Mast Cells and the Pancreas in Human Type 1 and Type 2 Diabetes
Abstract
:1. Introduction
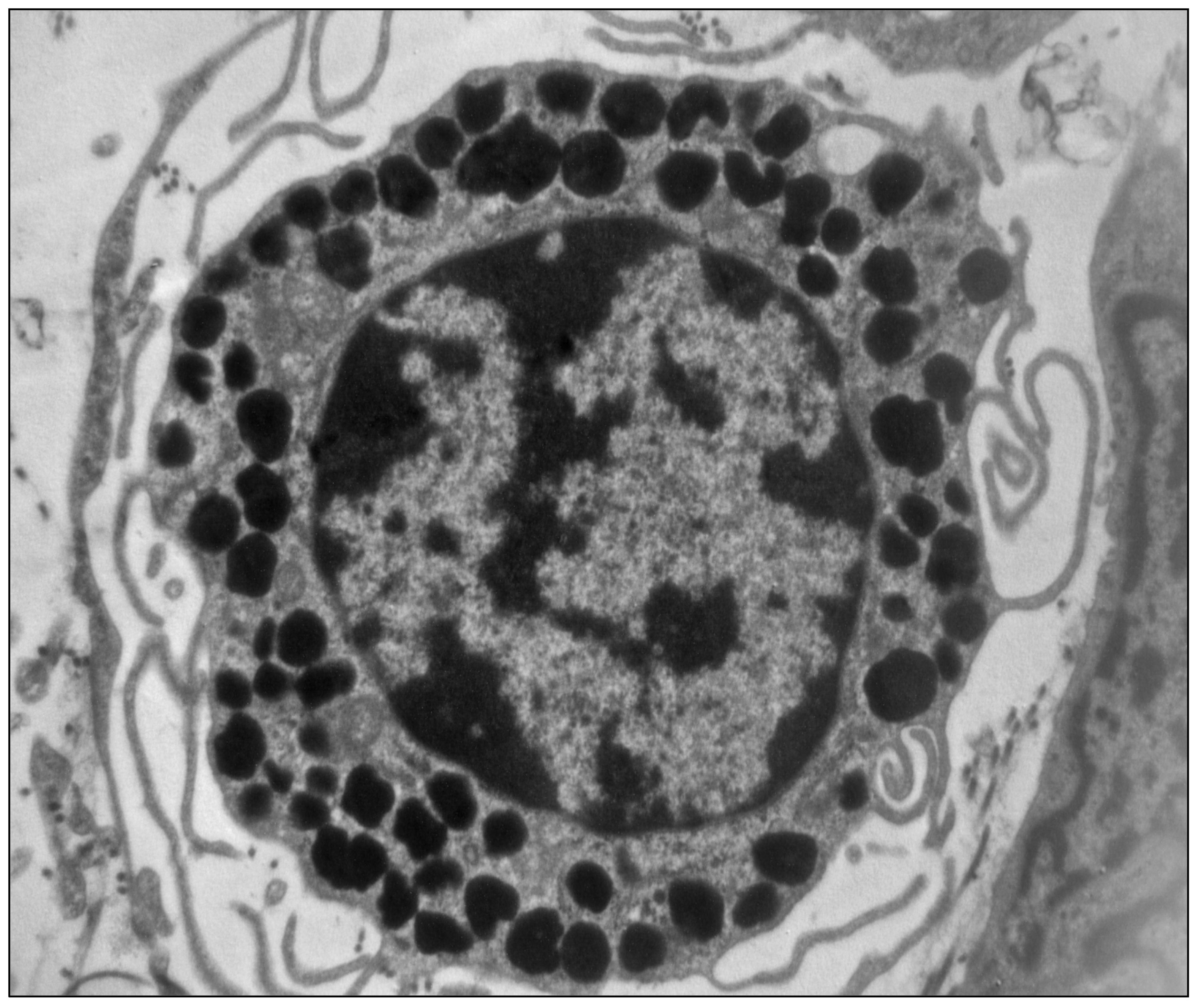
2. The Mast Cells
3. Something on Diabetes Mellitus
- (1)
- Type 1 diabetes, in most patients caused by autoimmune destruction of pancreatic beta cells (type 1A) and in some cases of non-autoimmune, unknown origin (type 1B or idiopathic, also associated with permanent insulinopenia);
- (2)
- Type 2 diabetes, caused by variable degrees of beta cell functional mass loss, often in the background of reduced insulin sensitivity;
- (3)
- Specific types of diabetes, due to several different causes;
- (4)
- Gestational diabetes.
