Gradual Response of Cyanobacterial Thylakoids to Acute High-Light Stress—Importance of Carotenoid Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and High-Light Treatment
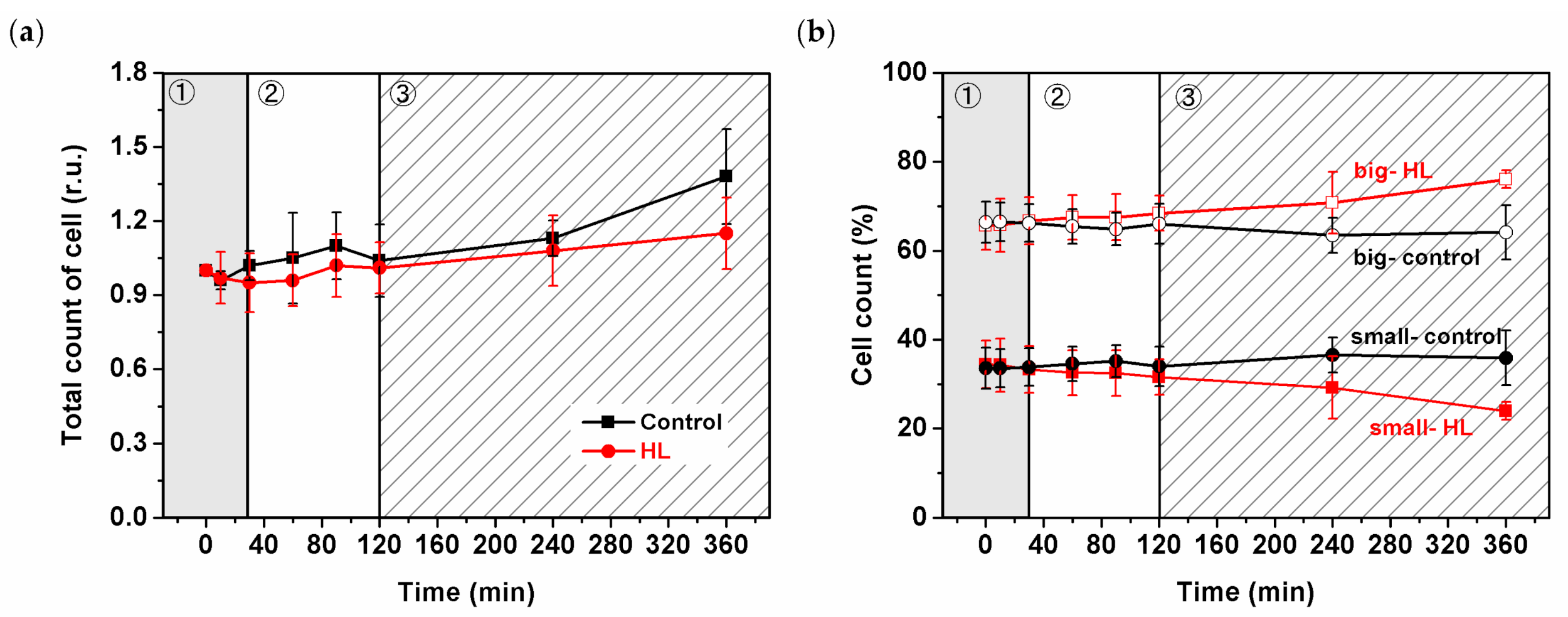
2.2. Characterization of Cell Sizes, Types and Counts
2.3. Biochemical Analysis of Pigment–Protein Complexes
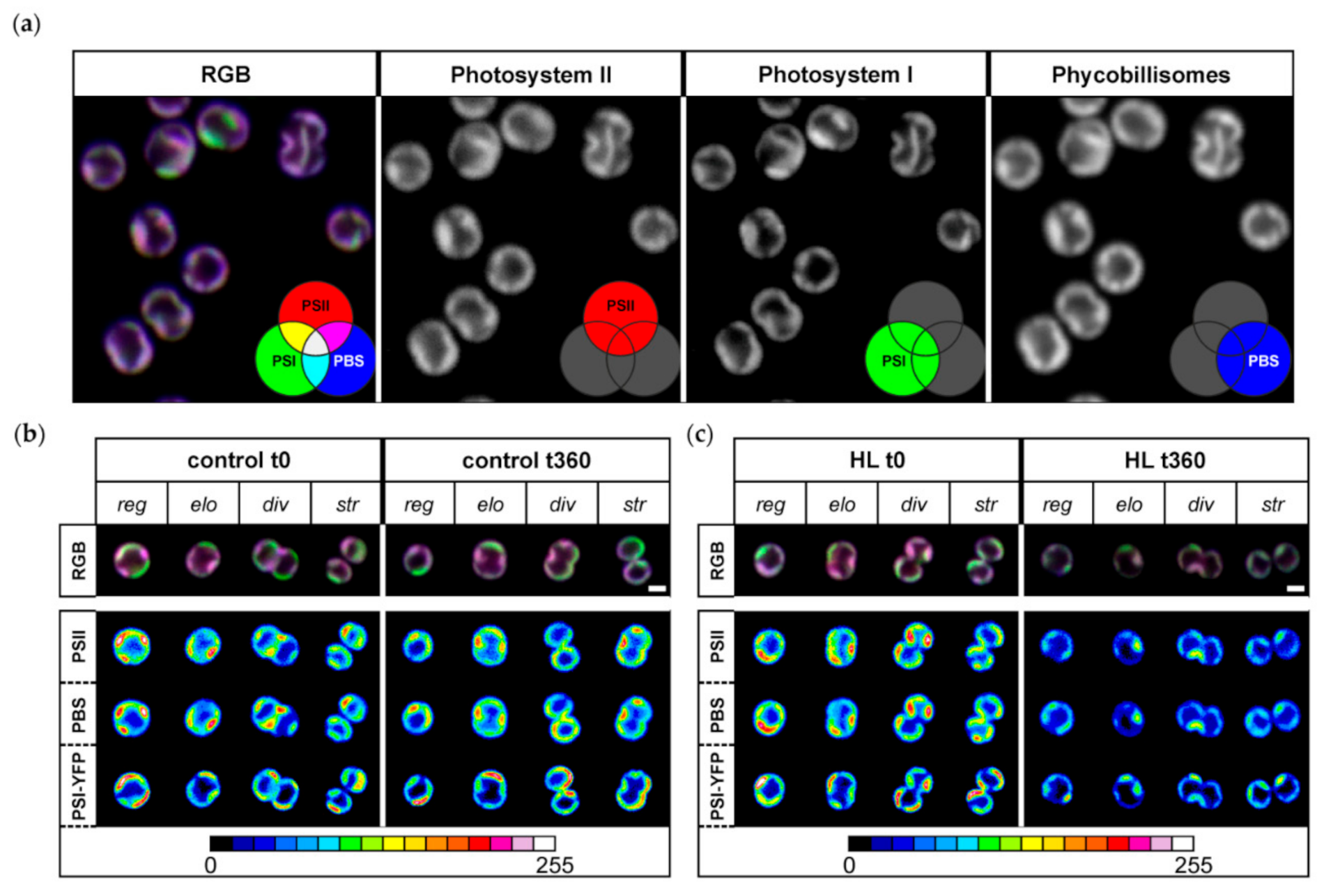
2.4. Confocal Microscopy
2.5. Measurements of Variable Chlorophyll a Fluorescence
2.6. Fluorescence and Absorption Spectroscopy
2.7. Characterization of Pigment Composition
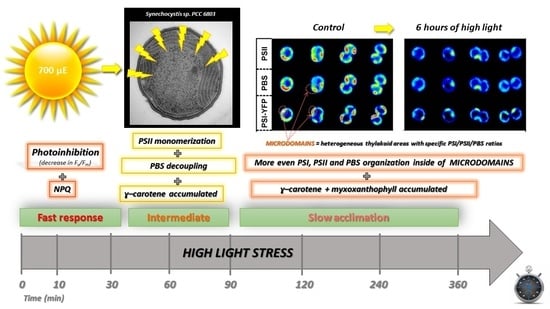
3. Results
3.1. High-Light Treatment and Cyanobacterial Cell Size
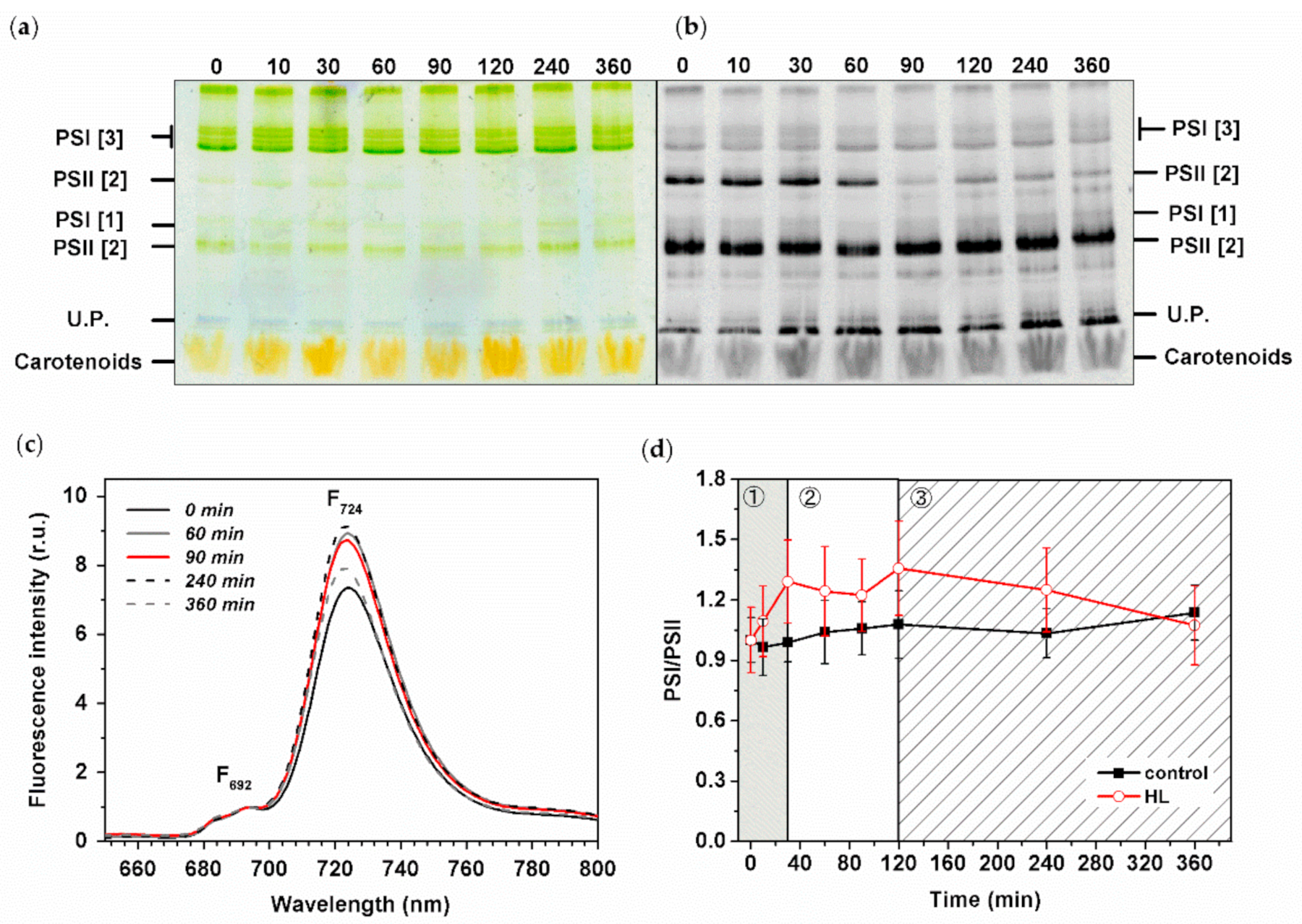
3.2. Changes in Organization of Pigment–Protein Complexes during HL Treatment
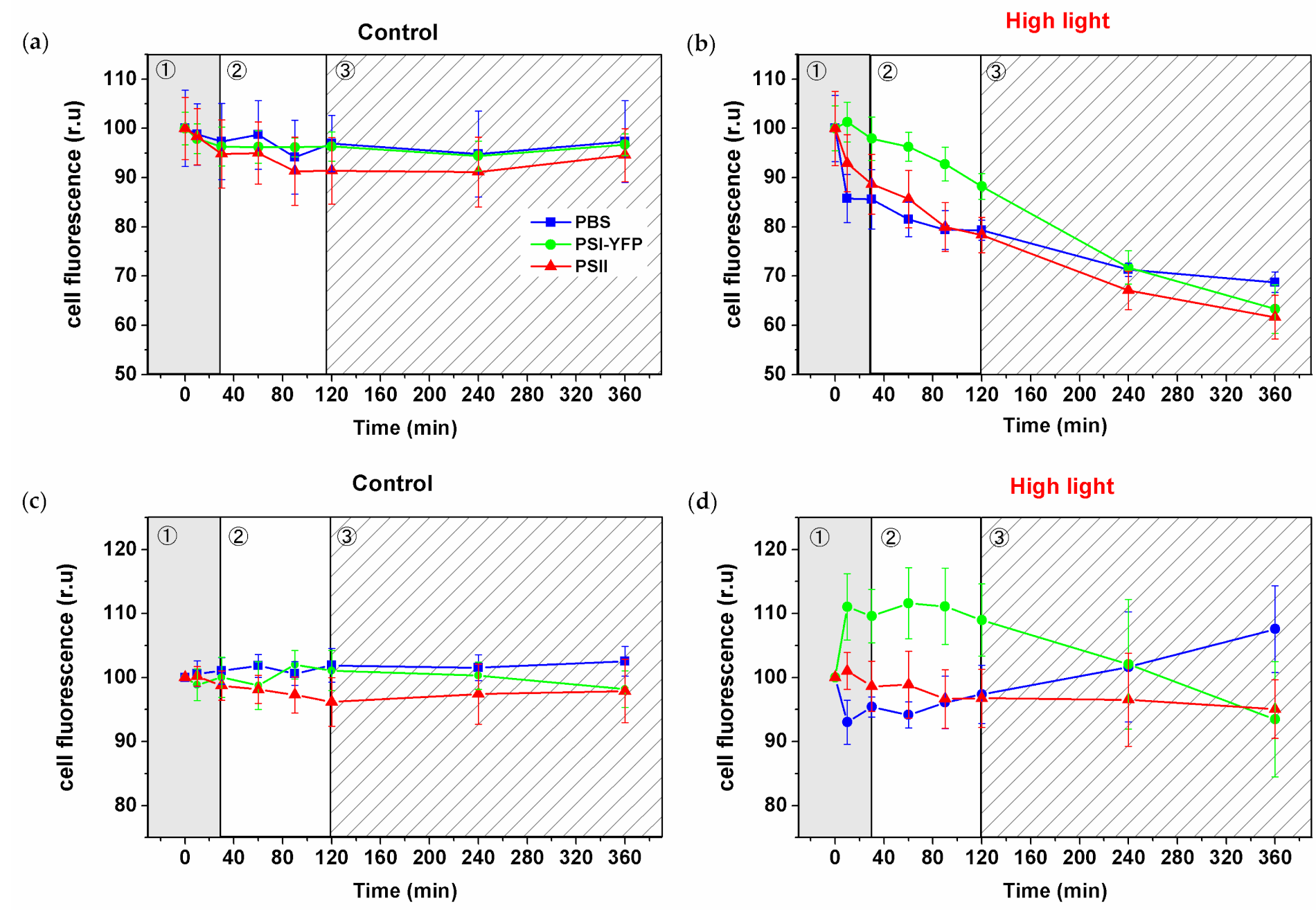
3.3. Changes in the Single-Cell Fluorescence during HL Stress
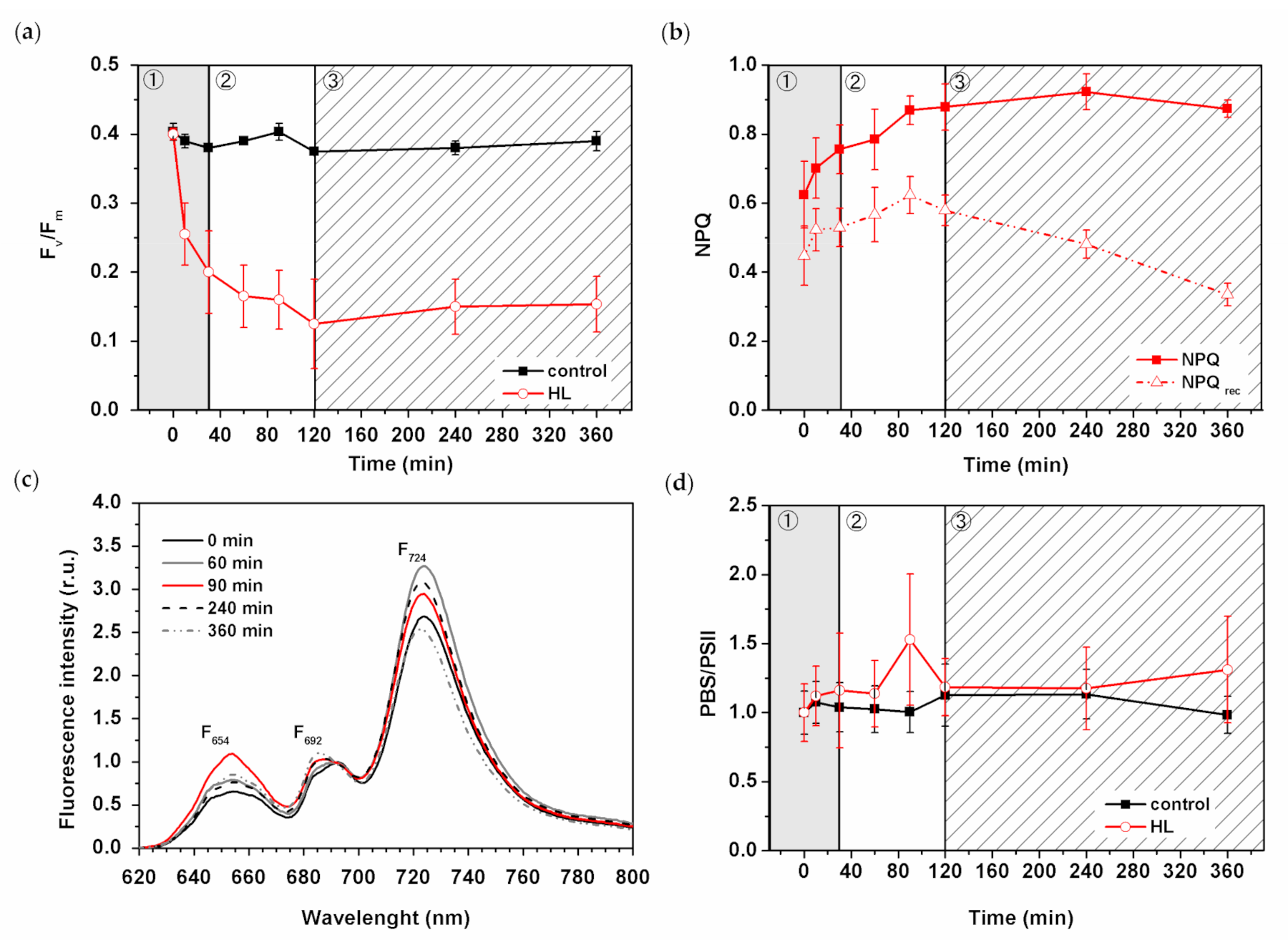
3.4. Effect of HL Treatment on Photosystem II Photochemistry
3.5. Effect of HL Treatment on PPCs Levels
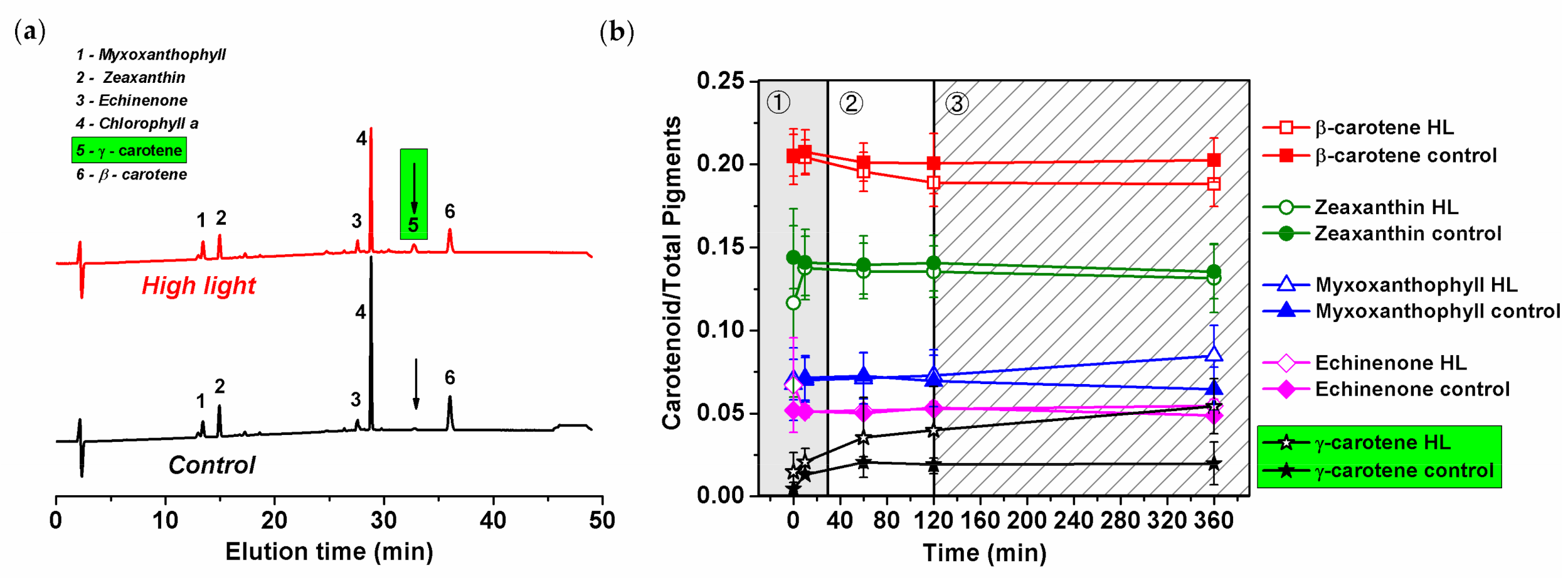
3.6. HL Effect on Pigment Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strašková, A.; Steinbach, G.; Konert, G.; Kotabová, E.; Komenda, J.; Tichý, M.; Kaňa, R. Pigment-protein complexes are organized into stable microdomains in cyanobacterial thylakoids. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1860. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Young, A.J.; Pascal, A.A.; Horton, P. The Effects of lllumination on the Xanthophyll Composition of the Photosystem II Light-Harvesting Complexes of Spinach Thylakoid Membranes. Plant Physiol 1994, 104, 227–234. [Google Scholar] [CrossRef]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Grigoryeva, N.; Chistyakova, L. Fluorescence Microscopic Spectroscopy for Investigation and Monitoring of Biological Diversity and Physiological State of Cyanobacterial Cultures. Cyanobacteria. Rij. IntechOpen 2018, 11–43. [Google Scholar] [CrossRef]
