CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Semen Samples
2.2. Ethical Approval
2.3. Spermatozoa Isolation by Density Gradient Centrifugation
2.4. Total RNA Preparation
2.5. RNA Expression Analysis by One-Step Evagreen qRT-PCR
2.6. PCR Primer Design
2.7. Protein Extraction and Western Blot Analysis
2.8. Asthenozoospermic Patient Treatment
2.9. Functional Annotation for circRNA/miRNA and Target miRNA Interaction
2.10. Statistical Analysis
3. Results
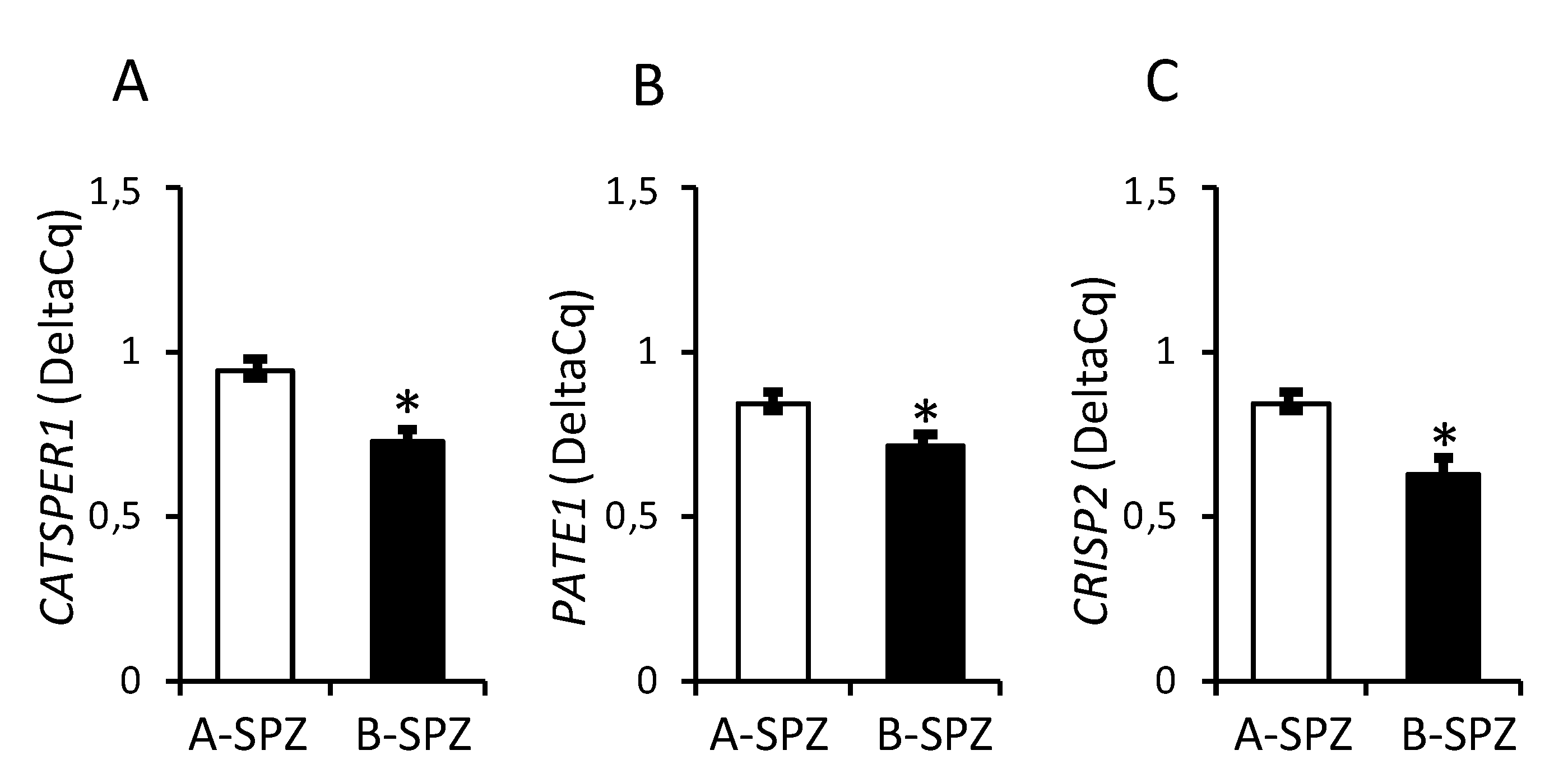
3.1. Expression of CATSPER1, PATE1 and CRISP2 in Normozoospermic Sperm
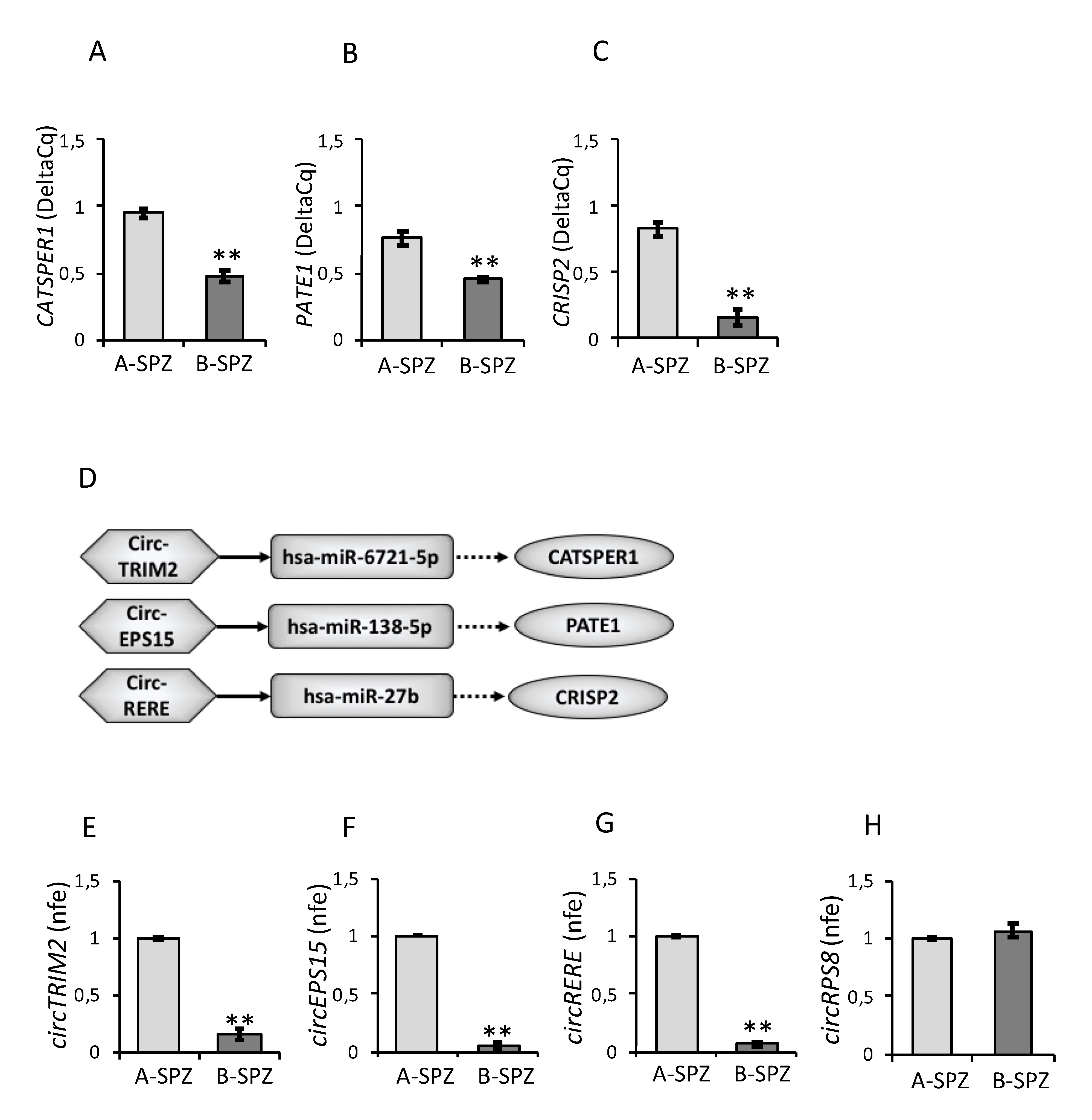
3.2. Expression of CATSPER1, PATE1, CRISP2 and circRNAs in Asthenozoospermic Sperm
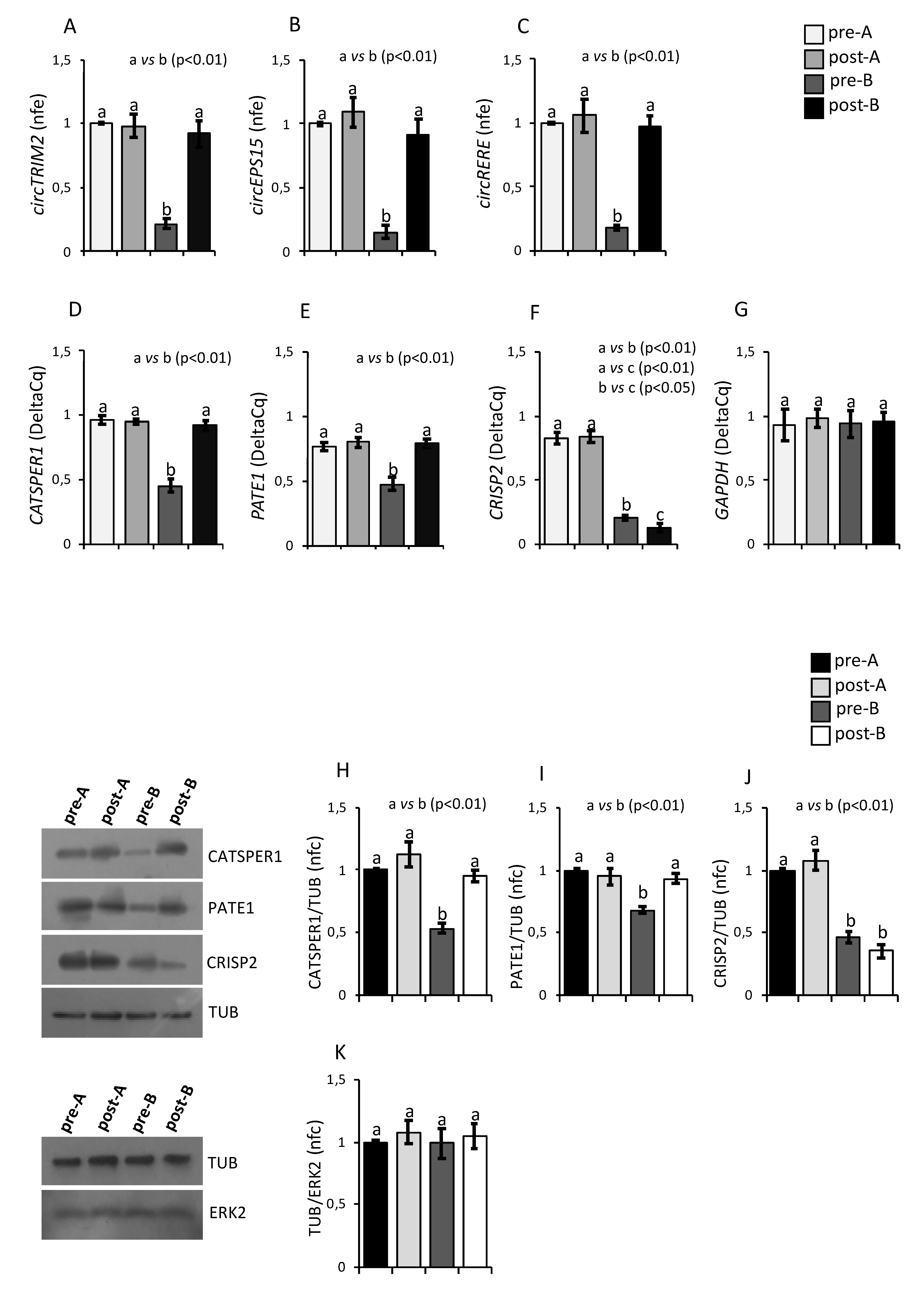
3.3. Expression of CATSPER1, PATE1, CRISP2 and circRNAs in Asthenozoospermic Sperm after an Oral Amino Acid Supplementation
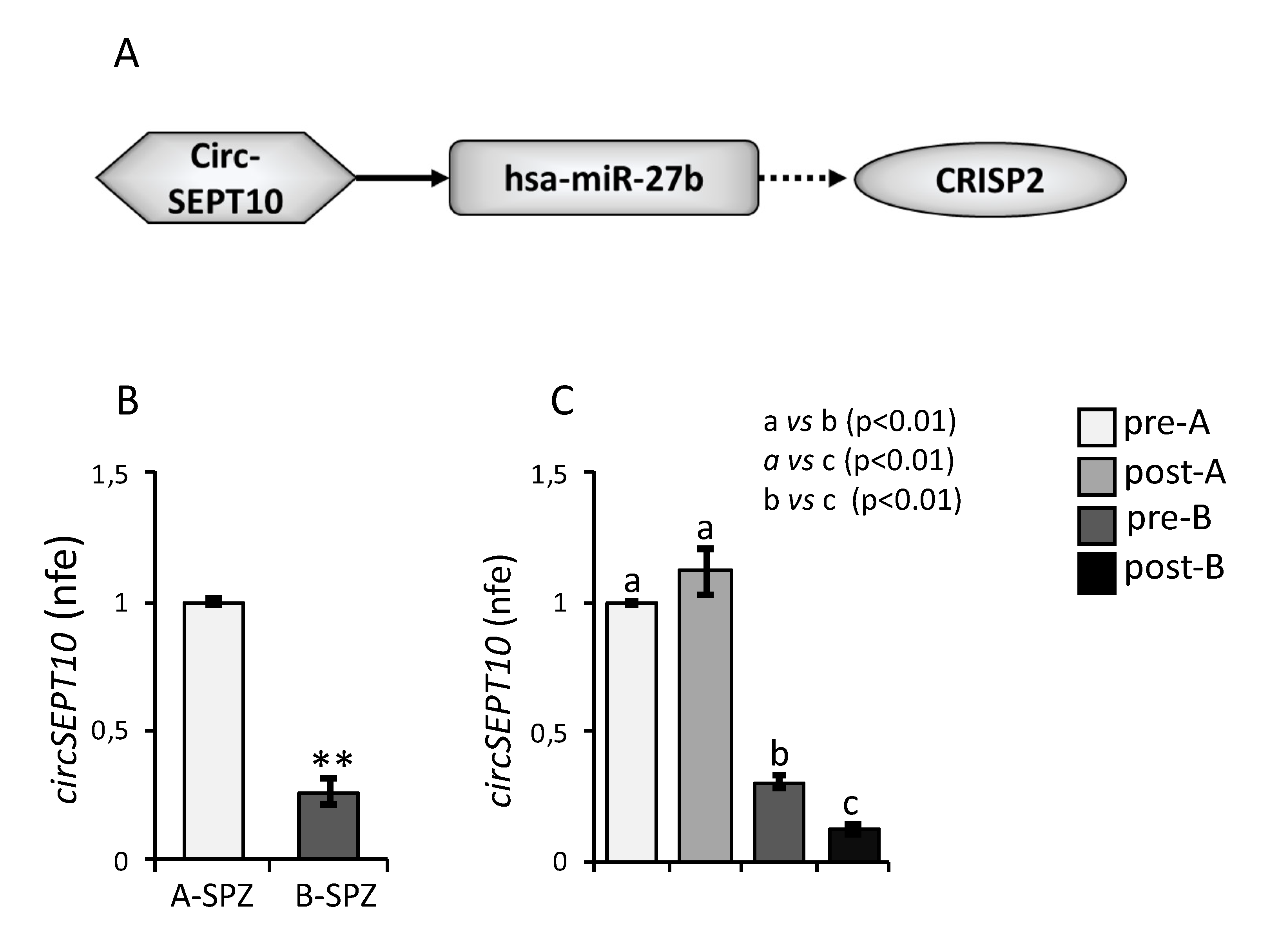
3.4. Expression of circSEPT10 in Asthenozoospermic Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human, Semen and Sperm-Cervical Mucus Interaction, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Vaughan, D.A.; Sakkas, D. Sperm selection methods in the 21st century. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Ng, V.; Yeung, W.S.; Ho, P.C. Profiles of sperm morphology and motility after discontinuous multiple-step Percoll density gradient centrifugation. Andrologia 1996, 28, 127–131. [Google Scholar] [CrossRef]
- Brahem, S.; Mehdi, M.; Elghezal, H.; Saad, A. Semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity. Andrologia 2011, 43, 196–202. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef] [PubMed]
- Canale, D.; Giorgi, P.M.; Gasperini, M.; Pucci, E.; Barletta, D.; Gasperi, M.; Martino, E. Inter and intra-individual variability of sperm morphology after selection with three different techniques: Layering, swimup from pellet and percoll. J. Endocrinol. Investig. 1994, 17, 729–732. [Google Scholar] [CrossRef]
