KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease
Abstract
:1. Introduction
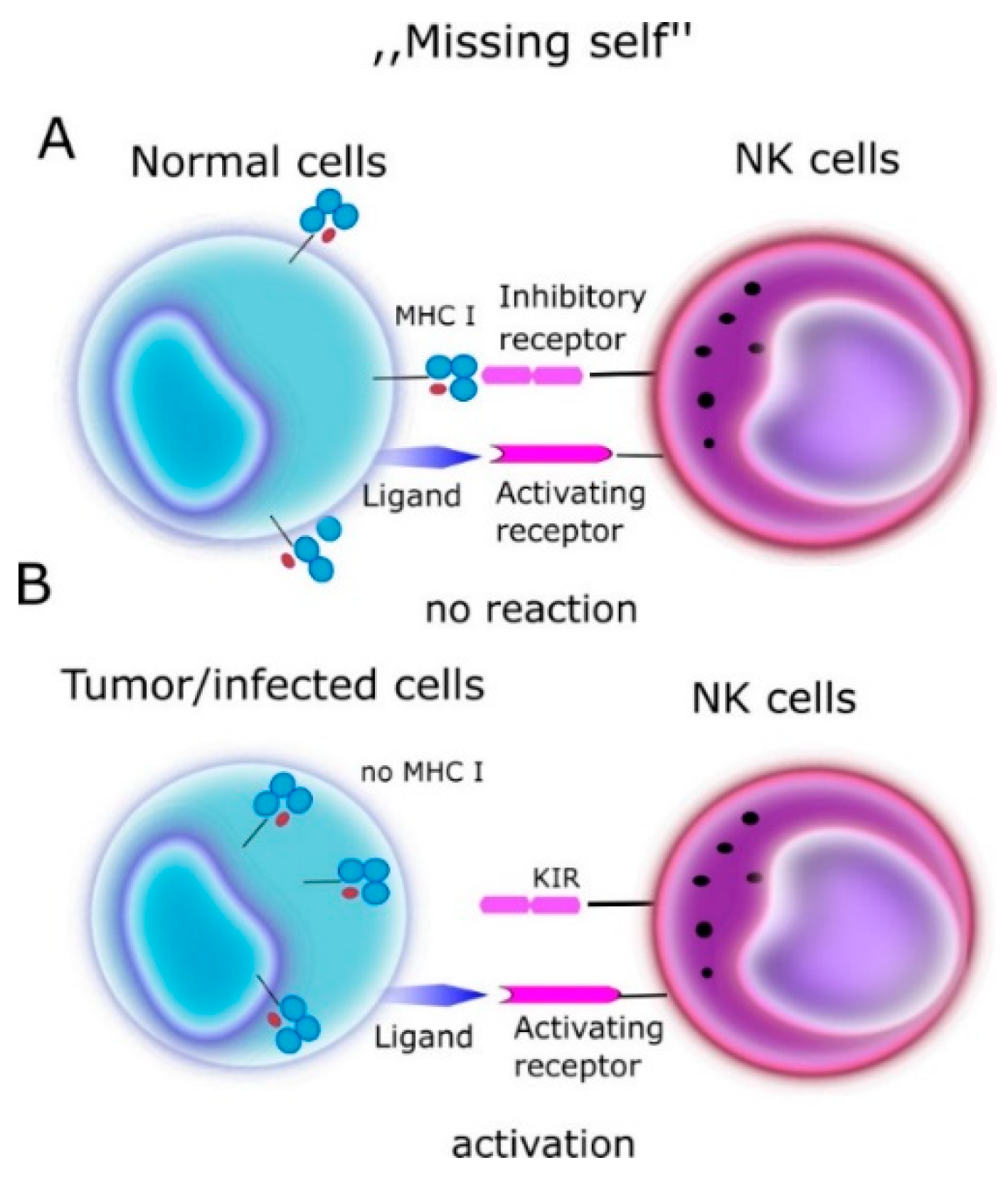
2. NK (Natural Killer Cells)
- Spontaneous cytotoxic reaction through release of perforin, granzyme B.
- Antibody dependent cytotoxicity (ADCC). NK cells express the CD16 receptor (FcγRIIIa), which binds Fc fragment of IgG class antibodies inducing NK cell activation and degranulation leading to destruction of the target cell.
- Receptor/ligand interactions via Fas/FasL, TNF/TNFR coupling and triggering of the apoptosis in the target cell.