- Konert, G.; Steinbach, G.; Canonico, M.; Kaňa, R. Protein arrangement factor: A new photosynthetic parameter characterizing the organization of thylakoid membrane proteins. Physiol Plant. 2019, 166, 264–277. [Google Scholar] [CrossRef]
- Canonico, M.; Konert, G.; Kaňa, R. Plasticity of Cyanobacterial Thylakoid Microdomains Under Variable Light Conditions. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Andersson, B.; Anderson, J.M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 1980, 593, 427–440. [Google Scholar] [CrossRef]
- Albertsson, P. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 2001, 6, 349–358. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Visualizing the dynamic structure of the plant photosynthetic membrane. Nat. Plants 2015, 1, 15161. [Google Scholar] [CrossRef]
- Vermaas, W.F.J.; Timlin, J.A.; Jones, H.D.T.; Sinclair, M.B.; Nieman, L.T.; Hamad, S.W.; Melgaard, D.K.; Haaland, D.M. In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 4050–4055. [Google Scholar] [CrossRef]
- Sherman, D.M.; Troyan, T.A.; Sherman, L.A. Localization of membrane-proteins in the cyanobacterium synechococcus sp pcc7942-radial asymmetry in the photosynthetic complexes. Plant Physiol. 1994, 106, 251–262. [Google Scholar] [CrossRef][Green Version]
- Huokko, T.; Ni, T.; Dykes, G.F.; Simpson, D.M.; Brownridge, P.; Conradi, F.D.; Beynon, R.J.; Nixon, P.J.; Mullineaux, C.W.; Zhang, P.; et al. Probing the biogenesis pathway and dynamics of thylakoid membranes. Nat. Commun. 2021, 12, 3475. [Google Scholar] [CrossRef]
- Mahbub, M.; Hemm, L.; Yang, Y.; Kaur, R.; Carmen, H.; Engl, C.; Huokko, T.; Riediger, M.; Watanabe, S.; Liu, L.N.; et al. mRNA localization, reaction centre biogenesis and thylakoid membrane targeting in cyanobacteria. Nat. Plants 2020, 6, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, C.W.; Liu, L.-N. Membrane Dynamics in Phototrophic Bacteria. Annu. Rev. Microbiol. 2020, 74, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Sarcina, M.; Bouzovitis, N.; Mullineaux, C.W. Mobilization of photosystem II induced by intense red light in the cyanobacterium Synechococcus sp PCC7942. Plant Cell 2006, 18, 457–464. [Google Scholar] [CrossRef]