4. Mast Cells in the Pancreas of Type 1 and Type 2 Diabetes
5. Mast Cells in the Pathophysiology of Type 1 Diabetes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrlich, P. Beiträge zut Theorie und Praxis der histologischen Färbung. Inaugural Dissertation. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 1878. [Google Scholar]
- Wong, G.W.; Zhuo, L.; Kimata, K.; Lam, B.K.; Satoh, N.; Stevens, R.L. Ancient origin of mast cells. Biochem. Biophys. Res. Commun. 2014, 451, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. From The Cover: Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, Y.; Oboki, K.; Ito, A. Molecular Mechanisms of Mast Cell Development. Immunol. Allergy Clin. N. Am. 2006, 26, 387–405. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Ekoff, M.; Grootens, J.; Löf, L.; Amini, R.-M.; Hagberg, H.; Ungerstedt, J.S.; Olsson-Strömberg, U.; Nilsson, G. KIT signaling is dispensable for human mast cell progenitor development. Blood 2017, 130, 1785–1794. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef] [Green Version]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endo-thelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental Origin and Functional Specialization of Mast Cell Subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Cildir, G.; Pant, H.; Lopez, A.F.; Tergaonkar, V. The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J. Exp. Med. 2017, 214, 2491–2506. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Schaffer, F. Positive and Negative Signals in Mast Cell Activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Pucillo, C. What we know (and don’t know) about the biology and functions of mast cells and basophils. Immunol. Rev. 2018, 282, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Kawakami, Y.; Kasakura, K.; Kawakami, T. Recent advances in mast cell activation and regulation. F1000Research 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Haidl, I.D.; Marshall, J.S. Human Mast Cell Activation with Viruses and Pathogen Products. Methods Mol. Biol. 2015, 1220, 179–201. [Google Scholar] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein–coupled receptor X2 on mast cell—mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Blokhuis, B.R.; Garssen, J.; Redegeld, F.A. Non-IgE mediated mast cell activation. Eur. J. Pharmacol. 2016, 778, 33–43. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S.; Weng, Z. The “missing link” in autoimmunity and autism: Extracellular mitochon-drial components secreted from activated live mast cells. Autoimmun. Rev. 2013, 12, 1136–1142. [Google Scholar] [CrossRef]
- Tsai, M.; Grimbaldeston, M.; Galli, S.J. Mast Cells and Immunoregulation/Immunomodulation. Adv. Exp. Med. Biol. 2011, 716, 186–211. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef]
- Liu, J.; Fu, T.; Song, F.; Xue, Y.; Xia, C.; Liu, P.; Wang, H.; Zhong, J.; Li, Q.; Chen, J.; et al. Mast Cells Participate in Corneal Development in Mice. Sci. Rep. 2015, 5, 17569. [Google Scholar] [CrossRef] [Green Version]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of Mast Cells and Importance of Their Tryptase and Chymase Serine Proteases in Inflammation and Wound Healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Le Gall, S.; Renault, N.; et al. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Lyons, D.O.; Pullen, N.A. Beyond IgE: Alternative Mast Cell Activation Across Different Disease States. Int. J. Mol. Sci. 2020, 21, 1498. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Kasprick, A.; Petersen, F. Revisiting the role of mast cells in autoimmunity. Autoimmun. Rev. 2015, 14, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.J.; Malone, D.G.; Jicinsky, J.; Chen, M.; Ory, P.; Engber, W.; Graziano, F.M. Human Synovial Mast Cell Involvement in Rheumatoid Arthritis and Osteoarthritis. Relationship to Disease Type, Clinical Activity, and Antirheumatic Therapy. Arthritis Rheum. 1991, 34, 1116–1124. [Google Scholar] [CrossRef]
- Gotis-Graham, I.; Smith, M.D.; Parker, A.; McNeil, H.P. Synovial mast cell responses during clinical improvement in early rheu-matoid arthritis. Ann. Rheum. Dis. 1998, 57, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Mishiro, T.; Takahara, S.; Yokoi, H.; Hamada, D.; Yukata, K.; Takata, Y.; Goto, T.; Egawa, H.; Yasuoka, S.; et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin. Rheumatol. 2007, 26, 1284–1292. [Google Scholar] [CrossRef]