- Natali, I. Sperm Preparation Techniques for Artificial Insemination—Comparison of Sperm Washing, Swim Up, and Density Gradient Centrifugation Methods. In Dalam: Manafi M, Penyunting. Artificial Insemination in Farm Animals; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Malvezzi, H.; Sharma, R.; Agarwal, A.; Abuzenadah, A.M.; Abu-Elmagd, M. Sperm quality after density gradient centrifugation with three commercially available media: A controlled trial. Reprod. Biol. Endocrinol. 2014, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, C.M.; Abramsson, L.; Holm, S.E.; Bjurulf, E. Bacterial contamination and sperm recovery after semen preparation by density gradient centrifugation using silane-coated silica particles at different g forces. Hum. Reprod. 2000, 15, 662–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnon, F.; Ouchchane, L.; Pons-Rejraji, H.; Artonne, C.; Farigoule, M.; Janny, L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2045–2057. [Google Scholar] [CrossRef]
- Coutton, C.; Fissore, R.A.; Palermo, G.D.; Stouffs, K.; Toure, A. Male infertility: Genetics, mechanism, and therapies. Biomed. Res. Int. 2016, 2016, 7372362. [Google Scholar] [CrossRef]
- Ford, W.C. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum. Reprod. 2006, 12, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, S.K.; Gupta, N.; Sankhwar, S.N.; Rajender, S. Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS ONE 2015, 10, e0127007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avenarius, M.R.; Hildebrand, M.S.; Zhang, Y.; Meyer, N.C.; Smith, L.L.; Kahrizi, K.; Najmabadi, H.; Smith, R.J. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 2009, 84, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.J.; Liu, X.; Han, J.L.; Wang, Y.W.; Jin, S.H.; Liu, X.X.; Liu, J.; Wang, W.T.; Wang, W.J. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorramkhorshid, H.R. Expression Analysis of the CRISP2, CATSPER1, PATE1 and SEMG1 in the Sperm of Men with Idiopathic Asthenozoospermia. J. Reprod. Infertil. 2019, 20, 70–75. [Google Scholar]
- Loux, S.C.; Crawford, K.R.; Ing, N.H.; González-Fernández, L.; Macías-García, B.; Love, C.C.; Varner, D.D.; Velez, I.C.; Choi, Y.H.; Hinrichs, K. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol. Reprod. 2013, 89, 123. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.M.; Ding, X.P.; Wang, T.; Mu, X.M.; Chen, Z.Y. Association of polymorphisms in PATE1 gene with idiopathic asthenozoospermia in Sichuan, China. J. Reprod. Immunol. 