TRIM22. A Multitasking Antiviral Factor
Abstract
:1. Introduction
2. TRIM22 Expression and Protein Localization
3. HIV-1
3.1. Life Cycle
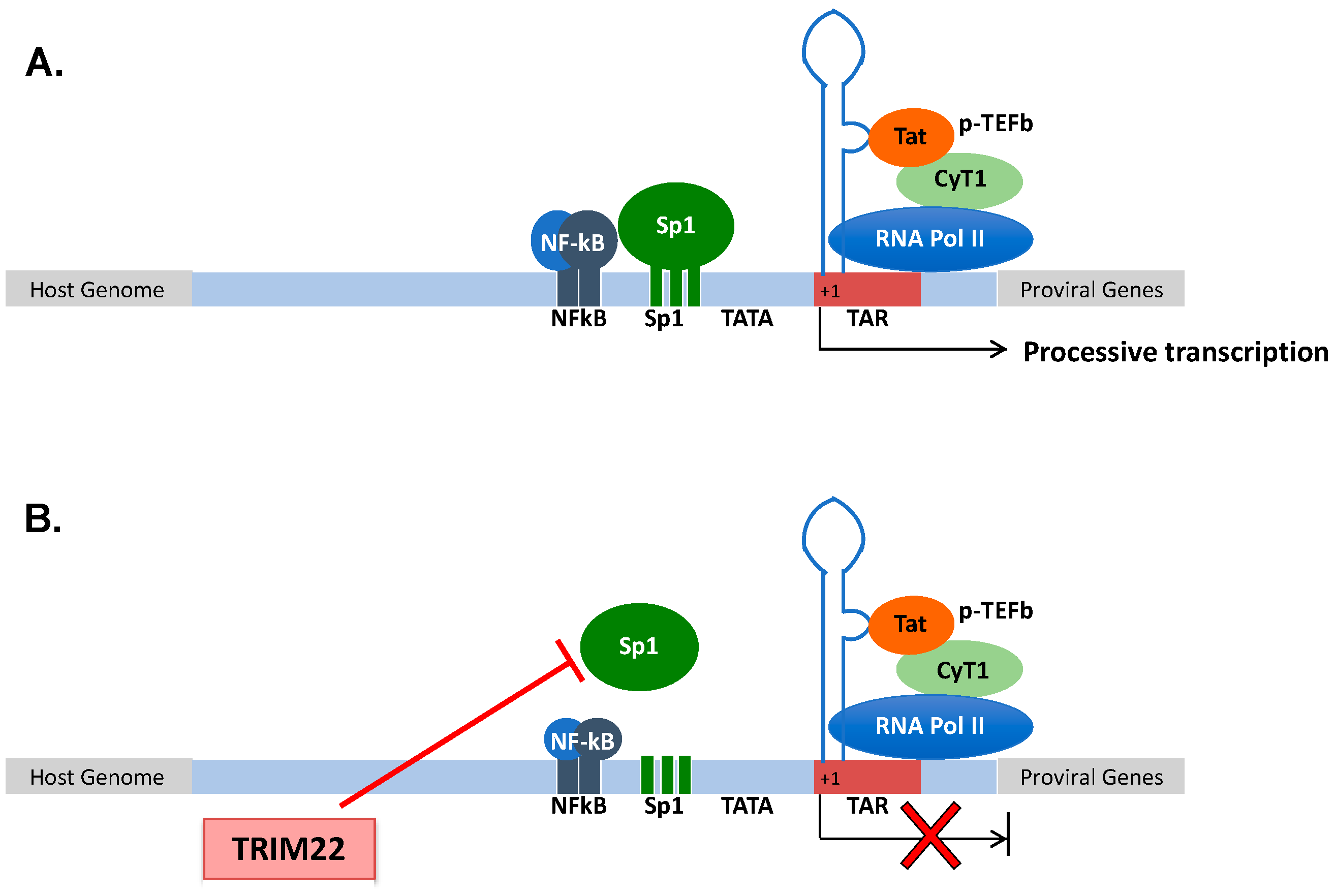
3.2. TRIM22 Restriction of HIV-1
4. Influenza A Virus (IAV)
4.1. IAV Infection
4.2. Mechanism of IAV Restriction by TRIM22
4.3. TRIM22 and IAV Evolution
5. Other RNA and DNA Viruses
| Family | Virus | Genome | Mechanism of Restriction | Ref. |
|---|---|---|---|---|
| Retroviridae | HIV-1 | ssRNA (+) | Gag trafficking, transcriptional silencing | [63,66] |
| Ortomyxoviridae | IAV | ssRNA (−) | Ubiquitination of NP | [86,89] |
| Picornaviridae | EMCV | ssRNA (+) | Ubiquitination of 3C protease | [87] |
| Flaviviridae | HCV | ssRNA (+) | Ubiquitination of NS5A | [102] |
| Pneumoviridae | RSV | ssRNA (−) | ND | [113] |
| Hepadnaviridae | HBV | dsDNA | Transcriptional repression | [31] |
| Herpesviridae | HSV-1 | dsDNA | Epigenetic silencing | [114] |
| Herpesviridae | EBV | dsDNA | LMP1 induces TRIM22 | [112,114] |
| Herpesviridae | KSHV | dsDNA | LANA induces TRIM22 | [115] |
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Shows, T.B.; Sakaguchi, A.Y.; Naylor, S.L.; Goedell, D.V.; Lawn, R.M. Clustering of leukocyte and fibroblast interferon genes of human chromosome 9. Science 1982, 218, 373–374. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- van Gent, M.; Sparrer, K.M.J.; Gack, M.U. TRIM Proteins and Their Roles in Antiviral Host Defenses. Annu. Rev. Virol. 2018, 5, 385–405. [Google Scholar] [CrossRef]
- van Tol, S.; Hage, A.; Giraldo, M.I.; Bharaj, P.; Rajsbaum, R. The TRIMendous Role of TRIMs in Virus-Host Interactions. Vaccines 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reymond, A.; Meroni, G.; Fantozzi, A.; Merla, G.; Cairo, S.; Luzi, L.; Riganelli, D.; Zanaria, E.; Messali, S.; Cainarca, S.; et al. The tripartite motif family identifies cell compartments. EMBO J. 2001, 20, 2140–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Inoue, S. TRIM proteins as RING finger E3 ubiquitin ligases. Adv. Exp. Med. Biol. 2012, 770, 27–37. [Google Scholar] [CrossRef]
- Meroni, G.; Diez-Roux, G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005, 27, 1147–1157. [Google Scholar] [CrossRef]
- Sanchez, J.G.; Okreglicka, K.; Chandrasekaran, V.; Welker, J.M.; Sundquist, W.I.; Pornillos, O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proc. Natl. Acad. Sci. USA 2014, 111, 2494–2499. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.A.; de Bono, B.; Trowsdale, J. Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology 2005, 116, 411–417. [Google Scholar] [CrossRef]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [Green Version]
- James, L.C.; Keeble, A.H.; Khan, Z.; Rhodes, D.A.; Trowsdale, J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc. Natl. Acad. Sci. USA 2007, 104, 6200–6205. [Google Scholar] [CrossRef] [Green Version]
- Short, K.M.; Cox, T.C. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J. Biol. Chem. 2006, 281, 8970–8980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernet, C.; Boretto, J.; Mattei, M.G.; Takahashi, M.; Jack, L.J.; Mather, I.H.; Rouquier, S.; Pontarotti, P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J. Mol. Evol. 1993, 37, 600–612. [Google Scholar] [CrossRef]