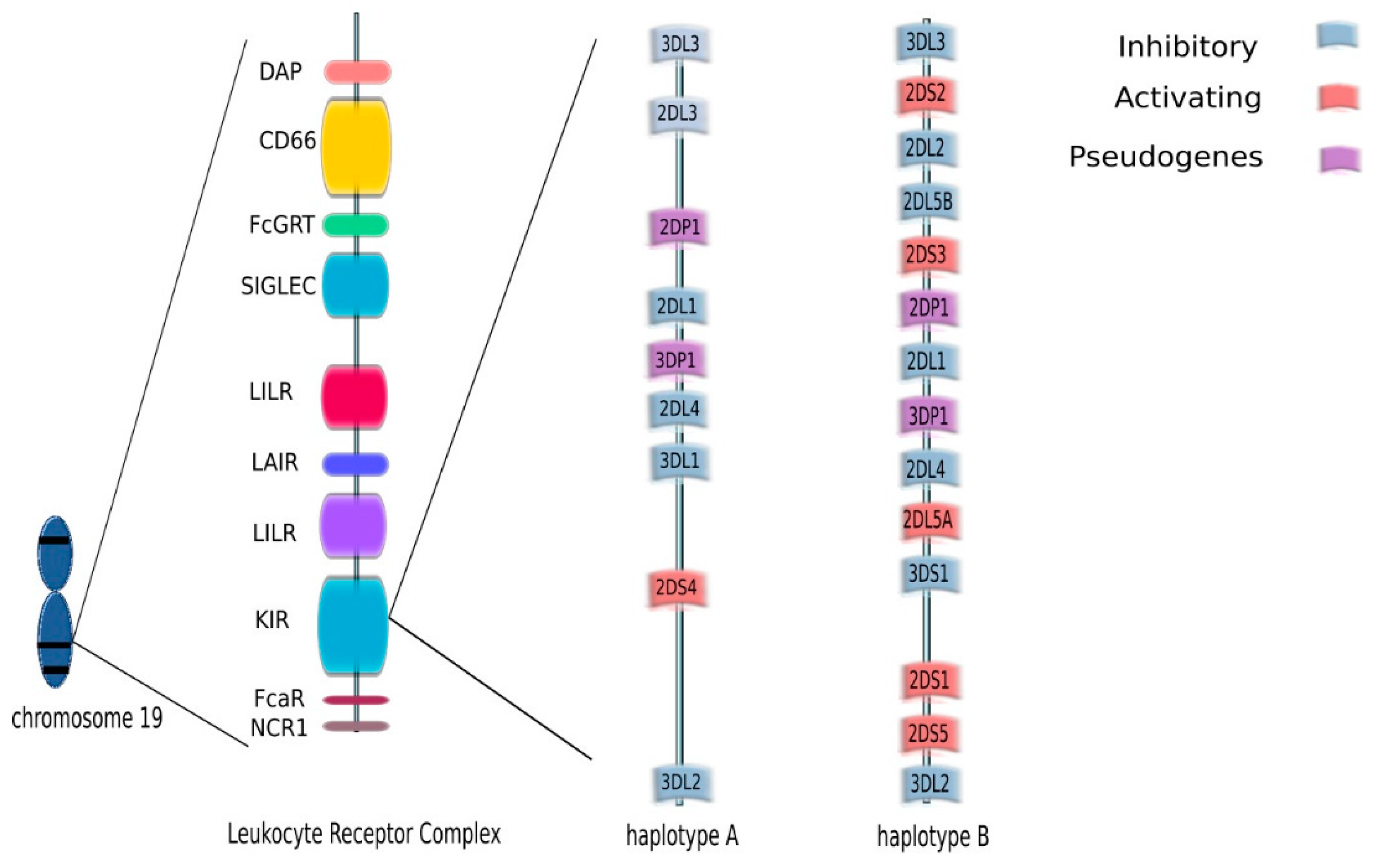
3. KIR (Killer Immunoglobulin-Like Receptors)
4. KIR Typing Methods
5. NK, KIR and Hematologic Diseases
6. NK, KIR and Solid Organ Transplantation
7. KIR/HLA Interaction and Association with Viral Infections after Transplantation
8. KIR/HLA Interaction in Pregnancy Complication
9. KIR/HLA Interaction in Solid Cancer
10. Therapy
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morris, P.J.; Batchelor, J.R.; Festenstein, H. Matching for HLA in transplantation. Br. Med. Bull. 1978, 34, 259–262. [Google Scholar] [CrossRef]
- Garcia, C.A.; Robinson, J.; Madrigal, J.A.; Marsh, S.G.E. Natural killer cell receptors: Functional roles. Inmunologia 2003, 22, 190–202. [Google Scholar]
- Saeki, Y.; Ishiyama, K.; Ishida, N.; Tanaka, Y.; Ohdan, H. Role of Natural Killer Cells in the Innate Immune System After Intraportal Islet Transplantation in Mice. Transplant. Proc. 2017, 49, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Gill, R.G.; Mehrad, B. The natural killer cell activating receptor, NKG2D, is critical to antibody-dependent chronic rejection in heart transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021. [Google Scholar] [CrossRef]
- López-Botet, M.; Vilches, C.; Redondo-Pachón, D.; Muntasell, A.; Pupuleku, A.; Yélamos, J.; Pascual, J.; Crespo, M. Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front. Immunol. 2017, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Freud, A.G.; Caligiuri, M.A. Location and cellular stages of natural killer cell development. Trends Immunol. 2013, 34, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e13. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [Green Version]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Shah, M.H.; Turner, M.J.; VanDeusen, J.B.; Whitman, S.P.; Cooper, M.A.; Suzuki, K.; Wechser, M.; Goodsaid, F.; Caligiuri, M.A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J. Immunol. 1999, 162, 4511–4520. [Google Scholar] [PubMed]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 hampers tumor surveillance of liver-resident and conventional NK cells by disrupting PI3K signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, H.Y.; Cornelius, D.C.; Guzik, T.J.; Delles, C. Natural killer cells in placentation and cancer: Implications for hypertension during pregnancy. Placenta 2017, 56, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kärre, K.; Ljunggren, H.G.; Piontek, G.; Kissling, R. Pillars Article: Selective Rejection of H—2-Deficient Lymphoma Variants Suggests Alternative Immune Defence Strategy. Nature 1986, 391, 675–678. [Google Scholar] [CrossRef]
- Quispe-Tintaya, W. NK cell education in human health and disease. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Nakimuli, A.; Chazara, O.; Hiby, S.E.; Farrell, L.; Tukwasibwe, S.; Jayaraman, J.; Traherne, J.A.; Trowsdale, J.; Colucci, F.; Lougee, E.; et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc. Natl. Acad. Sci. USA 2015, 112, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Shastry, A.; Sedimbi, S.K.; Rajalingam, R.; Nikitina-Zake, L.; Rumba, I.; Wigzell, H.; Sanjeevi, C.B. Combination of KIR 2DL2 and HLA-C1 (Asn80) confers susceptibility to type 1 diabetes in Latvians. Int. J. Immunogenet. 2008, 35, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Gang, M.; Wong, P.; Berrien-Elliott, M.M.; Fehniger, T.A. Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol. 2020, 57, 185–193. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T cell– and B cell–independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef]
- Paust, S.; Gill, H.S.; Wang, B.-Z.; Flynn, M.P.; Moseman, E.A.; Senman, B.; Szczepanik, M.; Telenti, A.; Askenase, P.W.; Compans, R.W.; et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010, 11, 1127–1135. [Google Scholar] [CrossRef]
- Brillantes, M.; Beaulieu, A.M. Memory and Memory-Like NK Cell Responses to Microbial Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roosnek, E.; Schneider, P.; Huard, B. Human NK cells can control CMV infection in the absence of T cells To the editor: Heparan sulfate proteoglycans, Fc receptors, and DC suppression. Blood 2008, 112, 914–916. [Google Scholar] [CrossRef]