2016, 118, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Zhou, Q.Z.; Lyu, X.M.; Zhu, T.; Chen, Z.J.; Chen, M.K.; Xia, H.; Wang, C.Y.; Qi, T.; Li, X.; et al. The expression of cysteine-rich secretory protein 2 (CRISP2) and its specific regulator miR-27b in the spermatozoa of patients with asthenozoospermia. Biol. Reprod. 2015, 92, 28. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cardenas, C.; Montoya, F.; Navarrete, F.A.; Hernandez-Cruz, A.; Corkidi, G.; Visconti, P.E.; Darszon, A. Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biol. Reprod. 2018, 99, 1010–1021. [Google Scholar] [CrossRef]
- Lim, S.; Kierzek, M.; O’Connor, A.E.; Brenker, C.; Merriner, D.J.; Okuda, H.; Volpert, M.; Gaikwad, A.; Bianco, D.; Potter, D.; et al. CRISP2 Is a Regulator of Multiple Aspects of Sperm Function and Male Fertility. Endocrinology 2019, 160, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.M.; Scanlon, M.J.; Swarbrick, J.; Curtis, S.; Gallant, E.; Dulhunty, A.F.; O’Bryan, M.K. The cysteine-rich secretory protein domain of Tpx-1 is related to ion channel toxins and regulates ryanodine receptor Ca2+ signaling. J. Biol. Chem. 2006, 281, 4156–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.P.; Rajender, S. CatSper channel, sperm function and male fertility. Reprod. Biomed. Online 2015, 30, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Brukman, N.G.; Miyata, H.; Torres, P.; Lombardo, D.; Caramelo, J.J.; Ikawa, M.; Da Ros, V.G.; Cuasnicú, P.S. Fertilization defects in sperm from Cysteine-rich secretory protein 2 (risp2) knockout mice: Implications for fertility disorders. Mol. Hum. Reprod. 2016, 22, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.; Wolff, C.A.; Suarez, S.S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 2009, 21, 345–350. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.H.; Bindereif, A. Exon circularization requires canonical splice signals. Cell. Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, M.; Barbagallo, D.; Chioccarelli, T.; Manfrevola, F.; Cobellis, G.; Di Pietro, C.; Brex, D.; Battaglia, R.; Fasano, S.; Ferraro, B.; et al. CircNAPEPLD is expressed in human and murine spermatozoa and physically interacts with oocyte miRNAs. RNA Biol. 2019, 16, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Chioccarelli, T.; Pierantoni, R.; Manfrevola, F.; Porreca, V.; Fasano, S.; Chianese, R.; Cobellis, G. Histone Post-Translational Modifications and CircRNAs in Mouse and Human Spermatozoa: Potential Epigenetic Marks to Assess Human Sperm Quality. J. Clin. Med. 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.W.; Li, H.M.; Qing, X.R.; Huang, D.H.; Li, H.G. Identification and characterization of human testis derived circular RNAs and their existence in seminal plasma. Sci. Rep. 2016, 6, 39080. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Han, M.; Cheng, L.; Chen, J.; Zhang, Z.; Shen, T.; Wang, M.; Wen, B.; Ni, T.; Han, C. Expression dynamics, relationships, and transcriptional regulations of diverse transcripts in mouse spermatogenic cells. RNA Biol. 2016, 13, 1011–1024. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Xie, X.; Li, M.; Shi, J.; Zhou, J.J.; Knox, K.S.; Wang, T.; Chen, Q.; Gu, W. Rat BodyMap transcriptomes reveal unique circular RNA features across tissue types and developmental stages. RNA 2018, 24, 1443–1456. [Google Scholar] [CrossRef] [Green Version]
- Ge, P.; Zhang, J.; Zhou, L.; Lv, M.Q.; Li, Y.X.; Wang, J.; Zhou, D.X. CircRNA expression profile and functional analysis in testicular tissue of patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Chioccarelli, T.; Manfrevola, F.; Ferraro, B.; Sellitto, C.; Cobellis, G.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Expression Patterns of Circular RNAs in High Quality and Poor Quality Human Spermatozoa. Front. Endocrinol. 2019, 10, 435. [Google Scholar] [CrossRef]
- Manfrevola, F.; Chioccarelli, T.; Cobellis, G.; Fasano, S.; Ferraro, B.; Sellitto, C.; Marella, G.; Pierantoni, R.; Chianese, R. CircRNA Role and circRNA-Dependent Network (ceRNET) in Asthenozoospermia. Front. Endocrinol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Gòdia, M.; Castelló, A.; Rocco, M.; Cabrera, B.; Rodríguez-Gil, J.E.; Balasch, S.; Lewis, C.; Sánchez, A.; Clop, A. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility. Sci. Rep. 2020, 10, 79–85. [Google Scholar] [CrossRef]