- Henry, J.; Mather, I.H.; McDermott, M.F.; Pontarotti, P. B30.2-like domain proteins: Update and new insights into a rapidly expanding family of proteins. Mol. Biol. Evol. 1998, 15, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.; Schultz, J.; Bork, P. SPRY domains in ryanodine receptors (Ca(2+)-release channels). Trends Biochem. Sci. 1997, 22, 193–194. [Google Scholar] [CrossRef]
- D’Cruz, A.A.; Babon, J.J.; Norton, R.S.; Nicola, N.A.; Nicholson, S.E. Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity. Protein Sci. 2013, 22, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stremlau, M.; Perron, M.; Welikala, S.; Sodroski, J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J. Virol. 2005, 79, 3139–3145. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.L.; Emerman, M.; Malik, H.S. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007, 3, e197. [Google Scholar] [CrossRef] [Green Version]
- Lian, Q.; Sun, B. Interferons command Trim22 to fight against viruses. Cell. Mol. Immunol. 2017, 14, 794–796. [Google Scholar] [CrossRef] [Green Version]
- Vicenzi, E.; Poli, G. The interferon-stimulated gene TRIM22: A double-edged sword in HIV-1 infection. Cytokine Growth Factor Rev. 2018, 40, 40–47. [Google Scholar] [CrossRef]
- Tissot, C.; Mechti, N. Molecular cloning of a new interferon-induced factor that represses human immunodeficiency virus type 1 long terminal repeat expression. J. Biol. Chem. 1995, 270, 14891–14898. [Google Scholar] [CrossRef] [Green Version]
- Hattlmann, C.J.; Kelly, J.N.; Barr, S.D. TRIM22: A Diverse and Dynamic Antiviral Protein. Mol. Biol. Int. 2012, 2012, 153415. [Google Scholar] [CrossRef] [PubMed]
- Carthagena, L.; Bergamaschi, A.; Luna, J.M.; David, A.; Uchil, P.D.; Margottin-Goguet, F.; Mothes, W.; Hazan, U.; Transy, C.; Pancino, G.; et al. Human TRIM gene expression in response to interferons. PLoS ONE 2009, 4, e4894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Wang, Y.; Xu, W.; Duan, Z.; Xiong, S. A 5’ extended IFN-stimulating response element is crucial for IFN-gamma-induced tripartite motif 22 expression via interaction with IFN regulatory factor-1. J. Immunol. 2010, 185, 2314–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.E.; Laimins, L.A. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J. Virol. 2000, 74, 4174–4182. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.H.; Park, E.S.; Kim, D.H.; Cho, K.C.; Kim, K.P.; Park, Y.K.; Ahn, S.H.; Park, S.H.; Kim, K.H.; Kim, C.W.; et al. Suppression of interferon-mediated anti-HBV response by single CpG methylation in the 5’-UTR of TRIM22. Gut 2018, 67, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Herr, A.M.; Dressel, R.; Walter, L. Different subcellular localisations of TRIM22 suggest species-specific function. Immunogenetics 2009, 61, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Duan, Z.; Xu, W.; Xiong, S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology 2009, 50, 424–433. [Google Scholar] [CrossRef]
- Sivaramakrishnan, G.; Sun, Y.; Tan, S.K.; Lin, V.C. Dynamic localization of tripartite motif-containing 22 in nuclear and nucleolar bodies. Exp. Cell Res. 2009, 315, 1521–1532. [Google Scholar] [CrossRef]
- Duan, Z.; Gao, B.; Xu, W.; Xiong, S. Identification of TRIM22 as a RING finger E3 ubiquitin ligase. Biochem. Biophys. Res. Commun. 2008, 374, 502–506. [Google Scholar] [CrossRef]