- Cichocki, F.; Wu, C.Y.; Zhang, B.; Felices, M.; Tesi, B.; Tuininga, K.; Dougherty, P.; Taras, E.; Hinderlie, P.; Blazar, B.R.; et al. ARID5B regulates metabolic programming in human adaptive NK cells. J. Exp. Med. 2018, 215, 2379–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Astarloa-Pando, G.; Zenarruzabeitia, O.; Borrego, F. Modulating NK cell metabolism for cancer immunotherapy. Semin. Hematol. 2020, 57, 213–224. [Google Scholar] [CrossRef]
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.; Highton, A.J.; Peine, S.; Sauter, J.; Schmidt, A.H.; Bunders, M.J.; Altfeld, M.; Körner, C. Natural Killer Cell Education Is Associated With a Distinct Glycolytic Profile. Front. Immunol. 2018, 9, 3020. [Google Scholar] [CrossRef] [Green Version]
- Naeimi Kararoudi, M.; Tullius, B.P.; Chakravarti, N.; Pomeroy, E.J.; Moriarity, B.S.; Beland, K.; Colamartino, A.B.L.; Haddad, E.; Chu, Y.; Cairo, M.S.; et al. Genetic and epigenetic modification of human primary NK cells for enhanced antitumor activity. Semin. Hematol. 2020, 57, 201–212. [Google Scholar] [CrossRef]
- Zitti, B.; Bryceson, Y.T. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 2018, 42, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef]
- Vandenhaute, J.; Wouters, C.H.; Matthys, P. Natural Killer Cells in Systemic Autoinflammatory Diseases: A Focus on Systemic Juvenile Idiopathic Arthritis and Macrophage Activation Syndrome. Front. Immunol. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Riffelmacher, T.; Kronenberg, M. Metabolic triggers of invariant natural killer T-cell activation during sterile autoinflammatory disease. Crit. Rev. Immunol. 2020, 40, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Lee, H.H.; Kang, H.; Cho, H. Cytokine-modulated natural killer cells differentially regulate the activity of the hepatitis C virus. Int. J. Mol. Sci. 2018, 19, 2771. [Google Scholar] [CrossRef] [Green Version]
- De Maria, A.; Fogli, M.; Mazza, S.; Basso, M.; Picciotto, A.; Costa, P.; Congia, S.; Mingari, M.C.; Moretta, L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur. J. Immunol. 2007, 37, 445–455. [Google Scholar] [CrossRef]
- Long, E.O.; Colonna, M.; Lanier, L.L. Inhibitory MHC class I receptors on NK and T cells: A standard nomenclature. Immunol. Today 1996, 17, 100. [Google Scholar] [CrossRef]
- Rajalingam, R. Diversity of Killer Cell Immunoglobulin-Like Receptors and Disease. Clin. Lab. Med. 2018, 38, 637–653. [Google Scholar] [CrossRef]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.E.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Biotec, M. Flow Cytometry Analysis of Whole-Blood NK Cells Expressing Single Killer Cell Immunoglobulin—Like Receptors. Available online: www.miltenyibiotec.com/UN-en/applications/nk-cells/killer-immunoglobulin-like-receptors-kirs-analysis.html (accessed on 4 July 2021).
- Björkström, N.; Fauriat, C.; Bryceson, Y.; Sandberg, J.; Ljunggren, H.-G.; Malmberg, K.-J. Analysis of the KIR Repertoire in Human NK Cells by Flow Cytometry. Methods Mol. Biol. 2010, 612, 353–364. [Google Scholar] [CrossRef]
- Crum, K.A.; Logue, S.E.; Curran, M.D.; Middleton, D. Development of a PCR-SSOP approach capable of defining the natural killer cell inhibitory receptor (KIR) gene sequence repertoires. Tissue Antigens 2000, 56, 313–326. [Google Scholar] [CrossRef]
- López-Hernández, R.; Campillo, J.A.; Legaz, I.; Valdés, M.; Salama, H.; Boix, F.; Hernández-Martínez, A.M.; Eguia, J.; González-Martínez, G.; Moya-Quiles, M.R.; et al. Killer immunoglobulin-like receptor repertoire analysis in a Caucasian Spanish cohort with inflammatory bowel disease. Microbiol. Immunol. 2016, 60, 787–792. [Google Scholar] [CrossRef]
- Gómez-Lozano, N.; Vilches, C. Genotyping of human killer-cell immunoglobulin-like receptor genes by polymerase chain reaction with sequence-specific primers: An update. Tissue Antigens 2002, 59, 184–193. [Google Scholar] [CrossRef]
- Ordóñez, D.; Moraru, M.; Gómez-Lozano, N.; Cisneros, E.; Vilches, C. KIR typing by non-sequencing methods: Polymerase-chain reaction with sequence-specific primers. Methods Mol. Biol. 2012, 882, 415–430. [Google Scholar] [CrossRef]