- Keller, D.W.; Polakoski, K.L. L-arginine stimulation of human sperm motility in vitro. Biol Reprod. 1975, 13, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.D.; Hernández, M.P. Effect of L-arginine and polyamines on sperm motility. Ginecol Obstet Mex. 1993, 61, 229–234. [Google Scholar] [PubMed]
- Perera, D.M.; Katz, M.; Heenbanda, S.R.; Marchant, S. Nitric oxide synthase inhibitor NG-monomethyl-L-arginine preserves sperm motility after swim-up. Fertil. Steril. 1996, 66, 830–833. [Google Scholar] [CrossRef]
- Patel, A.B.; Srivastava, S.; Phadke, R.S.; Govil, G. Arginine acts as a protective and reversal agent against glycolytic inhibitors in spermatozoa. Physiol. Chem. Phys. Med. NMR 1999, 31, 29–40. [Google Scholar]
- Morales, M.E.; Rico, G.; Bravo, C.; Tapia, R.; Alvarez, C.; Méndez, J.D. Progressive motility increase caused by L-arginine and polyamines in sperm from patients with idiopathic and diabetic asthenozoospermia. Ginecol. Obstet. Mex. 2003, 71, 297–303. [Google Scholar]
- Morgante, G.; Scolaro, V.; Tosti, C.; Di Sabatino, A.; Piomboni, P.; De Leo, V. Treatment with carnitine, acetyl carnitine, L-arginine and ginseng improves sperm motility and sexual health in men with asthenopermia. Minerva Urol. Nefrol. 2010, 62, 213–218. [Google Scholar]
- Stanislavov, R.; Rohdewald, P. Sperm quality in men is improved by supplementation with a combination of L-arginine, L-citrullin, roburins and Pycnogenol®. Minerva Urol Nefrol. 2014, 66, 217–223. [Google Scholar]
- Abd-Elrazek, A.M.; Ahmed-Farid, O.A.H. Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia 2018, 50, e12806. [Google Scholar] [CrossRef]
- Lambertos, A.; Ramos-Molina, B.; López-Contreras, A.J.; Cremades, A.; Peñafiel, R. New insights of polyamine metabolism in testicular physiology: A role of ornithine decarboxylase antizyme inhibitor 2 (AZIN2) in the modulation of testosterone levels and sperm motility. PLoS ONE 2018, 13, e0209202. [Google Scholar] [CrossRef]
