Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culturing
2.1.1. 2D Cell Cultures
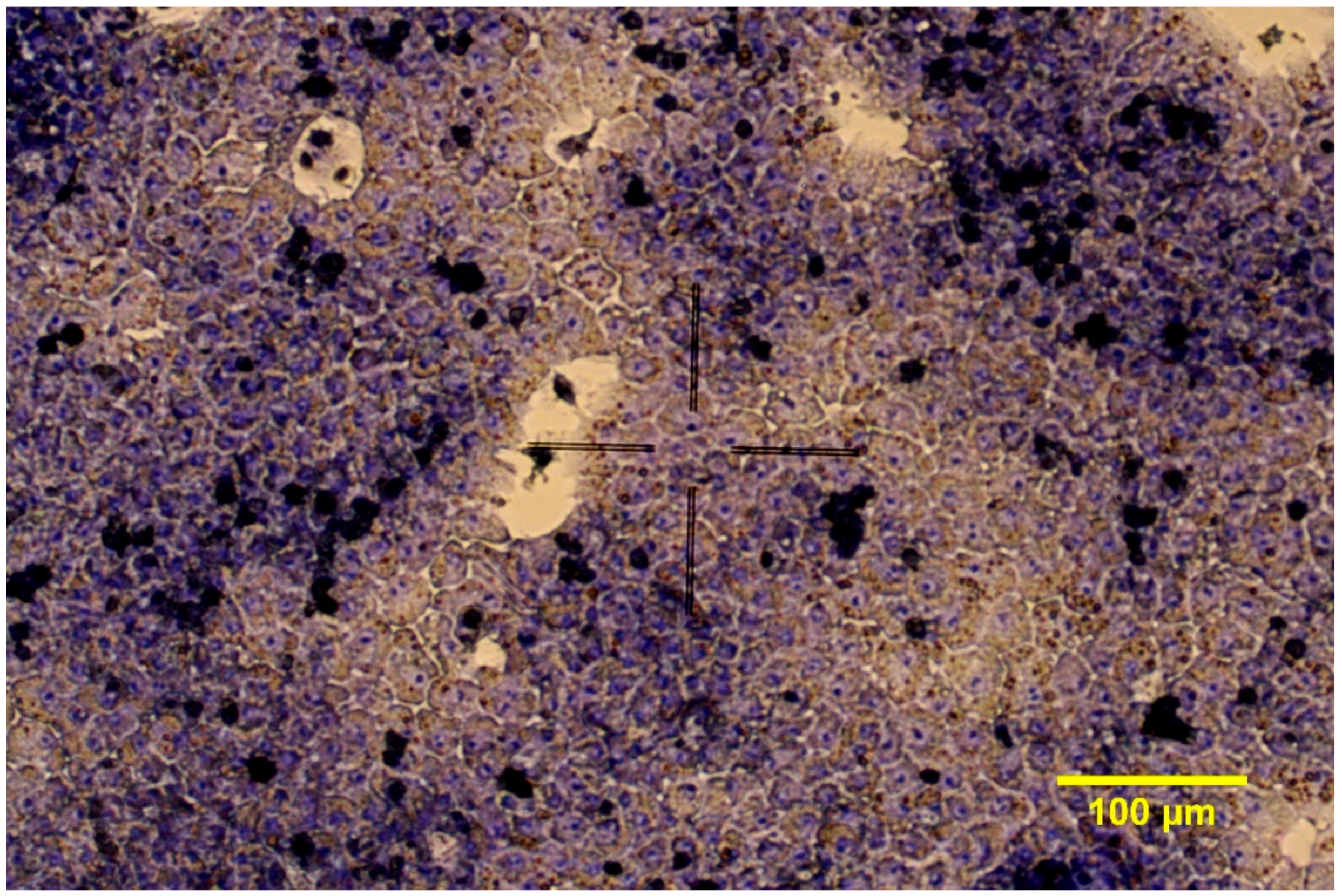
2.1.2. 3D Cell Cultures
2.2. Treatment of Cultured Cells
2.2.1. Study 1
2.2.2. Study 2
2.3. Measurements
2.4. Statistical Analysis
3. Results
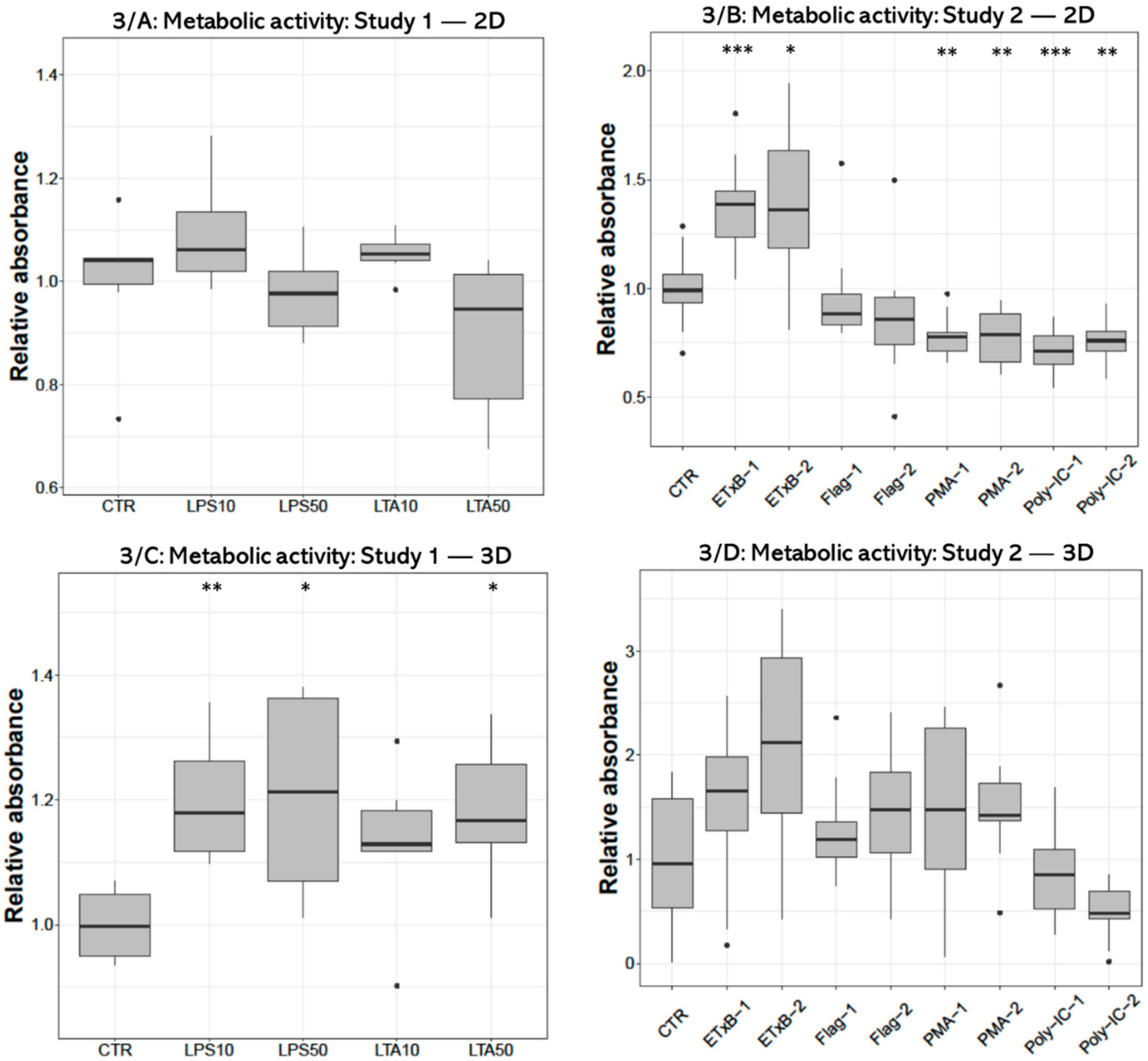
3.1. Metabolic Activity
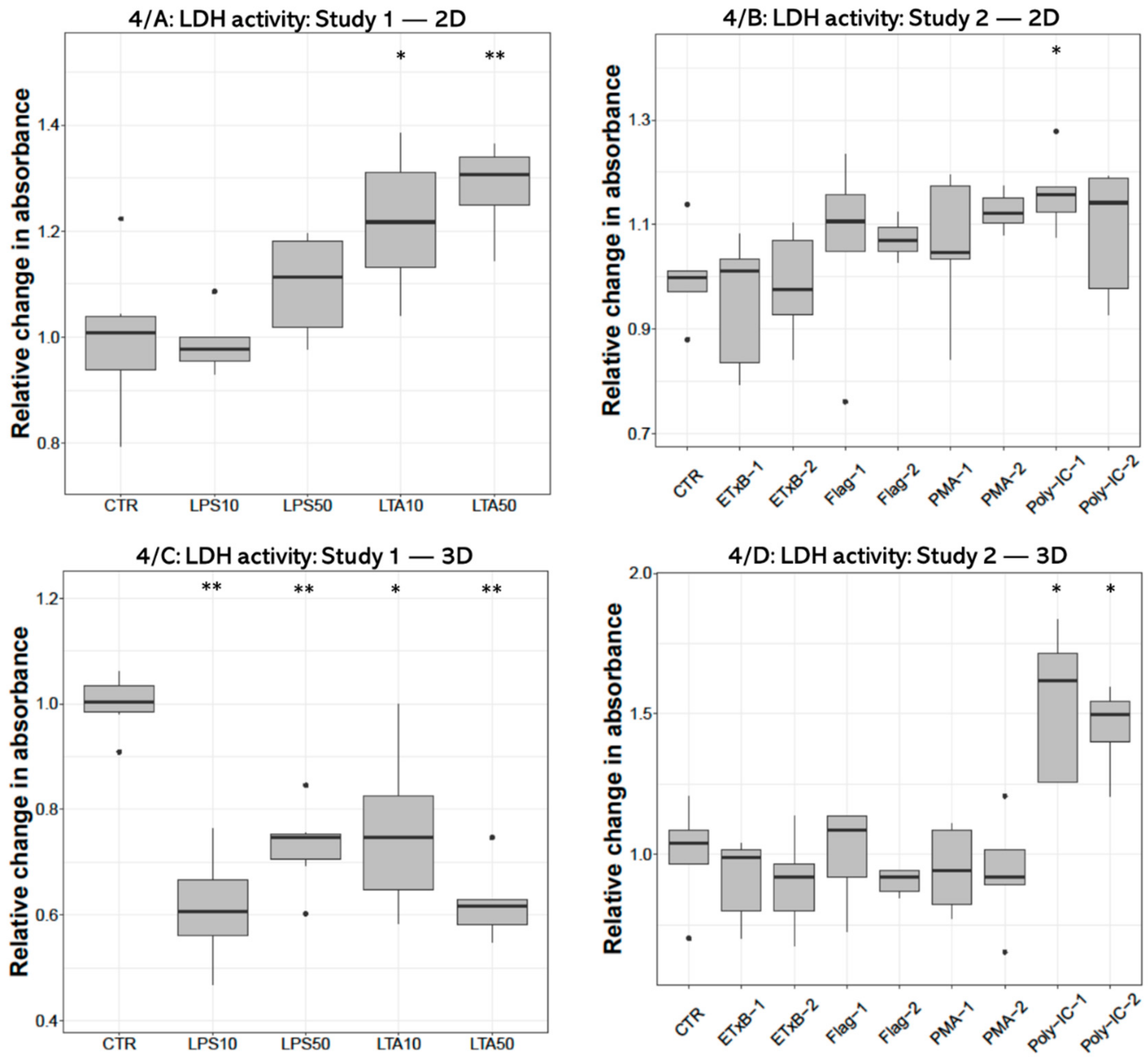
3.2. LDH Activity
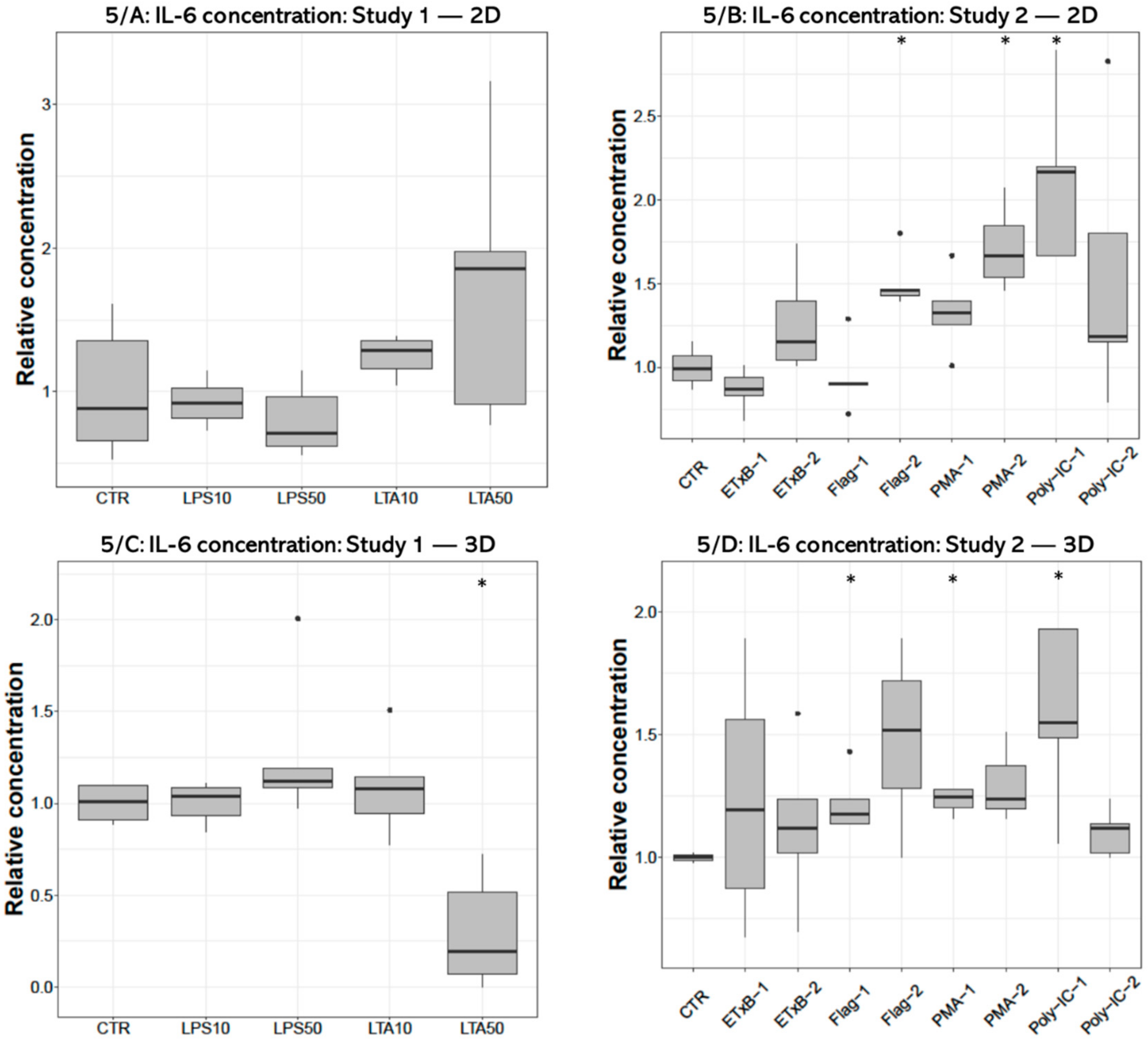
3.3. IL-6 Concentration
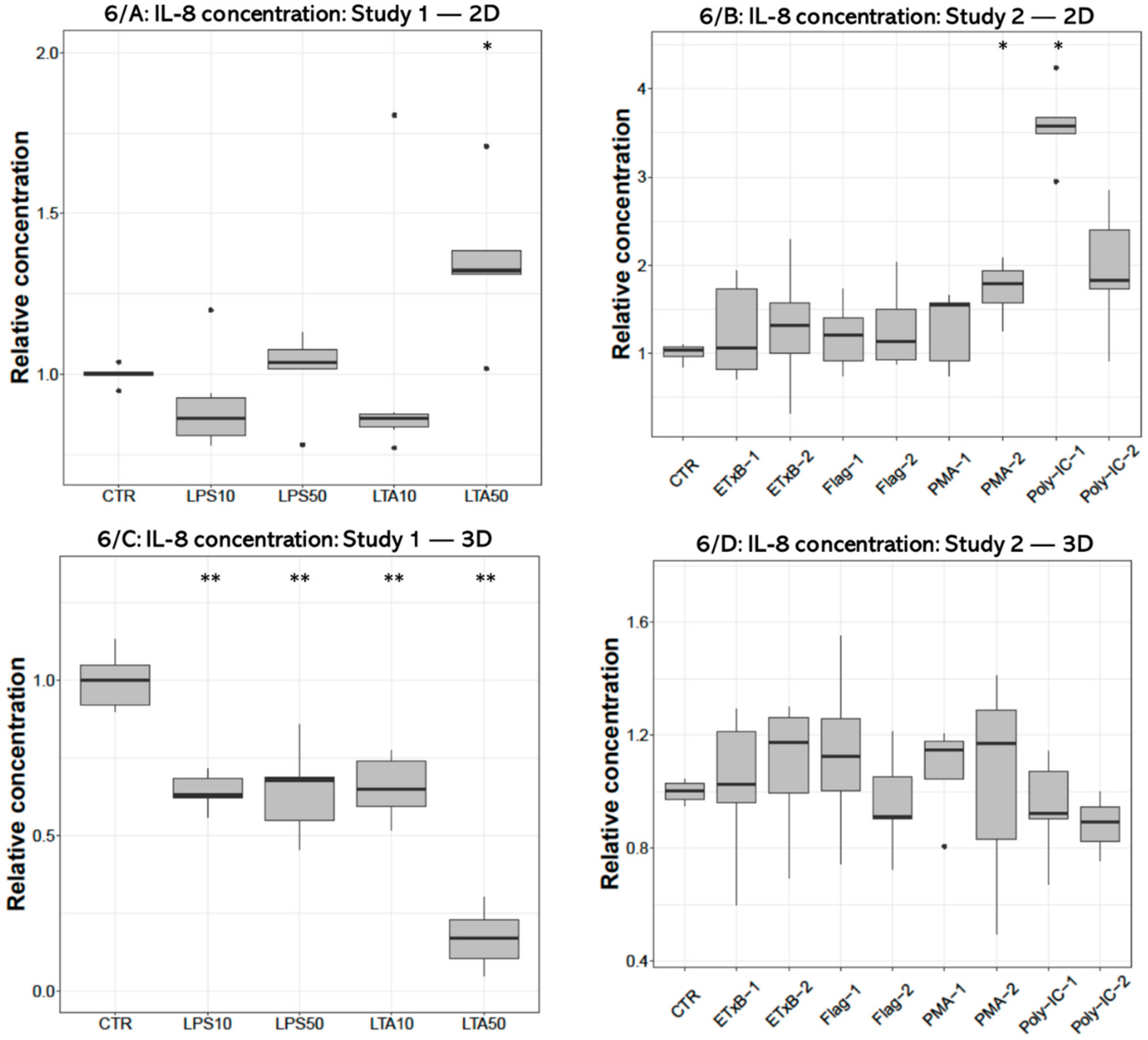
3.4. IL-8 Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brophy, C.M.; Luebke-Wheeler, J.L.; Amiot, B.P.; Khan, H.; Remmel, R.P.; Rinaldo, P.; Nyberg, S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology 2009, 49, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Jeong, W.-I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer Cell Metabolism and Function. J. Enzymol. Metab. 2015, 1, 101. [Google Scholar]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.; Vaezirad, M.M.; van Putten, J.P. Unique features of chicken Toll-like receptors. Dev. Comp. Immunol. 2013, 41, 316–323. [Google Scholar] [CrossRef]
- Kiziltas, S. Toll-like receptors in pathophysiology of liver diseases. World J. Hepatol. 2016, 8, 1354–1369. [Google Scholar] [CrossRef]
- Kannaki, T.; Reddy, M.; Shanmugam, M.; Verma, P.; Sharma, R. Chicken toll-like receptors and their role in immunity. World’s Poult. Sci. J. 2010, 66, 727–738. [Google Scholar] [CrossRef]
- Dickson, K.; Lehmann, C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int. J. Mol. Sci. 2019, 20, 4341. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, I. Role of Lipoteichoic Acid in Infection and Inflammation. The Lancet Infectious Diseases 2002, 2, 171–179. [Google Scholar] [CrossRef]
- Farnell, M.; He, H.; Kogut, M.H. Differential Activation of Signal Transduction Pathways Mediating Oxidative Burst by Chicken Heterophils in Response to Stimulation with Lipopolysaccharide and Lipoteichoic Acid. Inflammation 