- Tamary, E.; Kiss, V.; Nevo, R.; Adam, Z.; Bernat, G.; Rexroth, S.; Rogner, M.; Reich, Z. Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 319–327. [Google Scholar] [CrossRef]
- Steinbach, G.; Kaňa, R. Automated microscopy: Macro language controlling a confocal microscope and its external illumination–adaptation for photosynthetic organisms. Microsc. Microanal. 2016, 22, 258–263. [Google Scholar] [CrossRef]
- Kirilovsky, D. Modulating energy arriving at photochemical reaction centers: Orange carotenoid protein-related photoprotection and state transitions. Photosynth. Res. 2015, 126, 3–17. [Google Scholar] [CrossRef]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition-a historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Kaňa, R.; Prášil, O. Mechanisms Modulating Energy Arriving at Reaction Centers in Cyanobacteria. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 471–501. [Google Scholar] [CrossRef]
- Kirilovsky, D. Photoprotection in cyanobacteria: The orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth. Res. 2007, 93, 7–16. [Google Scholar] [CrossRef]
- Kok, B. On the Inhibition of Photosynthesis by Intense Light. Biochim Biophys. Acta 1956, 21, 234–244. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of Photosystem I: Its Physiological Significance in the Chilling Sensitivity of Plants. Plant Cell Physiol. 1996, 37, 239–247. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [CrossRef]
- Keren, N.; Krieger-Liszkay, A. Photoinhibition: Molecular mechanisms and physiological significance. Physiol. Plant. 2011, 142, 1–5. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ. 2003, 26, 505–512. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjarvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta 2012, 1817, 258–265. [Google Scholar] [CrossRef]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Komenda, J.; Sobotka, R.; Nixon, P.J. Assembling and maintaining the Photosystem II complex in chloroplasts and cyanobacteria. Curr. Opin. Plant Biol. 2012, 15, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Prasil, O.; Adir, N.; Ohad, I. Dynamics of photosystem II: Mechanism of photoinhibition and recovery processes. Top. Photosynth. 1992, 11, 295–348. [Google Scholar]