- Chen, J.Q.; Li, Y.S.; Li, Z.J.; Lu, H.X.; Zhu, P.Q.; Li, C.M. Dietary l-arginine supplementation improves semen quality and libido of boars under high ambient temperature. Animal 2018, 12, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Li, Z.; Li, C. Mitochondrial OXPHOS is involved in the protective effects of L-arginine against heat-induced low sperm motility of boar. J. Therm. Biol. 2019, 84, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pelloni, M.; Gallo, M.; Coltrinari, G.; Lombardo, F.; Lenzi, A.; Gandini, L. Sperm glyceraldehyde 3-phosphate dehydrogenase gene expression in asthenozoospermic spermatozoa. Asian J. Androl. 2017, 19, 409–413. [Google Scholar] [PubMed]
- Chawan, V.; Yevate, S.; Gajbhiye, R.; Kulkarni, V.; Parte, P. Acetylation/deacetylation and microtubule associated proteins influence flagellar axonemal stability and sperm motility. Biosci. Rep. 2020, 40, BSR20202442. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Carrell, D.T. The sperm epigenome and potential implications for the developing embryo. Reproduction 2012, 143, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev. Cell. 2018, 46, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.; Jodar, M.; Oliva, R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 2018, 24, 535–555. [Google Scholar] [CrossRef] [Green Version]
- Gross, N.; Strillacci, M.G.; Peñagaricano, F.; Khatib, H. Characterization and functional roles of paternal RNAs in 2–4 cell bovine embryos. Sci. Rep. 2019, 9, 203–247. [Google Scholar] [CrossRef] [Green Version]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell. Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U. Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front. Cell. Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef]
- Cescon, M.; Chianese, R.; Tavares, R.S. Environmental Impact on Male (In) Fertility via Epigenetic Route. J. Clin. Med. 2020, 9, 2520. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Lambard, S.; Said, L.; Saad, A.; Galeraud-Denis, I. RNA dynamics of fertile and infertile spermatozoa. Biochem. Soc. Trans. 2007, 35, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Jodar, M.; Kalko, S.; Castillo, J.; Ballescà, J.L.; Oliva, R. Differential RNAs in the sperm cells of asthenozoospermic patients. Hum. Reprod. 2012, 27, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Chen, W.; Jiang, Y.; He, Z. Regulation of long non-coding RNAs and circular RNAs in spermatogonial stem cells. Reproduction 2019, 158, R15–R25. [Google Scholar] [CrossRef]