2003, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J.; Williams, R.; Hirst, T.R.; Williams, N.A. The B Subunit of Escherichia coli Heat-Labile Enterotoxin Induces Both Caspase-Dependent and -Independent Cell Death Pathways in CD8+ T Cells. Infect. Immun. 2004, 72, 5850–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, M.H.; Iqbal, M.; He, H.; Philbin, V.; Kaiser, P.; Smith, A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005, 29, 791–807. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Yu, Y.; Krishna, U.S.; Israel, D.A.; Lyons, S.L.; Peek, J.R.M.; Peek, R.M. Helicobacter pylori Flagellin Evades Toll?Like Receptor 5–Mediated Innate Immunity. J. Infect. Dis. 2004, 189, 1914–1920. [Google Scholar] [CrossRef] [Green Version]
- Fliegmann, J.; Felix, G. Immunity: Flagellin seen from all sides. Nat. Plants 2016, 2, 16136. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Jeon, S.; Hui, Z.; Kim, Y.; Lim, W.; Kim, C.; Choi, H.; Kim, O. Anti-inflammatory effects of zinc in PMA-treated human gingival fibroblast cells. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e180–e187. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Wang, C.; Qu, L.; Deng, J.; Wang, H.; Qin, Z. Resolution of PMA-Induced Skin Inflammation Involves Interaction of IFN-γand ALOX15. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Farnell, M.; Kogut, M.H. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 177–184. [Google Scholar] [CrossRef]
- Li, K.; Chen, Z.; Kato, N.; Gale, M.; Lemon, S.M. Distinct Poly(I-C) and Virus-activated Signaling Pathways Leading to Interferon-β Production in Hepatocytes. J. Biol. Chem. 2005, 280, 16739–16747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Funami, K.; Tanabe, M.; Oshiumi, H.; Shingai, M.; Seto, Y.; Yamamoto, A.; Seya, T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 2003, 171, 3154–3162. [Google Scholar] [CrossRef] [Green Version]
- Aviagen Ross Broiler Management Handbook 2018. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 16 November 2020).