- Ashouri, E.; Ghaderi, A.; Reed, E.F.; Rajalingam, R. A novel duplex SSP–PCR typing method for KIR gene profiling. Tissue Antigens 2009, 74, 62–67. [Google Scholar] [CrossRef]
- Russnak, R.; King, E.; Ly, N.; Quinto, K.; Antovich, Z. 117-P: KIR TYPING WITH LINKSEQTM, A REAL-TIME PCR DETECTION SYSTEM. Hum. Immunol. 2013, 74, 130. [Google Scholar] [CrossRef]
- Jayaraman, J.; Kirgizova, V.; Di, D.; Johnson, C.; Jiang, W.; Traherne, J.A. qKAT: Quantitative Semi-automated Typing of Killer-cell Immunoglobulin-like Receptor Genes. J. Vis. Exp. 2019, e58646. [Google Scholar] [CrossRef]
- Closa, L.; Vidal, F.; Herrero, M.J.; Caro, J.L. Design and Validation of a Multiplex KIR and HLA Class I Genotyping Method Using Next Generation Sequencing. Front. Immunol. 2018, 9, 2991. [Google Scholar] [CrossRef]
- Rocha, V.; Locatelli, F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant. 2008, 41, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Tian, Z. Which is better, HLA-matched sibling or haploidentical transplantation? Cell. Mol. Immunol. 2021, 18, 1347. [Google Scholar] [CrossRef]
- Solomon, S.R.; Aubrey, M.T.; Zhang, X.; Piluso, A.; Freed, B.M.; Brown, S.; Jackson, K.C.; Morris, L.E.; Holland, H.K.; Solh, M.M.; et al. Selecting the Best Donor for Haploidentical Transplant: Impact of HLA, Killer Cell Immunoglobulin-Like Receptor Genotyping, and Other Clinical Variables. Biol. Blood Marrow Transplant. 2018, 24, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Dehn, J.; Spellman, S.; Hurley, C.K.; Shaw, B.E.; Barker, J.N.; Burns, L.J.; Confer, D.L.; Eapen, M.; Fernandez-Vina, M.; Hartzman, R.; et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: Guidelines from the NMDP/CIBMTR. Blood 2019, 134, 924–934. [Google Scholar] [CrossRef]
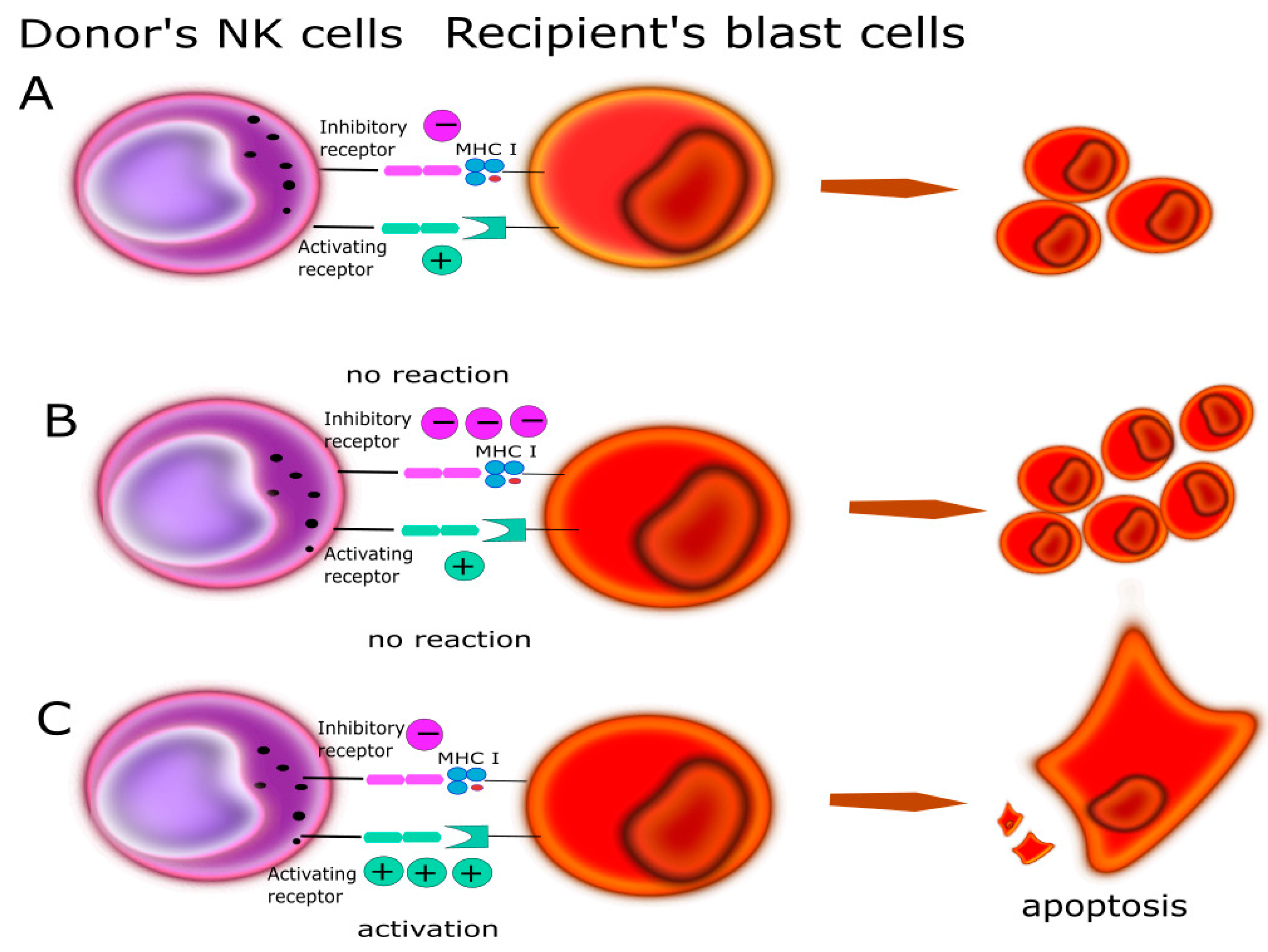
- Velardi, A.; Ruggeri, L.; Mancusi, A.; Burchielli, E.; Perruccio, K.; Aversa, F.; Martelli, M.F.; Immunobiology Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Clinical impact of natural killer cell reconstitution after allogeneic hematopoietic transplantation. Semin. Immunopathol. 2008, 30, 489–503. [Google Scholar] [CrossRef]
- Ruggeri, L.; Mancusi, A.; Capanni, M.; Urbani, E.; Carotti, A.; Aloisi, T.; Stern, M.; Pende, D.; Perruccio, K.; Burchielli, E.; et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: Challenging its predictive value. Blood 2007, 110, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Mavers, M.; Bertaina, A. High-risk leukemia: Past, present, and future role of NK cells. J. Immunol. Res. 2018, 2018, 1586905. [Google Scholar] [CrossRef] [Green Version]
- Long, E.O. Negative signaling by inhibitory receptors: The NK cell paradigm. Immunol. Rev. 2008, 224, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Graczyk-Pol, E.; Rogatko-Koros, M.; Nestorowicz, K.; Gwozdowicz, S.; Mika-Witkowska, R.; Pawliczak, D.; Zubala, M.; Szlendak, U.; Witkowska, A.; Tomaszewska, A.; et al. Role of donor HLA class I mismatch, KIR-ligand mismatch and HLA:KIR pairings in hematological malignancy relapse after unrelated hematopoietic stem cell transplantation. HLA 2018, 92, 42–46. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell aloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verneris, M.R.; Miller, J.S.; Hsu, K.C.; Wang, T.; Sees, J.A.; Paczesny, S.; Rangarajan, H.; Lee, D.A.; Spellman, S.R.; Lee, S.J. Investigation of donor KIR content and matching in children undergoing hematopoietic cell transplantation for acute leukemia. Blood Adv. 2020, 4, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Y.; Yu, X.X.; Xu, Z.L.; Cao, X.H.; Huo, M.R.; Zhao, X.S.; Chang, Y.J.; Wang, Y.; Zhang, X.H.; Xu, L.P.; et al. Donor and host coexpressing KIR ligands promote NK education after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019, 3, 4312–4325. [Google Scholar] [CrossRef] [Green Version]
- Weisdorf, D.; Cooley, S.; Wang, T.; Trachtenberg, E.; Vierra-Green, C.; Spellman, S.A.; Sees, J.; Spahn, A.; Vogel, J.; Fehniger, T.A.; et al. KIR B donors improve the outcome for AML patients given reduced intensity conditioning and unrelated donor transplantation. Blood Adv. 2020, 4, 740–754. [Google Scholar] [CrossRef] [Green Version]
