAGAAGGAATGC-3′ | ||
| IL-8 | Forward: 5′-TGGGAACAAGAGGGCATCTG-3′ | [43] |
| Reverse: 5′-CCACCACTGCATCAAATTCATG-3′ | ||
| CCL2 | Forward: 5′-TTCATTCCCCAAGGGCTCGCTCA-3′ | [42] |
| Reverse: 5′-AGCACAGATCTCCTTGGCCACAA-3′ | ||
| TNFα | Forward: 5′-CCTGGGGAACTCTTCCCTCTGGGG-3′ | * |
| Reverse: 5′-CAGGCGCCACCACGCTCTTC-3′ | ||
| TBP | Forward: 5′-TGC ACA GGA GCC AAG AGT GAA-3′ | [48] |
| Reverse: 5′-CAC ATC ACA GCT CCC CAC CA-3′ | ||
| YWHAZ | Forward: 5′-CCGCCAGGACAAACCAGTAT-3′ | [48] |
| Reverse: 5′-CAC ATC ACA GCT CCC CAC CA-3′ | ||
| TOP1 | Forward: 5′-GATGAACCTGAAGATGATGGC-3′ | [48] |
| Reverse: 5′-TCAGCATCATCCTCATCTCG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lye, P.; Dunk, C.E.; Zhang, J.; Wei, Y.; Nakpu, J.; Hamada, H.; Imperio, G.E.; Bloise, E.; Matthews, S.G.; Lye, S.J. ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth. Cells 2021, 10, 1724. https://doi.org/10.3390/cells10071724
Lye P, Dunk CE, Zhang J, Wei Y, Nakpu J, Hamada H, Imperio GE, Bloise E, Matthews SG, Lye SJ. ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth. Cells. 2021; 10(7):1724. https://doi.org/10.3390/cells10071724
Chicago/Turabian StyleLye, Phetcharawan, Caroline E. Dunk, Jianhong Zhang, Yanxing Wei, Jittanan Nakpu, Hirotaka Hamada, Guinever E. Imperio, Enrrico Bloise, Stephen G. Matthews, and Stephen J. Lye. 2021. "ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth" Cells 10, no. 7: 1724. https://doi.org/10.3390/cells10071724
APA StyleLye, P., Dunk, C. E., Zhang, J., Wei, Y., Nakpu, J., Hamada, H., Imperio, G. E., Bloise, E., Matthews, S. G., & Lye, S. J. (2021). ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth. Cells, 10(7), 1724. https://doi.org/10.3390/cells10071724