- Sivaramakrishnan, G.; Sun, Y.; Rajmohan, R.; Lin, V.C. B30.2/SPRY domain in tripartite motif-containing 22 is essential for the formation of distinct nuclear bodies. FEBS Lett. 2009, 583, 2093–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajaste-Rudnitski, A.; Marelli, S.S.; Pultrone, C.; Pertel, T.; Uchil, P.D.; Mechti, N.; Mothes, W.; Poli, G.; Luban, J.; Vicenzi, E. TRIM22 inhibits HIV-1 transcription independently of its E3 ubiquitin ligase activity, Tat, and NF-kappaB-responsive long terminal repeat elements. J. Virol. 2011, 85, 5183–5196. [Google Scholar] [CrossRef] [Green Version]
- Everett, R.D.; Chelbi-Alix, M.K. PML and PML nuclear bodies: Implications in antiviral defence. Biochimie 2007, 89, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Accolla, R.S. Tripartite Motif 22 and Class II Transactivator Restriction Factors: Unveiling Their Concerted Action against Retroviruses. Front. Immunol. 2017, 8, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpet, A.; Kleijwegt, C.; Roubille, S.; Juillard, F.; Jacquet, K.; Texier, P.; Lomonte, P. PML nuclear bodies and chromatin dynamics: Catch me if you can! Nucleic Acids Res. 2020, 48, 11890–11912. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Tosi, G.; Turrini, F.; Poli, G.; Vicenzi, E.; Accolla, R.S. Tripartite Motif-Containing Protein 22 Interacts with Class II Transactivator and Orchestrates Its Recruitment in Nuclear Bodies Containing TRIM19/PML and Cyclin T1. Front. Immunol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlani, G.; Turrini, F.; Poli, G.; Vicenzi, E.; Accolla, R. P-D2 TRIM22 binds to CIITA and sequesters it into nuclear bodies containing TRIM19/PML and Cyclin T1: Implications for HIV-1 infection. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 77, 59. [Google Scholar] [CrossRef]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef]
- Goodsell, D.S. Illustrations of the HIV life cycle. Curr. Top. Microbiol. Immunol. 2015, 389, 243–252. [Google Scholar] [CrossRef]
- Rice, A.P. The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies. Curr. Pharm. Des. 2017, 23, 4098–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkhout, B.; Silverman, R.H.; Jeang, K.T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 1989, 59, 273–282. [Google Scholar] [CrossRef]
- Feng, S.; Holland, E.C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature 1988, 334, 165–167. [Google Scholar] [CrossRef]
- Cullen, B.R. Regulation of HIV-1 gene expression. FASEB J. 1991, 5, 2361–2368. [Google Scholar] [CrossRef] [Green Version]
- Khoury, G.; Darcis, G.; Lee, M.Y.; Bouchat, S.; Van Driessche, B.; Purcell, D.F.J.; Van Lint, C. The Molecular Biology of HIV Latency. Adv. Exp. Med. Biol. 2018, 1075, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Dahabieh, M.S.; Battivelli, E.; Verdin, E. Understanding HIV latency: The road to an HIV cure. Annu. Rev. Med. 2015, 66, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microbiol. 2016, 14, 55–60. [Google Scholar] [CrossRef]
- Nchioua, R.; Bosso, M.; Kmiec, D.; Kirchhoff, F. Cellular Factors Targeting HIV-1 Transcription and Viral RNA Transcripts. Viruses 2020, 12, 495. [Google Scholar] [CrossRef]
- Jimenez-Guardeno, J.M.; Apolonia, L.; Betancor, G.; Malim, M.H. Immunoproteasome activation enables human TRIM5alpha restriction of HIV-1. Nat. Microbiol. 2019, 4, 933–940. [Google Scholar] [CrossRef]