- Alberti, C.; Cochella, L. A framework for understanding the roles of miRNAs in animal development. Development 2017, 144, 2548–2559. [Google Scholar] [CrossRef] [Green Version]
- Khazaie, Y.; Nasr Esfahani, M.H. MicroRNA and male infertility: A potential for diagnosis. Int. J. Fertil. Steril. 2014, 8, 113–118. [Google Scholar]
- Wang, C.; Yang, C.; Chen, X.; Yao, B.; Yang, C.; Zhu, C.; Li, L.; Wang, J.; Li, X.; Shao, Y.; et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin. Chem. 2011, 57, 1722–1731. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.H.; Zhou, Q.Z.; Yang, J.K.; Lyu, X.M.; Bian, J.; Guo, W.B.; Chen, Z.J.; Xia, M.; Xia, H.; Qi, T.; et al. MicroRNA-27a-mediated repression of cysteine-rich secretory protein 2 translation in asthenoteratozoospermic patients. Asian J. Androl. 2017, 19, 591–595. [Google Scholar] [PubMed]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorram Khorshid, H.R. MicroRNA profiling in spermatozoa of men with unexplained asthenozoospermia. Andrologia 2019, 51, e13284. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chang, S.; Xia, W.; Wang, X.; Zhang, C.; Cheng, L.; Liu, X.; Chen, L.; Shi, Q.; Huang, J.; et al. Circular RNAs from BOULE play conserved roles in protection against stress-induced fertility decline. Sci. Adv. 2020, 6, eabb7426. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, L.; Marchiani, S.; Vicini, E.; Muciaccia, B.; Cambi, M.; Pellegrini, S.; Forti, G.; Muratori, M.; Baldi, E. Quantification of CatSper1 expression in human spermatozoa and relation to functional parameters. Hum. Reprod. 2015, 30, 1532–1544. [Google Scholar] [CrossRef]
- Hamatani, T. Human spermatozoal RNAs. Fertil Steril. 2012, 97, 275–281. [Google Scholar] [CrossRef]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell. 2018, 46, 481–494. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Desai, P.; Coutinho, E.; Govil, G. Mechanism of action of L-arginine on the vitality of spermatozoa is primarily through increased biosynthesis of nitric oxide. Biol. Reprod. 2006, 74, 954–958. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef] [PubMed]