- Komenda, J.; Tichy, M.; Prásil, O.; Knoppová, J.; Kuviková, S.; de Vries, R.; Nixon, P.J. The exposed N-terminal tail of the D1 subunit is required for rapid D1 degradation during photosystem II repair in Synechocystis sp PCC 6803. Plant Cell 2007, 19, 2839–2854. [Google Scholar] [CrossRef]
- Komenda, J. Role of two forms of the D1 protein in the recovery from photoinhibition of photosystem II in the cyanobacterium Synechococcus PCC 7942. Biochim. Biophys. Acta 2000, 1457, 243–252. [Google Scholar] [CrossRef]
- Stoitchkova, K.; Zsiros, O.; Jávorfi, T.; Páli, T.; Andreeva, A.; Gombos, Z.; Garab, G. Heat- and light-induced reorganizations in the phycobilisome antenna of Synechocystis sp. PCC 6803. Thermo-optic effect. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 750–756. [Google Scholar] [CrossRef]
- Kaňa, R.; Prášil, O.; Komárek, O.; Papageorgiou, G.; Govindjee. Spectral characteristic of fluorescence induction in a model cyanobacterium, Synechococcus sp (PCC 7942). Biochim. Biophys. Acta 2009, 1787, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Oquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. MMBR 1998, 62, 667–683. [Google Scholar] [CrossRef]
- Cser, K.; Vass, I. Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim. Biophys. Acta 2007, 1767, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Stadnichuk, I.N.; Yanyushin, M.F.; Bernát, G.; Zlenko, D.V.; Krasilnikov, P.M.; Lukashev, E.P.; Maksimov, E.G.; Paschenko, V.Z. Fluorescence quenching of the phycobilisome terminal emitter LCM from the cyanobacterium Synechocystis sp. PCC 6803 detected in vivo and in vitro. J. Photochem. Photobiol. B Biol. 2013, 125, 137–145. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Kerfeld, C.A. The Orange Carotenoid Protein: A blue-green light photoactive protein. Photochem. Photobiol. Sci. 2013, 12, 1135–1143. [Google Scholar] [CrossRef]