- Yuan, T.; Yao, W.; Tokunaga, K.; Yang, R.; Sun, B. An HIV-1 capsid binding protein TRIM11 accelerates viral uncoating. Retrovirology 2016, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Yang, T.; Luo, Y.; Wu, L.; Jiang, Y.; Song, Z.; Pan, T.; Liu, B.; Liu, G.; Liu, J.; et al. TRIM28 promotes HIV-1 latency by SUMOylating CDK9 and inhibiting P-TEFb. Elife 2019, 8. [Google Scholar] [CrossRef]
- Ali, H.; Mano, M.; Braga, L.; Naseem, A.; Marini, B.; Vu, D.M.; Collesi, C.; Meroni, G.; Lusic, M.; Giacca, M. Cellular TRIM33 restrains HIV-1 infection by targeting viral integrase for proteasomal degradation. Nat. Commun. 2019, 10, 926. [Google Scholar] [CrossRef]
- Ohainle, M.; Kim, K.; Komurlu Keceli, S.; Felton, A.; Campbell, E.; Luban, J.; Emerman, M. TRIM34 restricts HIV-1 and SIV capsids in a TRIM5alpha-dependent manner. PLoS Pathog. 2020, 16, e1008507. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.A.; Tardif, K.; Mansky, L.M. Anti-HIV-1 activity of Trim 37. J. Gen. Virol. 2014, 95, 960–967. [Google Scholar] [CrossRef] [Green Version]
- Bouazzaoui, A.; Kreutz, M.; Eisert, V.; Dinauer, N.; Heinzelmann, A.; Hallenberger, S.; Strayle, J.; Walker, R.; Rubsamen-Waigmann, H.; Andreesen, R.; et al. Stimulated trans-acting factor of 50 kDa (Staf50) inhibits HIV-1 replication in human monocyte-derived macrophages. Virology 2006, 356, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Wu, J.; Sung, D.Y.; Thompson, W.; Powell, M.; McCarrey, J.; Gibbs, R.; Walker, W. SP1 transcription factors in male germ cell development and differentiation. Mol. Cell Endocrinol. 2007, 270, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.P.; Pan, T.; Zhang, N.; Guo, J.R.; Yang, B.W.; Zhang, D.; Li, J.; Xu, K.; Meng, Z.; He, H. Sp1 promotes dental pulp stem cell osteoblastic differentiation through regulating noggin. Mol. Cell Probes 2020, 50, 101504. [Google Scholar] [CrossRef] [PubMed]
- Obad, S.; Brunnstrom, H.; Vallon-Christersson, J.; Borg, A.; Drott, K.; Gullberg, U. Staf50 is a novel p53 target gene conferring reduced clonogenic growth of leukemic U-937 cells. Oncogene 2004, 23, 4050–4059. [Google Scholar] [CrossRef] [Green Version]
- Deniaud, E.; Baguet, J.; Mathieu, A.L.; Pages, G.; Marvel, J.; Leverrier, Y. Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 2006, 25, 7096–7105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malewicz, M.; Perlmann, T. Function of transcription factors at DNA lesions in DNA repair. Exp. Cell Res. 2014, 329, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrini, F.; Marelli, S.; Kajaste-Rudnitski, A.; Lusic, M.; Van Lint, C.; Das, A.T.; Harwig, A.; Berkhout, B.; Vicenzi, E. HIV-1 transcriptional silencing caused by TRIM22 inhibition of Sp1 binding to the viral promoter. Retrovirology 2015, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Turrini, F.; Saliu, F.; Forlani, G.; Das, A.T.; Van Lint, C.; Accolla, R.S.; Berkhout, B.; Poli, G.; Vicenzi, E. Interferon-inducible TRIM22 contributes to maintenance of HIV-1 proviral latency in T cell lines. Virus Res. 2019, 269, 197631. [Google Scholar] [CrossRef] [Green Version]