- Cooley, S.; Trachtenberg, E.; Bergemann, T.L.; Saeteurn, K.; Klein, J.; Chap, T.L.; Marsh, S.G.E.; Guethlein, L.A.; Parham, P.; Miller, J.S.; et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009, 113, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Marsh, S.G.E.; Spellman, S.; Haagenson, M.D.; Saeturn, K.; Ladner, M.; et al. Donor Killer Cell Ig-like Receptor B Haplotypes, Recipient HLA-C1, and HLA-C Mismatch Enhance the Clinical Benefit of Unrelated Transplantation for Acute Myelogenous Leukemia. J. Immunol. 2014, 192, 4592–4600. [Google Scholar] [CrossRef]
- Wanquet, A.; Bramanti, S.; Harbi, S.; Fürst, S.; Legrand, F.; Faucher, C.; Granata, A.; Calmels, B.; Lemarie, C.; Picard, C.; et al. Killer Cell Immunoglobulin-Like Receptor–Ligand Mismatch in Donor versus Recipient Direction Provides Better Graft-versus-Tumor Effect in Patients with Hematologic Malignancies Undergoing Allogeneic T Cell–Replete Haploidentical Transplantation Followed b. Biol. Blood Marrow Transplant. 2018, 24, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Guo, X.; Yuan, L.; Gao, J.; Huo, L.; Li, Q. Killer cell immunoglobulin-like receptor gene cluster predisposes to susceptibility to B-cell acute lymphoblastic leukemia in Chinese children. Int. J. Clin. Exp. Pathol. 2020, 13, 536–542. [Google Scholar] [PubMed]
- Li, Y.; Wang, T.; Hu, X.; Zhang, H.; Chen, L.; Bao, X.; He, J. Study of KIR gene expression at the mRNA level in specific donor-derived NK cells after allogeneic HSCT. Immunogenetics 2020, 72, 135–141. [Google Scholar] [CrossRef]
- Solloch, U.V.; Schefzyk, D.; Schäfer, G.; Massalski, C.; Kohler, M.; Pruschke, J.; Heidl, A.; Schetelig, J.; Schmidt, A.H.; Lange, V.; et al. Estimation of German KIR Allele Group Haplotype Frequencies. Front. Immunol. 2020, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Alice, K.; Chen, C.C.; Marçais, A.; Barba, T.; Mathias, V.; Sicard, A.; Rabeyrin, M.; Racapé, M.; Duong-Van-Huyen, J.P.; Bruneval, P.; et al. Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat. Commun. 2019, 10, 5350. [Google Scholar] [CrossRef]
- La Manna, G.; Corsini, S.; Iannelli, S.; Cappuccilli, M.L.; Comai, G.; Iorio, M.; Todeschini, P.; Carretta, E.; Scolari, M.P.; Bontadini, A.; et al. Influence of the immunogenetic KIR and HLA systems on long-term renal transplant outcome. Ann. Transplant. 2013, 18, 611–621. [Google Scholar] [CrossRef]
- Littera, R.; Piredda, G.; Argiolas, D.; Lai, S.; Congeddu, E.; Ragatzu, P.; Melis, M.; Carta, E.; Michittu, M.B.; Valentini, D.; et al. KIR and their HLA Class I ligands: Two more pieces towards completing the puzzle of chronic rejection and graft loss in kidney transplantation. PLoS ONE 2017, 12, e0180831. [Google Scholar] [CrossRef] [Green Version]
- Thabut, G.; Mal, H. Outcomes after lung transplantation. J. Thorac. Dis. 2017, 9, 2684–2691. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, D.R.; Lanier, L.L.; Greenland, J.R. Natural killer cells in lung transplantation. Thorax 2019, 74, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel-van Erp, J.M.; van de Graaf, E.A.; Paantjens, A.W.M.; van Ginkel, W.G.J.; Schellekens, J.; van Kessel, D.A.; van den Bosch, J.M.M.; Otten, H.G. The Killer Immunoglobulin-Like Receptor (KIR) Group A Haplotype is Associated With Bronchiolitis Obliterans Syndrome After Lung Transplantation. J. Hear. Lung Transplant. 2008, 27, 995–1001. [Google Scholar] [CrossRef]