- Siefermann-Harms, D. The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol. Plant. 1987, 69, 561–568. [Google Scholar] [CrossRef]
- Daddy, S.; Zhan, J.; Jantaro, S.; He, C.; He, Q.; Wang, Q. A novel high light-inducible carotenoid-binding protein complex in the thylakoid membranes of Synechocystis PCC 6803. Sci. Rep. 2015, 5, 9480. [Google Scholar] [CrossRef]
- Kuthanová Trsková, E.; Belgio, E.; Yeates, A.M.; Sobotka, R.; Ruban, A.V.; Kana, R. Antenna proton sensitivity determines photosynthetic light harvesting strategy. J. Exp. Bot. 2018, 69, 4483–4493. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Cogdell, R. The function of pigments in chloroplasts. Plant Pigment. 1988, 183–230. [Google Scholar]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid. Res. 2013, 52, 539–561. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Xanthophylls as modulators of membrane protein function. Arch. Biochem. Biophys. 2010, 504, 78–85. [Google Scholar] [CrossRef]
- Kaňa, R.; Kotabová, E.; Kopečná, J.; Trsková, E.; Belgio, E.; Sobotka, R.; Ruban, A.V. Violaxanthin inhibits nonphotochemical quenching in light-harvesting antennae of Chromera velia. FEBS Lett. 2016, 590, 1076–1085. [Google Scholar] [CrossRef]
- Hirschberg, J.; Chamovitz, D. Carotenoids in Cyanobacteria. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 559–579. [Google Scholar] [CrossRef]
- Zakar, T.; Herman, E.; Vajravel, S.; Kovacs, L.; Knoppová, J.; Komenda, J.; Domonkos, I.; Kis, M.; Gombos, Z.; Laczko-Dobos, H. Lipid and carotenoid cooperation-driven adaptation to light and temperature stress in Synechocystis sp. PCC6803. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 337–350. [Google Scholar] [CrossRef]
- Tóth, T.N.; Chukhutsina, V.; Domonkos, I.; Knoppová, J.; Komenda, J.; Kis, M.; Lénárt, Z.; Garab, G.; Kovács, L.; Gombos, Z.; et al. Carotenoids are essential for the assembly of cyanobacterial photosynthetic complexes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766–778. [Google Scholar]