- Rivellese, F.; Nerviani, A.; Rossi, F.W.; Marone, G.; Matucci-Cerinic, M.; De Paulis, A.; Pitzalis, C. Mast cells in rheumatoid arthritis: Friends or foes? Autoimmun. Rev. 2017, 16, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takayanagi, H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 2006, 18, 419–426. [Google Scholar] [CrossRef] [PubMed]
- McFarland, H.F.; Martin, R. Multiple sclerosis: A complicated picture of autoimmunity. Nat. Immunol. 2007, 8, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, N.; Zappulla, J.P.; Lauwers-Cances, V.; Uro-Coste, E.; Delisle, M.-B.; Clanet, M.; Montagne, L.; Van Der Valk, P.; Bö, L.; Liblau, R.S. Mast cell transcripts are increased within and outside multiple sclerosis lesions. J. Neuroimmunol. 2008, 195, 176–185. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Cao, Y. Role of Mast Cells in the Pathogenesis of Multiple Sclerosis and Experimental Autoimmune En-cephalomyelitis. Clin. Rev. Allergy Immunol. 2017, 52, 436–445. [Google Scholar] [CrossRef]
- Rozniecki, J.J.; Hauser, S.L.; Stein, M.; Lincoln, R.; Theoharides, T.C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995, 37, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Redegeld, F.A.; Bischoff, S.C.; Gibbs, B.F.; Maurer, M. Mast Cells as Drivers of Disease and Therapeutic Targets. Trends Immunol. 2018, 39, 151–162. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsai, M.; Galli, S.J. Mast cell–derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Sibilano, R.; Starkl, P.; Roers, A.; Grimbaldeston, M.A.; Tsai, M.; Gaudenzio, N.; Galli, S.J. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2017, 2, 92900. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.Y.; John, A.L.S.; Abraham, S.N. Mast Cell Interleukin-10 Drives Localized Tolerance in Chronic Bladder Infection. Immunity 2013, 38, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Gabryšová, L.; Howes, A.; Saraiva, M.; O’Garra, A. The Regulation of IL-10 Expression. Curr. Top. Microbiol. Immunol. 2014, 380, 157–190. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Marchetti, P.; Suleiman, M.; De Luca, C.; Baronti, W.; Bosi, E.; Tesi, M.; Marselli, L. A direct look at the dysfunction and pathology of the beta cells in human type 2 diabetes. Semin. Cell Dev. Biol. 2020, 103, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Gaglia, J.; Bonner-Weir, S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 249–256. [Google Scholar] [CrossRef]
- Marselli, L.; Suleiman, M.; Masini, M.; Campani, D.; Bugliani, M.; Syed, F.; Martino, L.; Focosi, D.; Scatena, F.; Olimpico, F.; et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 2014, 57, 362–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaghan, M.K.; Polonsky, K.S. Insulin secretion in vivo. In Joslin’s Diabetes Mellitus; Kahn, G., Weir, G.C., King, G.L., Jacobson, A.M., Moses, A.C., Smith, R.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 109–124. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2020, 162, 108086. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the Interna-tional Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [Green Version]
- Godlewski, M. The mast cells of the digestive tract and of the pancreas. Arch. Mal. Appar. Dig. Mal. Nutr. 1959, 48, 1187–1192. [Google Scholar] [PubMed]
- Westermark, P. Mast cells in the islets of langerhans in insular amyloidosis. Virchows Arch. 1971, 354, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mlac, M.; Melato, M.; Marin, G. Mast cells in the islets of Langerhans. A study of their behaviour in connection with diabetes and with insular amyloidosis. Virchows Arch. Pathol. Anat. Histol. 1975, 365, 213–220. [Google Scholar] [CrossRef]
- Esposito, I.; Friess, H.; Kappeler, A.; Shrikhande, S.; Kleeff, J.; Ramesh, H.; Zimmermann, A.; Büchler, M.W. Mast cell distribution and activation in chronic pancreatitis. Hum. Pathol. 2001, 32, 1174–1183. [Google Scholar] [CrossRef]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Goyal, H.; Mishra, A. Food-Induced Acute Pancreatitis. Dig. Dis. Sci. 2017, 62, 3287–3297. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hwang, R.F.; Logsdon, C.D.; Ullrich, S.E. Dynamic Mast Cell—Stromal Cell Interactions Promote Growth of Pancreatic Cancer. Cancer Res. 2013, 73, 3927–3937. [Google Scholar] [CrossRef] [Green Version]