- Mackei, M.; Molnár, A.; Nagy, S.; Pál, L.; Kővágó, C.; Gálfi, P.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Effects of Acute Heat Stress on a Newly Established Chicken Hepatocyte—Nonparenchymal Cell Co-Culture Model. Animals 2020, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Development of a chicken ileal explant culture model for measurement of gut inflammation induced by lipopolysaccharide. Poult. Sci. 2017, 96, 3096–3103. [Google Scholar] [CrossRef]
- Kalaiyarasu, S.; Bhatia, S.; Mishra, N.; Sood, R.; Kumar, M.; Senthilkumar, D.; Bhat, S.; Prakash, M.D. Elevated level of pro inflammatory cytokine and chemokine expression in chicken bone marrow and monocyte derived dendritic cells following LPS induced maturation. Cytokine 2016, 85, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J.; Pitman, R.S.; Jimi, E.; Soriani, M.; Hirst, T.R.; Ghosh, S.; Rincon, M.; Williams, N.A. CD8+ T Cell Apoptosis Induced by Escherichia coli Heat-labile Enterotoxin B Subunit Occurs via a Novel Pathway Involving NF-κB-dependent Caspase Activation. Eur. J. Immunol. 2002, 32, 1737–1747. [Google Scholar] [CrossRef]
- Turcanu, V.; Hirst, T.R.; Williams, N.A. Modulation of human monocytes by Escherichia coli heat-labile enterotoxin B-subunit; altered cytokine production and its functional consequences. Immunology 2002, 106, 316–325. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Isobe, N.; Yoshimura, Y. Effects of different TLR ligands on the expression of proinflammatory cytokines and avian β-defensins in the uterine and vaginal tissues of laying hens. Vet. Immunol. Immunopathol. 2014, 162, 132–141. [Google Scholar] [CrossRef]
- Kamimura, T.; Isobe, N.; Yoshimura, Y. Effects of inhibitors of transcription factors, nuclear factor-κB and activator protein 1, on the expression of proinflammatory cytokines and chemokines induced by stimulation with Toll-like receptor ligands in hen vaginal cells. Poult. Sci. 2017, 96, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.K.; Tseng, H.; Souza, G.R. Assembly of Hepatocyte Spheroids Using Magnetic 3D Cell Culture for CYP450 Inhibition/Induction. Int. J. Mol. Sci. 2017, 18, 1085. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal Stem Cells Support Migration, Extracellular Matrix Invasion, Proliferation, and Survival of Endothelial Cells In Vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef]
- Seno, K.; Munakata, Y.; Sano, M.; Kawahara-Miki, R.; Takahashi, H.; Ohkuchi, A.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Aggregation of Human Trophoblast Cells into Three-Dimensional Culture System Enhances Anti-Inflammatory Characteristics through Cytoskeleton Regulation. Int. J. Mol. Sci. 2018, 19, 2322. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, H.; Li, H.; Wu, Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J. Cell. Mol. Med. 2017, 21, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. AMS Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Corrò, C.; Novellasdemunt, L.; Li, V.S. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Tsunedomi, R.; Hazama, S.; Nagano, H.; Yoshida, T.; Shibutani, M.; Ichikawa, R.; et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials 2020, 237, 119823. [Google Scholar] [CrossRef] [PubMed]
- Abugomaa, A.; Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Mori, T.; Uchide, T.; Azakami, D.; Fukushima, R.; Yoshida, T.; et al. Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture. Sci. Rep. 2020, 10, 9393. [Google Scholar] [CrossRef] [PubMed]
- Abugomaa, A.; Elbadawy, M. Patient-derived organoid analysis of drug resistance in precision medicine: Is there a value? Expert Rev. Precis. Med. Drug Dev. 2020, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Elbadawy, M.; Sato, Y.; Mori, T.; Goto, Y.; Hayashi, K.; Yamanaka, M.; Azakami, D.; Uchide, T.; Fukushima, R.; Yoshida, T.; et al. Anti-tumor effect of trametinib in bladder cancer organoid and the underlying mechanism. Cancer Biol. Ther. 2021, 1–15. [Google Scholar] [CrossRef]
- Elbadawy, M.; Abugomaa, A.; Yamawaki, H.; Usui, T.; Sasaki, K. Development of Prostate Cancer Organoid Culture Models in Basic Medicine and Translational Research. Cancers 2020, 12, 777. [Google Scholar] [CrossRef] [Green Version]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackei, M.; Orbán, K.; Molnár, A.; Pál, L.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Cellular Effects of T-2 Toxin on Primary Hepatic Cell Culture Models of Chickens. Toxins 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mátis, G.; Kulcsár, A.; Petrilla, J.; Talapka, P.; Neogrády, Z. Porcine hepatocyte-Kupffer cell co-culture as an in vitro model for testing the efficacy of anti-inflammatory substances. J. Anim. Physiol. Anim. Nutr. 2016, 101, 201–207. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Jackson, S.; Haycock, J.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef]