- Tichý, M.; Bečková, M.; Kopečná, J.; Noda, J.; Sobotka, R.; Komenda, J. Strain of Synechocystis PCC 6803 with Aberrant Assembly of Photosystem II Contains Tandem Duplication of a Large Chromosomal Region. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Strašková, A.; Knoppová, J.; Komenda, J. Isolation of the cyanobacterial YFP-tagged photosystem I using GFP-Trap®. Photosynthetica 2018, 56, 300–305. [Google Scholar] [CrossRef]
- Wittig, I.; Karas, M.; Schägger, H. High Resolution Clear Native Electrophoresis for In-gel Functional Assays and Fluorescence Studies of Membrane Protein Complexes. Mol. &Amp; Cell. Proteom. 2007, 6, 1215–1225. [Google Scholar] [CrossRef]
- Kaňa, R.; Kotabová, E.; Komárek, O.; Šedivá, B.; Papageorgiou, G.C.; Govindjee; Prášil, O. The slow S to M fluorescence rise in cyanobacteria is due to a state 2 to state 1 transition. Biochim. Biophys. Acta 2012, 1817, 1237–1247. [Google Scholar] [CrossRef]
- Kaňa, R. Application of spectrally resolved fluorescence induction to study light-induced nonphotochemical quenching in algae. Photosynthetica 2018, 56, 132–138. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Pazderník, M.; Mareš, J.; Pilný, J.; Sobotka, R. The antenna-like domain of the cyanobacterial ferrochelatase can bind chlorophyll and carotenoids in an energy-dissipative configuration. J. Biol. Chem. 2019, 294, 11131–11143. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Steinbach, G.; Schubert, F.; Kana, R. Cryo-imaging of photosystems and phycobilisomes in Anabaena sp PCC 7120 cells. J. Photochem. Photobiol. B-Biol. 2015, 152, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; McCaffery, S.; Anderson, J.M. Photoinhibition and D1 protein-degradation in peas acclimated to different growth irradiances. Plant Physiol. 1993, 103, 835–843. [Google Scholar] [CrossRef]