- Martino, L.; Masini, M.; Bugliani, M.; Marselli, L.; Suleiman, M.; Boggi, U.; Nogueira, T.C.; Filipponi, F.; Occhipinti, M.; Campani, D.; et al. Mast cells infiltrate pancreatic islets in human type 1 diabetes. Diabetologia 2015, 58, 2554–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, P.; Pirozzi, N.M.; Wolters, A.H.G.; Kuipers, J.; Kusmartseva, I.; Atkinson, M.A.; Campbell-Thompson, M.; Giepmans, B.N.G. Large-scale electron microscopy database for human type 1 diabetes. Nat. Commun. 2020, 11, 2475. [Google Scholar] [CrossRef]
- Hessner, M.J.; Wang, X.; Meyer, L.; Geoffrey, R.; Jia, S.; Fuller, J.; Lernmark, A.; Ghosh, S. Involvement of eotaxin, eosinophils, and pancreatic predisposition in development of type 1 diabetes mellitus in the BioBreeding rat. J. Immunol. 2004, 173, 6993–7002. [Google Scholar] [CrossRef] [Green Version]
- Geoffrey, R.; Jia, S.; Kwitek, A.; Woodliff, J.; Ghosh, S.; Lernmark, Å.; Wang, X.; Hessner, M.J. Evidence of a Functional Role for Mast Cells in the Development of Type 1 Diabetes Mellitus in the BioBreeding Rat. J. Immunol. 2006, 177, 7275–7286. [Google Scholar] [CrossRef] [Green Version]
- Betto, E.; Usuelli, V.; Mandelli, A.; Badami, E.; Sorini, C.; Capolla, S.; Danelli, L.; Frossi, B.; Guarnotta, C.; Ingrao, S.; et al. Mast cells contribute to autoimmune diabetes by releasing interleukin-6 and failing to acquire a tolerogenic IL-10 + phenotype. Clin. Immunol. 2017, 178, 29–38. [Google Scholar] [CrossRef]
- Carlos, D.; Yaochite, J.N.; Rocha, F.A.; Toso, V.D.; Malmegrim, K.C.; Ramos, S.G.; Jamur, M.C.; Oliver, C.; Camara, N.O.; Andrade, M.V.; et al. Mast cells control insulitis and increase Treg cells to confer protection against STZ-induced type 1 diabe-tes in mice. Eur. J. Immunol. 2015, 45, 2873–2885. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, D.A.; Fu, W.; Schonefeldt, S.; Feyerabend, T.B.; Ortiz-Lopez, A.; Lampi, Y.; Liston, A.; Mathis, D.; Rodewald, H.R. Type 1 di-abetes in NOD mice unaffected by mast cell deficiency. Diabetes 2014, 63, 3827–3834. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Lopez-Perez, D.; Redruello-Romero, A.; Garcia-Rubio, J.; Arana, C.; Garcia-Escudero, L.A.; Tamayo, F.; Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Salmeron, J.; Blanco, A.; et al. In Patients With Obesity, the Number of Adipose Tissue Mast Cells Is Significantly Lower in Subjects with Type 2 Diabetes. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-F.; Xiao, Y.; Sun, L. A Glimpse of the Mechanisms Related to Renal Fibrosis in Diabetic Nephropathy. Adv. Exp. Med. Biol. 2019, 1165, 49–79. [Google Scholar] [CrossRef] [PubMed]

| RODENT | ||
| Characteristic | CTMCs | MMC |
| Size | Larger (10–20 μm) | Smaller (5–10 μm) |
| Granule neutral proteases | Chymase, Tryptase, Proteinase 5, Carboxypeptidase A | Chymase |
| Histamine content | High | Low |
| HUMAN | ||
| Characteristic | MCT | MCTC |
| Distribution | Predominant subtype in small intestinal mucosa and alveoli | Predominant subtype in skin and small intestinal submucosa |
| Granule neutral proteases | Tryptase | Tryptase, chymase, carboxypeptidase, cathepsin G |
| Category | Essential Features |
|---|---|
| Type 1 diabetes | Immune-mediated death of pancreatic beta cells (Type 2) Conspicous/absolute insulin deficiency Includes LADA (Latent Autoimmune Diabetes of Adulthood) Includes idiopatic type 1 diabetes (Type 1B) * |
| Type 2 diabetes | Combination of beta cell dysfunction and death Varying degrees of insulin resistance |
| Specific types | Heterogenous group Mostly associated with beta cell dysfunction/loss Due to genetic or acquired causes |
| Gestational diabetes | Onset during pregnancy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masini, M.; Suleiman, M.; Novelli, M.; Marselli, L.; Marchetti, P.; De Tata, V. Mast Cells and the Pancreas in Human Type 1 and Type 2 Diabetes. Cells 2021, 10, 1875. https://doi.org/10.3390/cells10081875
Masini M, Suleiman M, Novelli M, Marselli L, Marchetti P, De Tata V. Mast Cells and the Pancreas in Human Type 1 and Type 2 Diabetes. Cells. 2021; 10(8):1875. https://doi.org/10.3390/cells10081875
Chicago/Turabian StyleMasini, Matilde, Mara Suleiman, Michela Novelli, Lorella Marselli, Piero Marchetti, and Vincenzo De Tata. 2021. "Mast Cells and the Pancreas in Human Type 1 and Type 2 Diabetes" Cells 10, no. 8: 1875. https://doi.org/10.3390/cells10081875