- Greenland, J.R.; Sun, H.; Calabrese, D.; Chong, T.; Singer, J.P.; Kukreja, J.; Hays, S.R.; Golden, J.A.; Caughey, G.H.; Venstrom, J.M.; et al. HLA Mismatching Favoring Host-Versus-Graft NK Cell Activity Via KIR3DL1 Is Associated With Improved Outcomes Following Lung Transplantation. Am. J. Transplant. 2017, 17, 2192–2199. [Google Scholar] [CrossRef] [Green Version]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Fosby, B.; Næss, S.; Hov, J.R.; Traherne, J.; Boberg, K.M.; Trowsdale, J.; Foss, A.; Line, P.D.; Franke, A.; Melum, E.; et al. HLA variants related to primary sclerosing cholangitis influence rejection after liver transplantation. World J. Gastroenterol. 2014, 20, 3986–4000. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, K.H.; Park, H.S.; Ryu, J.H.; Lim, J.; Kim, Y.; Na, G.H.; Kim, D.G.; Oh, E.J. Human leukocyte antigen-C genotype and killer immunoglobulin-like receptor-ligand matching in Korean living donor liver transplantation. Ann. Lab. Med. 2017, 37, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Legaz, I.; López-Álvarez, M.R.; Campillo, J.A.; Moya-Quiles, M.R.; Bolarín, J.M.; De La Peña, J.; Salgado, G.; Gimeno, L.; García-Alonso, A.M.; Muro, M.; et al. KIR gene mismatching and KIR/C ligands in liver transplantation: Consequences for short-term liver allograft injury. Transplantation 2013, 95, 1037–1044. [Google Scholar] [CrossRef]
- Deborska-Materkowska, D.; Perkowska-Ptasinska, A.; Sadowska-Jakubowicz, A.; Gozdowska, J.; Ciszek Michałand Pazik, J.; Ostaszewska, A.; Kosieradzki, M.; Nowak, J.; Durlik, M. Killer immunoglobulin-like receptor 2DS2 (KIR2DS2), KIR2DL2-HLA-C1, and KIR2DL3 as genetic markers for stratifying the risk of cytomegalovirus infection in kidney transplant recipients. Int. J. Mol. Sci. 2019, 20, 546. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg, E. NIH Public Access. Bone 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Van Duin, D.; Avery, R.K.; Hemachandra, S.; Yen-Lieberman, B.; Zhang, A.; Jain, A.; Butler, R.S.; Barnard, J.; Schold, J.D.; Fung, J.; et al. KIR and HLA interactions are associated with control of primary CMV infection in solid organ transplant recipients. Am. J. Transplant. 2014, 14, 156–162. [Google Scholar] [CrossRef]
- Behrendt, C.E.; Nakamura, R.; Forman, S.J.; Zaia, J.A. Donor killer immunoglobulin-like receptor genes and reactivation of cytomegalovirus after HLA-matched hematopoietic stem-cell transplantation: HLA-C allotype is an essential cofactor. Front. Immunol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Hadaya, K.; Hönger, G.; Martin, P.Y.; Steiger, J.; Hess, C.; Villard, J. Telomeric rather than centromeric activating KIR genes protect from cytomegalovirus infection after kidney transplantation. Am. J. Transplant. 2011, 11, 1302–1307. [Google Scholar] [CrossRef] [Green Version]
- Schmied, L.; Terszowski, G.; Gonzalez, A.; Schmitter, K.; Hirsch, H.H.; Garzoni, C.; Van Delden, C.; Boggian, K.; Mueller, N.J.; Berger, C.; et al. Protection from varicella zoster in solid organ transplant recipients carrying killer cell immunoglobulin-like receptor b haplotypes. Transplantation 2015, 99, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- Nowak, I.; Wilczyńska, K.; Wilczyński, J.R.; Malinowski, A.; Radwan, P.; Radwan, M.; Kuśnierczyk, P. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch. Immunol. Ther. Exp. (Warsz.) 2017, 65, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Kofod, L.; Lindhard, A.; Hviid, T.V.F. Implications of uterine NK cells and regulatory T cells in the endometrium of infertile women. Hum. Immunol. 2018, 79, 693–701. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, Y.; Ni, X.; Tong, X.; Xu, X.; Dong, Z.; Sun, R.; Tian, Z.; Wei, H. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017, 47, 1100–1113.e6. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Wang, H.; Zhang, B.; Kang, Y.; Guo, Q.; Xiao, H.; Yang, H.; Liao, S. Maternal natural killer cell immunoglobulin receptor genes and human leukocyte antigen-C ligands influence recurrent spontaneous abortion in the han Chinese population. Exp. Ther. Med. 2018, 15, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Morin, S.J.; Treff, N.R.; Tao, X.; Scott, R.T.; Franasiak, J.M.; Juneau, C.R.; Maguire, M.; Scott, R.T. Combination of uterine natural killer cell immunoglobulin receptor haplotype and trophoblastic HLA-C ligand influences the risk of pregnancy loss: A retrospective cohort analysis of direct embryo genotyping data from euploid transfers. Fertil. Steril. 2017, 107, 677–683.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, S.; Yasin Ahmadi, S.A.; Shahsavar, F. The Relationship of Maternal KIR and Parental HLA-C Genes With Risk of Recurrent Spontaneous Abortion: A Regional Study in Lorestan Province, Iran. Crescent J. Med. Biol. Sci. 2018, 5, 194–197. [Google Scholar]