- Martins, B.M.C.; Tooke, A.K.; Thomas, P.; Locke, J.C.W. Cell size control driven by the circadian clock and environment in cyanobacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E11415–E11424. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, P.; Hellingwerf, K.J. Sustained Circadian Rhythms in Continuous Light in Synechocystis sp. PCC6803 Growing in a Well-Controlled Photobioreactor. PLoS ONE 2015, 10, e0127715. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Garab, G.; Adams, W.W., III; Govindjee. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume XXXVIII, p. 649. [Google Scholar] [CrossRef]
- Boulay, C.; Wilson, A.; D’Haene, S.; Kirilovsky, D. Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11620–11625. [Google Scholar] [CrossRef]
- Gwizdala, M.; Wilson, A.; Kirilovsky, D. In Vitro Reconstitution of the Cyanobacterial Photoprotective Mechanism Mediated by the Orange Carotenoid Protein in Synechocystis PCC 6803. Plant Cell 2011, 23, 2631–2643. [Google Scholar] [CrossRef]
- Kopecna, J.; Komenda, J.; Bucinska, L.; Sobotka, R. Long-term acclimation of the cyanobacterium Synechocystis sp. PCC 6803 to high light is accompanied by an enhanced production of chlorophyll that is preferentially channeled to trimeric photosystem I. Plant Physiol. 2012, 160, 2239–2250. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Kaňa, R.; Steinbach, G.; Sobotka, R.; Vámosi, G.; Komenda, J. Fast Diffusion of the Unassembled PetC1-GFP Protein in the Cyanobacterial Thylakoid Membrane. Life 2021, 11, 15. [Google Scholar] [CrossRef]
- Izuhara, T.; Kaihatsu, I.; Jimbo, H.; Takaichi, S.; Nishiyama, Y. Elevated Levels of Specific Carotenoids During Acclimation to Strong Light Protect the Repair of Photosystem II in Synechocystis sp. PCC 6803. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Rast, A.; Schaffer, M.; Albert, S.; Wan, W.; Pfeffer, S.; Beck, F.; Plitzko, J.M.; Nickelsen, J.; Engel, B.D. Biogenic regions of cyanobacterial thylakoids form contact sites with the plasma membrane. Nat. Plants 2019, 5, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Vajravel, S.; Kis, M.; Kłodawska, K.; Laczko-Dobos, H.; Malec, P.; Kovács, L.; Gombos, Z.; Toth, T.N. Zeaxanthin