- Hotter, D.; Bosso, M.; Jonsson, K.L.; Krapp, C.; Sturzel, C.M.; Das, A.; Littwitz-Salomon, E.; Berkhout, B.; Russ, A.; Wittmann, S.; et al. IFI16 Targets the Transcription Factor Sp1 to Suppress HIV-1 Transcription and Latency Reactivation. Cell Host Microbe 2019, 25, 858–872.e13. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.D.; Smiley, J.R.; Bushman, F.D. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 2008, 4, e1000007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghezzi, S.; Galli, L.; Kajaste-Rudnitski, A.; Turrini, F.; Marelli, S.; Toniolo, D.; Casoli, C.; Riva, A.; Poli, G.; Castagna, A.; et al. Identification of TRIM22 single nucleotide polymorphisms associated with loss of inhibition of HIV-1 transcription and advanced HIV-1 disease. Aids 2013, 27, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Franzoso, G.; Biswas, P.; Poli, G.; Carlson, L.M.; Brown, K.D.; Tomita-Yamaguchi, M.; Fauci, A.S.; Siebenlist, U.K. A family of serine proteases expressed exclusively in myelo-monocytic cells specifically processes the nuclear factor-kappa B subunit p65 in vitro and may impair human immunodeficiency virus replication in these cells. J. Exp. Med. 1994, 180, 1445–1456. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Osterhaus, A.D.; Rimmelzwaan, G.F.; Martina, B.E.; Bestebroer, T.M.; Fouchier, R.A. Influenza B virus in seals. Science 2000, 288, 1051–1053. [Google Scholar] [CrossRef]
- Manuguerra, J.C.; Hannoun, C. Natural infection of dogs by influenza C virus. Res. Virol. 1992, 143, 199–204. [Google Scholar] [CrossRef]
- Cox, N.J.; Subbarao, K. Global epidemiology of influenza: Past and present. Annu. Rev. Med. 2000, 51, 407–421. [Google Scholar] [CrossRef]
- Parrish, C.R.; Kawaoka, Y. The origins of new pandemic viruses: The acquisition of new host ranges by canine parvovirus and influenza A viruses. Ann. Rev. Microbiol. 2005, 59, 553–586. [Google Scholar] [CrossRef]
- Ghendon, Y. Influenza vaccines: A main problem in control of pandemics. Eur. J. Epidemiol. 1994, 10, 485–486. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. The persistent legacy of the 1918 influenza virus. N. Engl. J. Med. 2009, 361, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.L.; Webster, R.G. Pandemic influenza as a current threat. Curr. Top. Microbiol. Immunol. 2009, 333, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Palese, P. Is a Universal Influenza Virus Vaccine Possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Capua, I.; Kajaste-Rudnitski, A.; Bertoli, E.; Vicenzi, E. Pandemic vaccine preparedness--have we left something behind? PLoS Pathog. 2009, 5, e1000482. [Google Scholar] [CrossRef] [Green Version]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, I.; Melen, K.; Nyqvist, M.; Pirhonen, J.; Sareneva, T.; Matikainen, S. Inflammatory responses in influenza A virus infection. Vaccine 2000, 19 (Suppl. S1), S32–S37. [Google Scholar] [CrossRef]
- Brass, A.L.; Huang, I.C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef] [Green Version]
- Karlas, A.; Machuy, N.; Shin, Y.; Pleissner, K.P.; Artarini, A.; Heuer, D.; Becker, D.; Khalil, H.; Ogilvie, L.A.; Hess, S.; et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 2010, 463, 818–822. [Google Scholar] [CrossRef]