- Mansour, L.; Alkhuriji, A.; Babay, Z.A.; Alqadheeb, S.; Al-Khulaifi, F.; Al-Talhi, R.; Alomar, S. Association of killer immunoglobulin-like receptor and human leukocyte antigen class i ligand with recurrent abortion in Saudi Women. Genet. Test. Mol. Biomarkers 2020, 24, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.H.; Lin, A.; Chen, B.G.; Zhou, M.Y.; Dai, M.Z.; Chen, X.J.; Gan, L.H.; Zhu, M.; Shi, W.W.; Li, B.L. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am. J. Reprod. Immunol. 2007, 57, 233–242. [Google Scholar] [CrossRef]
- Kataoka, T.R.; Ueshima, C.; Hirata, M.; Haga, H.; Minamiguchi, S. Killer immunoglobulin-like receptor 2DL4 (Cd158d) regulates human mast cells both positively and negatively: Possible roles in pregnancy and cancer metastasis. Int. J. Mol. Sci. 2020, 21, 954. [Google Scholar] [CrossRef] [Green Version]
- Akbari, S.; Shahsavar, F.; Karami, R.; Yari, F.; Anbari, K.; Ahmadi, S.A.Y. Recurrent spontaneous abortion (RSA) and maternal kir genes: A comprehensive meta-analysis. J. Bras. Reprod. Assist. 2020, 24, 197–213. [Google Scholar] [CrossRef]
- Jobim, M.R.; Jobim, M.; Salim, P.H.; Portela, P.; Jobim, L.F.; Leistner-Segal, S.; Bittelbrunn, A.C.; Menke, C.H.; Biazús, J.V.; Roesler, R.; et al. Analysis of KIR gene frequencies and HLA class I genotypes in breast cancer and control group. Hum. Immunol. 2013, 74, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Hong, C.; Ma, Q.; Tan, F.; Liu, C.; Kuśnierczyk, P.; Li, C.; Shi, L.; Yao, Y. The association of HLA/KIR genes with non-small cell lung cancer (adenocarcinoma) in a Han Chinese population. J. Cancer 2019, 10, 4731–4738. [Google Scholar] [CrossRef] [PubMed]
- Al Omar, S.; Middleton, D.; Marshall, E.; Porter, D.; Xinarianos, G.; Raji, O.; Field, J.K.; Christmas, S.E. Associations between genes for killer immunoglobulin-like receptors and their ligands in patients with solid tumors. Hum. Immunol. 2010, 71, 976–981. [Google Scholar] [CrossRef]
- He, Y.; Bunn, P.A.; Zhou, C.; Chan, D. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget 2016, 7, 82104–82111. [Google Scholar] [CrossRef] [Green Version]
- Yousefinejad, F.; Jowkar, F.; Barani, S.; Jamali, E.; Mahmoudi, E.; Ramezani, A.; Maymand, E.M.; Ghaderi, A. Killer cell immunoglobulin-like receptors (KIRs) genotype and haplotype analysis in Iranians with non-melanoma Skin Cancers. Iran. Biomed. J. 2019, 23, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.E.; Ní Chorcora, C.; Dring, M.M.; Stallings, R.L.; O’Meara, A.; Gardiner, C.M. Increased frequencies of the killer immunoglobulin-like receptor genes KIR2DL2 and KIR2DS2 are associated with neuroblastoma. Tissue Antigens 2015, 86, 172–177. [Google Scholar] [CrossRef]
- Bao, X.; Hanson, A.L.; Madeleine, M.M.; Wang, S.S.; Schwartz, S.M.; Newell, F.; Pettersson-Kymmer, U.; Hemminki, K.; Tiews, S.; Steinberg, W.; et al. HLA and KIR associations of cervical neoplasia. J. Infect. Dis. 2018, 218, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Portela, P.; Jobim, L.F.; Salim, P.H.; Koff, W.J.; Wilson, T.J.; Jobim, M.R.; Schwartsmann, G.; Roesler, R.; Jobim, M. Analysis of KIR gene frequencies and HLA class I genotypes in prostate cancer and control group. Int. J. Immunogenet. 2012, 39, 423–428. [Google Scholar] [CrossRef]
- Morales-Estevez, C.; De la Haba-Rodriguez, J.; Manzanares-Martin, B.; Porras-Quintela, I.; Rodriguez-Ariza, A.; Moreno-Vega, A.; Ortiz-Morales, M.J.; Gomez-España, M.A.; Cano-Osuna, M.T.; Lopez-Gonzalez, J.; et al. KIR genes and their ligands predict the response to anti-EGFR monoclonal antibodies in solid tumors. Front. Immunol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-mortari, A.; Mcnearney, S.A.; Yun, G.H.; Fautsch, S.K.; Mckenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.N.; Baird, K.; Delbrook, C.P.; Fleisher, T.A.; Kohler, M.E.; Rampertaap, S.; Lemberg, K.; Hurley, C.K.; Kleiner, D.E.; Merchant, M.S.; et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015, 125, 784–792. [Google Scholar] [CrossRef]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Foon, K.A.; Whiteside, T.L.; Boyiadzis, M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol. Immunother. 2010, 59, 73–79. [Google Scholar] [CrossRef]