and echinenone modify the structure of photosystem I trimer in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Mohamed, H.E.; van de Meene, A.M.L.; Roberson, R.W.; Vermaas, W.F.J. Myxoxanthophyll Is Required for Normal Cell Wall Structure and Thylakoid Organization in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2005, 187, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Schäfer, L.; Sandmann, G. High-light-dependent upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803. J. Photochem. Photobiol. B Biol. 1999, 52, 14–18. [Google Scholar] [CrossRef]
- Kaňa, R. Mobility of photosynthetic proteins. Photosynth. Res. 2013, 116, 465–479. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Wilhelm, C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. USA 1999, 96, 8784–8789. [Google Scholar] [CrossRef]
- Böhme, K.; Wilhelm, C.; Goss, R. Light regulation of carotenoid biosynthesis in the prasinophycean alga Mantoniella squamata. Photochem. Photobiol. Sci. 2002, 1, 619–628. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canonico, M.; Konert, G.; Crepin, A.; Šedivá, B.; Kaňa, R. Gradual Response of Cyanobacterial Thylakoids to Acute High-Light Stress—Importance of Carotenoid Accumulation. Cells 2021, 10, 1916. https://doi.org/10.3390/cells10081916
Canonico M, Konert G, Crepin A, Šedivá B, Kaňa R. Gradual Response of Cyanobacterial Thylakoids to Acute High-Light Stress—Importance of Carotenoid Accumulation. Cells. 2021; 10(8):1916. https://doi.org/10.3390/cells10081916
Chicago/Turabian StyleCanonico, Myriam, Grzegorz Konert, Aurélie Crepin, Barbora Šedivá, and Radek Kaňa. 2021. "Gradual Response of Cyanobacterial Thylakoids to Acute High-Light Stress—Importance of Carotenoid Accumulation" Cells 10, no. 8: 1916. https://doi.org/10.3390/cells10081916
APA StyleCanonico, M., Konert, G., Crepin, A., Šedivá, B., & Kaňa, R. (2021). Gradual Response of Cyanobacterial Thylakoids to Acute High-Light Stress—Importance of Carotenoid Accumulation. Cells, 10(8), 1916. https://doi.org/10.3390/cells10081916