- Konig, R.; Stertz, S.; Zhou, Y.; Inoue, A.; Hoffmann, H.H.; Bhattacharyya, S.; Alamares, J.G.; Tscherne, D.M.; Ortigoza, M.B.; Liang, Y.; et al. Human host factors required for influenza virus replication. Nature 2010, 463, 813–817. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrell, L.; Lyall, J.W.; Tiley, L.S.; Fodor, E.; Vreede, F.T. The role and assembly mechanism of nucleoprotein in influenza A virus ribonucleoprotein complexes. Nat. Commun. 2013, 4, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, A.; Kajaste-Rudnitski, A.; Oteiza, A.; Nicora, L.; Towers, G.J.; Mechti, N.; Vicenzi, E. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J. Virol. 2013, 87, 4523–4533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldin, P.; Papon, L.; Oteiza, A.; Brocchi, E.; Lawson, T.G.; Mechti, N. TRIM22 E3 ubiquitin ligase activity is required to mediate antiviral activity against encephalomyocarditis virus. J. Gen. Virol. 2009, 90, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawaoka, Y. Influenza virus-host interactomes as a basis for antiviral drug development. Curr. Opin. Virol. 2015, 14, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, I.; Di Pietro, A.; Oteiza, A.; Ghitti, M.; Mechti, N.; Naffakh, N.; Vicenzi, E. Mutations Conferring Increased Sensitivity to Tripartite Motif 22 Restriction Accumulated Progressively in the Nucleoprotein of Seasonal Influenza A (H1N1) Viruses between 1918 and 2009. mSphere 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Pappas, L.; Foglierini, M.; Piccoli, L.; Kallewaard, N.L.; Turrini, F.; Silacci, C.; Fernandez-Rodriguez, B.; Agatic, G.; Giacchetto-Sasselli, I.; Pellicciotta, G.; et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014, 516, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Fujii, K.; Muramoto, Y.; Yamada, S.; Yamayoshi, S.; Takada, A.; Goto, H.; Horimoto, T.; Kawaoka, Y. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 2007, 81, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Chenavas, S.; Estrozi, L.F.; Slama-Schwok, A.; Delmas, B.; Di Primo, C.; Baudin, F.; Li, X.; Crepin, T.; Ruigrok, R.W. Monomeric nucleoprotein of influenza A virus. PLoS Pathog. 2013, 9, e1003275. [Google Scholar] [CrossRef]
- Marklund, J.K.; Ye, Q.; Dong, J.; Tao, Y.J.; Krug, R.M. Sequence in the influenza A virus nucleoprotein required for viral polymerase binding and RNA synthesis. J. Virol. 2012, 86, 7292–7297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thippamom, N.; Sreta, D.; Kitikoon, P.; Thanawongnuwech, R.; Poovorawan, Y.; Theamboonlers, A.; Suwannakarn, K.; Parchariyanon, S.; Damrongwatanapokin, S.; Amonsin, A. Genetic variations of nucleoprotein gene of influenza A viruses isolated from swine in Thailand. Virol. J. 2010, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, A.C.; de Mutsert, G.; Graus, Y.M.; Fouchier, R.A.; Sintnicolaas, K.; Osterhaus, A.D.; Rimmelzwaan, G.F. Sequence variation in a newly identified HLA-B35-restricted epitope in the influenza A virus nucleoprotein associated with escape from cytotoxic T lymphocytes. J. Virol. 2002, 76, 2567–2572. [Google Scholar] [CrossRef] [Green Version]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Manz, B.; Dornfeld, D.; Gotz, V.; Zell, R.; Zimmermann, P.; Haller, O.; Kochs, G.; Schwemmle, M. Pandemic influenza A viruses escape from restriction by human MxA through adaptive mutations in the nucleoprotein. PLoS Pathog. 2013, 9, e1003279. [Google Scholar] [CrossRef] [PubMed]