- Boieri, M.; Ulvmoen, A.; Sudworth, A.; Lendrem, C.; Collin, M.; Dickinson, A.M.; Kveberg, L.; Inngjerdingen, M. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology 2017, 6, e1274478. [Google Scholar] [CrossRef] [Green Version]
- Ravi, D.; Sarkar, S.; Purvey, S.; Passero, F.; Beheshti, A.; Chen, Y.; Mokhtar, M.; David, K.; Konry, T.; Evens, A.M. Interaction kinetics with transcriptomic and secretory responses of CD19-CAR natural killer-cell therapy in CD20 resistant non-hodgkin lymphoma. Leukemia 2020, 34, 1291–1304. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; André, P.; Paturel, C.; et al. A phase 1 study of lirilumab (antibody against killer immunoglobulinlike receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675–17688. [Google Scholar] [CrossRef] [Green Version]
- Yalniz, F.F.; Daver, N.; Rezvani, K.; Kornblau, S.; Ohanian, M.; Borthakur, G.; DiNardo, C.D.; Konopleva, M.; Burger, J.; Gasior, Y.; et al. A Pilot Trial of Lirilumab with or without Azacitidine for Patients with Myelodysplastic Syndrome. Clin. Lymphoma. Myeloma Leuk. 2018, 18, 658–663.e2. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Hofmeister, C.C.; Padmanabhan, S.; Suvannasankha, A.; Jagannath, S.; Abonour, R.; Bakan, C.; Andre, P.; Efebera, Y.; Tiollier, J.; et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012, 120, 4324–4333. [Google Scholar] [CrossRef]
- Bagot, M.; Porcu, P.; Marie-Cardine, A.; Battistella, M.; William, B.M.; Vermeer, M.; Whittaker, S.; Rotolo, F.; Ram-Wolff, C.; Khodadoust, M.S.; et al. IPH4102, a first-in-class anti-KIR3DL2 monoclonal antibody, in patients with relapsed or refractory cutaneous T-cell lymphoma: An international, first-in-human, open-label, phase 1 trial. Lancet. Oncol. 2019, 20, 1160–1170. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Mattei, J.; Bunn, P.A.J.; Zhou, C.; Chan, D. The combination of anti-KIR monoclonal antibodies with anti-PD-1/PD-L1 monoclonal antibodies could be a critical breakthrough in overcoming tumor immune escape in NSCLC. Drug Des. Devel. Ther. 2018, 12, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, D.; Brandt, L.; Felices, M.; Guldevall, K.; Lenvik, T.; Hinderlie, P.; Curtsinger, J.; Warlick, E.; Spellman, S.R.; Blazar, B.R.; et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2018, 2, 1459–1469. [Google Scholar] [CrossRef] [Green Version]
- Singer, H.; Kellner, C.; Lanig, H.; Aigner, M.; Stockmeyer, B.; Oduncu, F.; Schwemmlein, M.; Stein, C.; Mentz, K.; Mackensen, A.; et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J. Immunother. 2010, 33, 599–608. [Google Scholar] [CrossRef]


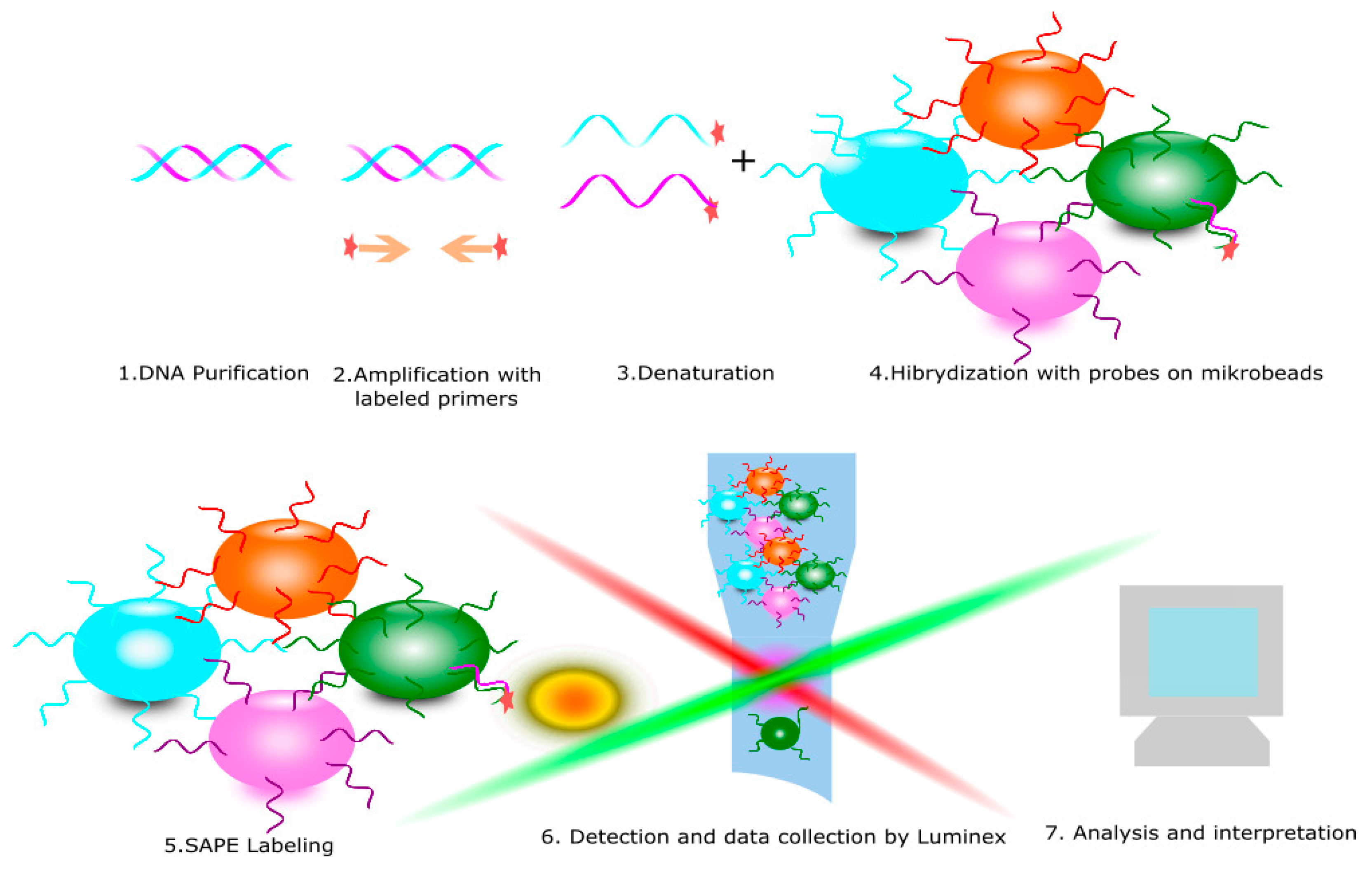
| Methods for KIR Typing | Based on KIR Expression on NK Cells | Based on DNA | Resolution Level | Quantitative | Qualitative | Time of Performing |
|---|---|---|---|---|---|---|
| Flow cytometry | + | − | − | + | + | <24 h |
| PCR SSO | − | + | intermediate | − | + | <24 h |
| PCR SSP | − | + | low | − | + | <24 h |
| Real time PCR | − | + | low | + | + | <24 h |
| NGS | − | + | high | − | + | >24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. https://doi.org/10.3390/cells10071777
Dębska-Zielkowska J, Moszkowska G, Zieliński M, Zielińska H, Dukat-Mazurek A, Trzonkowski P, Stefańska K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells. 2021; 10(7):1777. https://doi.org/10.3390/cells10071777
Chicago/Turabian StyleDębska-Zielkowska, Joanna, Grażyna Moszkowska, Maciej Zieliński, Hanna Zielińska, Anna Dukat-Mazurek, Piotr Trzonkowski, and Katarzyna Stefańska. 2021. "KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease" Cells 10, no. 7: 1777. https://doi.org/10.3390/cells10071777
APA StyleDębska-Zielkowska, J., Moszkowska, G., Zieliński, M., Zielińska, H., Dukat-Mazurek, A., Trzonkowski, P., & Stefańska, K. (2021). KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells, 10(7), 1777. https://doi.org/10.3390/cells10071777