| Gene Primers | Sequences 5′-3′ | Tm (°C) |
|---|---|---|
| CRISP2 S | TGCCATTATTGTCCTGCTGGT | 56 |
| CRISP2 AS | CATGTTCACAGCCAGTTGTATTCT | |
| CATSPER1 S | AAGGGCAATTTCAGAAACGCA | 57 |
| CATSPER1 AS | TCAAAGGCCAAGGATTGGGTTA | |
| PATE1 S | TCTGCTGCTTTAGGGCGTTAT | 57 |
| PATE1 AS | GGTGGCACATCCTACACYGA | |
| GAPDH S | TGCACCACCAACTGCTTAGC | 58 |
| GAPDH AS | GGCATGGACTGTGGTCATGAG | |
| PCNA S | TAAACCTGCAGAGCATGGAC | 53 |
| PCNA AS | GCCGGCGCATTTTAGTATTT | |
| circRERE S | CAGACCCAGTTATCAAGAACCGA | 54 |
| circRERE AS | GGGAGTTGTGGACCTAAGGG | |
| circEPS15 S | CCTTTTGTTGGCAATCTCTTCTC | 52 |
| circEPS15 AS | CGGCTCAGCTCTTCTCTAGC | |
| circTRIM2 S | TTGCCCAAACCACGATG | 52 |
| circTRIM2 AS | ACAGGACTTGGGATGTTGG | |
| circSEPT10 S | ACCCATACCAGGCACTATGA | 52 |
| circSEPT10 AS | TGAAAGAGCTGACTGGCTTG | |
| circRPS8 S | GTTGTGGCCGTCTTGGTCAC | 58 |
| circRPS8 AS | GGAGAGCAAGGCAAGTGAGG |
| Parameters Studied | Asthenozoospermic Men Pre-Treatment | Asthenozoospermic Men Post-Treatment |
|---|---|---|
| Age of patients | 28.63 ± 4.3 | 29.61 ± 5.8 |
| Semen total volume (mL) | 2.35 ± 0.44 | 2.74 ± 0.62 |
| Sperm concentration (×106/mL) | 31.32 ± 10.31 | 33.75 ± 11.82 |
| Total motility (%) | 23.21 ± 1.38 | 43.45 ± 2.42 * |
| Progressive motility (%) | 20.35 ± 2.27 | 28.73 ± 1.27 * |
| Sperm vitality (%) | 41.34 ± 5.32 | 46.92 ± 3.25 |
| Normal morphology (%) | 12.5 ± 3.3 | 15.9 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfrevola, F.; Ferraro, B.; Sellitto, C.; Rocco, D.; Fasano, S.; Pierantoni, R.; Chianese, R. CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen. Cells 2021, 10, 1956. https://doi.org/10.3390/cells10081956
Manfrevola F, Ferraro B, Sellitto C, Rocco D, Fasano S, Pierantoni R, Chianese R. CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen. Cells. 2021; 10(8):1956. https://doi.org/10.3390/cells10081956
Chicago/Turabian StyleManfrevola, Francesco, Bruno Ferraro, Carolina Sellitto, Domenico Rocco, Silvia Fasano, Riccardo Pierantoni, and Rosanna Chianese. 2021. "CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen" Cells 10, no. 8: 1956. https://doi.org/10.3390/cells10081956