- Dornfeld, D.; Petric, P.P.; Hassan, E.; Zell, R.; Schwemmle, M. Eurasian Avian-Like Swine Influenza A Viruses Escape Human MxA Restriction through Distinct Mutations in Their Nucleoprotein. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Cidoncha, M.; Killip, M.J.; Oliveros, J.C.; Asensio, V.J.; Fernandez, Y.; Bengoechea, J.A.; Randall, R.E.; Ortin, J. An unbiased genetic screen reveals the polygenic nature of the influenza virus anti-interferon response. J. Virol. 2014, 88, 4632–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobel Leonard, A.; McClain, M.T.; Smith, G.J.; Wentworth, D.E.; Halpin, R.A.; Lin, X.; Ransier, A.; Stockwell, T.B.; Das, S.R.; Gilbert, A.S.; et al. Deep Sequencing of Influenza A Virus from a Human Challenge Study Reveals a Selective Bottleneck and Only Limited Intrahost Genetic Diversification. J. Virol. 2016, 90, 11247–11258. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhao, X.; Sun, D.; Yang, L.; Chong, C.; Pan, Y.; Chi, X.; Gao, Y.; Wang, M.; Shi, X.; et al. Interferon alpha (IFNalpha)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5A. Cell Mol. Immunol. 2016, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Carocci, M.; Bakkali-Kassimi, L. The encephalomyocarditis virus. Virulence 2012, 3, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Tesh, R.B. The prevalence of encephalomyocarditis virus neutralizing antibodies among various human populations. Am. J. Trop. Med. Hyg. 1978, 27, 144–149. [Google Scholar] [CrossRef]
- Lavanchy, D. The global burden of hepatitis C. Liver Int. 2009, 29 (Suppl. S1), 74–81. [Google Scholar] [CrossRef] [PubMed]
- Moron-Lopez, S.; Gomez-Mora, E.; Salgado, M.; Ouchi, D.; Puertas, M.C.; Urrea, V.; Navarro, J.; Jou, A.; Perez, M.; Tural, C.; et al. Short-term Treatment With Interferon Alfa Diminishes Expression of HIV-1 and Reduces CD4+ T-Cell Activation in Patients Coinfected With HIV and Hepatitis C Virus and Receiving Antiretroviral Therapy. J. Infect. Dis. 2016, 213, 1008–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qashqari, H.; Al-Mars, A.; Chaudhary, A.; Abuzenadah, A.; Damanhouri, G.; Alqahtani, M.; Mahmoud, M.; El Sayed Zaki, M.; Fatima, K.; Qadri, I. Understanding the molecular mechanism(s) of hepatitis C virus (HCV) induced interferon resistance. Infect. Genet. Evol. 2013, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Lin, Y.; Quan, Y.; Xiao, X.; Zhang, R. TRIM22 inhibits respiratory syncytial virus replication by targeting JAK-STAT1/2 signaling. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Meng, J.; Stobart, C.C.; Hotard, A.L.; Moore, M.L. An Overview of Respiratory Syncytial Virus. PLoS Pathog. 2014, 10, e1004016. [Google Scholar] [CrossRef]
- Su, A.I.; Pezacki, J.P.; Wodicka, L.; Brideau, A.D.; Supekova, L.; Thimme, R.; Wieland, S.; Bukh, J.; Purcell, R.H.; Schultz, P.G.; et al. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 2002, 99, 15669–15674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, X.Y.; Ma, W.; Zhang, Y.; Zhao, H.; Deng, Y.; Yuan, W.; Wang, Y.; Li, Y.; Zhu, C.; Liu, M.; et al. Microarray analyses of differentially expressed human genes and biological processes in ECV304 cells infected with rubella virus. J. Med. Virol. 2007, 79, 1783–1791. [Google Scholar] [CrossRef]