- Walker, T.M.; Rhodes, P.C.; Westmoreland, C. The differential cytotoxicity of methotrexate in rat hepatocyte monolayer and spheroid cultures. Toxicol. Vitr. 2000, 14, 475–485. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Tacke, F. Relevance of Autophagy in Parenchymal and Non-Parenchymal Liver Cells for Health and Disease. Cells 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Perry, C.N.; Huang, C.; Iwai-Kanai, E.; Carreira, R.S.; Glembotski, C.C.; Gottlieb, R.A. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H470–H479. [Google Scholar] [CrossRef] [Green Version]
- Luhr, M.; Szalai, P.; Engedal, N. The Lactate Dehydrogenase Sequestration Assay—A Simple and Reliable Method to Determine Bulk Autophagic Sequestration Activity in Mammalian Cells. J. Vis. Exp. JoVE 2018, 137, e57971. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.D.; Lee, C.; Billips, B.K.; Habermacher, G.M.; Zhang, Q.; Liu, V.; Wong, L.Y.; Klumpp, D.J.; Thumbikat, P. The toll-like receptor pathway: A novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate 2008, 68, 223–229. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.-Y.; Qiao, J.; Lu, C.-Z.; Xiao, B.-G. Granulocyte-colony stimulating factor is involved in low-dose LPS-induced neuroprotection. Neurosci. Lett. 2009, 465, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Kulkarni, R. (Ravi); Sharif, S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev. Comp. Immunol. 2011, 35, 28–34. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, S.-M.; Suk, K.; Lee, W.-H. A Novel Pathway Responsible for Lipopolysaccharide-Induced Translational Regulation of TNF-α and IL-6 Expression Involves Protein Kinase C and Fascin. J. Immunol. 2011, 187, 6327–6334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnault, E.; Bonsergent, C.; Larcher, T.; Bed’Hom, B.; Vautherot, J.-F.; Delaleu, B.; Guigand, L.; Soubieux, D.; Marc, D.; Quéré, P. A novel chicken lung epithelial cell line: Characterization and response to low pathogenicity avian influenza virus. Virus Res. 2011, 159, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Ciraci, C.; Chang, G.; Hu, J.; Lamont, S.J. NLRC5 knockdown in chicken macrophages alters response to LPS and poly (I:C) stimulation. BMC Vet. Res. 2012, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keestra, A.M.; van Putten, J. Unique Properties of the Chicken TLR4/MD-2 Complex: Selective Lipopolysaccharide Activation of the MyD88-Dependent Pathway. J. Immunol. 2008, 181, 4354–4362. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin Alleviates Lipopolysaccharide-Induced Liver Inflammation in Chicken by Suppressing TLR4-Mediated NF-κB Pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardiiandBacillus subtilisB10 Modulate TLRs Mediated Signaling to Induce Immunity by Chicken BMDCs. J. Cell. Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rola-Pleszczynski, M.; Stankova, J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: Transcriptional and posttranscriptional mechanisms. Blood 1992, 80, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct Global Gene Expression Patterns and Functional Phenotypes. Tissue Eng. Part A 2008, 15, 559–567. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebők, C.; Tráj, P.; Vörösházi, J.; Mackei, M.; Papp, M.; Gálfi, P.; Neogrády, Z.; Mátis, G. Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells 2021, 10, 1910. https://doi.org/10.3390/cells10081910
Sebők C, Tráj P, Vörösházi J, Mackei M, Papp M, Gálfi P, Neogrády Z, Mátis G. Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells. 2021; 10(8):1910. https://doi.org/10.3390/cells10081910
Chicago/Turabian StyleSebők, Csilla, Patrik Tráj, Júlia Vörösházi, Máté Mackei, Márton Papp, Péter Gálfi, Zsuzsanna Neogrády, and Gábor Mátis. 2021. "Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures" Cells 10, no. 8: 1910. https://doi.org/10.3390/cells10081910
APA StyleSebők, C., Tráj, P., Vörösházi, J., Mackei, M., Papp, M., Gálfi, P., Neogrády, Z., & Mátis, G. (2021). Two Sides to Every Question: Attempts to Activate Chicken Innate Immunity in 2D and 3D Hepatic Cell Cultures. Cells, 10(8), 1910. https://doi.org/10.3390/cells10081910