- Zhang, J.; Das, S.C.; Kotalik, C.; Pattnaik, A.K.; Zhang, L. The latent membrane protein 1 of Epstein-Barr virus establishes an antiviral state via induction of interferon-stimulated genes. J. Biol. Chem. 2004, 279, 46335–46342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Reddi, T.S.; Merkl, P.E.; Lim, S.Y.; Letvin, N.L.; Knipe, D.M. Tripartite Motif 22 (TRIM22) protein restricts herpes simplex virus 1 by epigenetic silencing of viral immediate-early genes. PLoS Pathog. 2021, 17, e1009281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Tang, Q.; Maul, G.G.; Yuan, Y. Kaposi’s sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: Involvement of host cellular factors. J. Virol. 2008, 82, 2867–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection--natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.T.; Wu, J.Z.; Yang, G. Identification and characterization of multiple TRIM proteins that inhibit hepatitis B virus transcription. PLoS ONE 2013, 8, e70001. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Park, Y.K.; Shin, C.Y.; Park, S.H.; Ahn, S.H.; Kim, D.H.; Lim, K.H.; Kwon, S.Y.; Kim, K.P.; Yang, S.I.; et al. Hepatitis B virus inhibits liver regeneration via epigenetic regulation of urokinase-type plasminogen activator. Hepatology 2013, 58, 762–776. [Google Scholar] [CrossRef]
- Slagle, B.L.; Bouchard, M.J. Hepatitis B Virus X and Regulation of Viral Gene Expression. Cold Spring Harb. Perspect. Med. 2016, 6, a021402. [Google Scholar] [CrossRef] [Green Version]
- Carlson, A.; Norwitz, E.R.; Stiller, R.J. Cytomegalovirus infection in pregnancy: Should all women be screened? Rev. Obstet. Gynecol. 2010, 3, 172–179. [Google Scholar] [PubMed]
- Chang, Y.; Moore, P. Twenty years of KSHV. Viruses 2014, 6, 4258–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charman, M.; McFarlane, S.; Wojtus, J.K.; Sloan, E.; Dewar, R.; Leeming, G.; Al-Saadi, M.; Hunter, L.; Carroll, M.; Stewart, J.P.; et al. Constitutive TRIM22 expression within the respiratory tract identifies tissue-specific and cell-type dependent intrinsic immune barriers to influenza A virus infection. bioRxiv 2019, 679159. [Google Scholar] [CrossRef]
- Cagliani, R.; Forni, D.; Biasin, M.; Comabella, M.; Guerini, F.R.; Riva, S.; Pozzoli, U.; Agliardi, C.; Caputo, D.; Malhotra, S.; et al. Ancient and recent selective pressures shaped genetic diversity at AIM2-like nucleic acid sensors. Genome Biol. Evol. 2014, 6, 830–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggal, N.K.; Emerman, M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 2012, 12, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.S.; Hultquist, J.F.; Evans, D.T. The restriction factors of human immunodeficiency virus. J. Biol. Chem. 2012, 287, 40875–40883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malim, M.H.; Bieniasz, P.D. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb. Perspect. Med. 2012, 2, a006940. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagani, I.; Poli, G.; Vicenzi, E. TRIM22. A Multitasking Antiviral Factor. Cells 2021, 10, 1864. https://doi.org/10.3390/cells10081864
Pagani I, Poli G, Vicenzi E. TRIM22. A Multitasking Antiviral Factor. Cells. 2021; 10(8):1864. https://doi.org/10.3390/cells10081864
Chicago/Turabian StylePagani, Isabel, Guido Poli, and Elisa Vicenzi. 2021. "TRIM22. A Multitasking Antiviral Factor" Cells 10, no. 8: 1864. https://doi.org/10.3390/cells10081864
APA StylePagani, I., Poli, G., & Vicenzi, E. (2021). TRIM22. A Multitasking Antiviral Factor. Cells, 10(8), 1864. https://doi.org/10.3390/cells10081864





