Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases?
Abstract
:1. Introduction
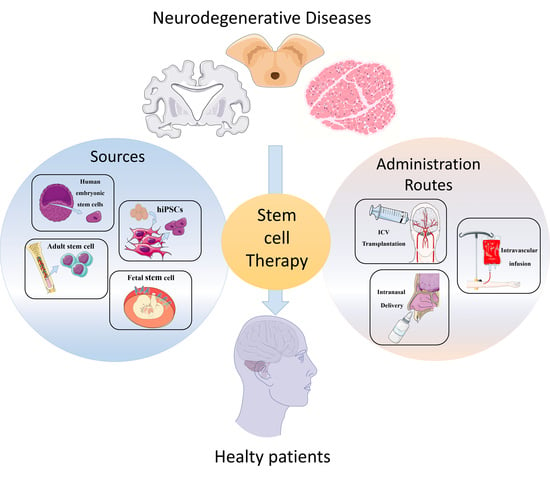
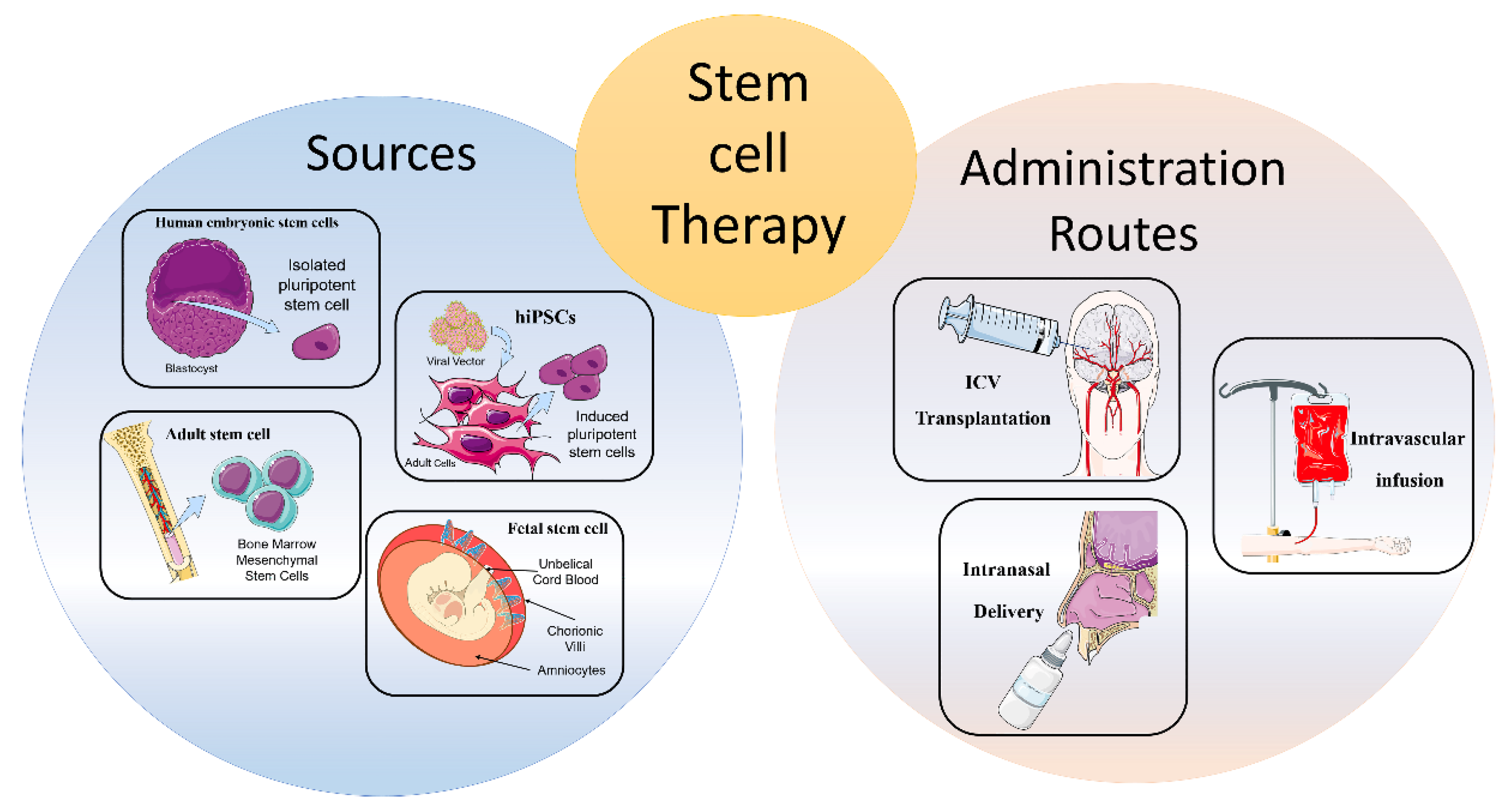
2. Cellular Therapies from Different Cell Sources
2.1. Human Embryonic Stem Cells
2.2. Human-Induced Pluripotent Stem Cells
2.3. Fetal Stem Cell
2.4. Adult Stem Cells
3. Routes of Administration
3.1. Intracerebral or Intracerebroventricular Transplantation
3.2. Intravascular Infusion
3.3. Intranasal Delivery
4. Immunomodulation
5. Stem Cell Therapy for AD
6. Stem Cell Therapy in ALS
7. Stem Cell Therapy in PD
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuchs, E.; Chen, T. A matter of life and death: Self-renewal in stem cells. EMBO Rep. 2013, 14, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Sakthiswary, R.; Raymond, A.A. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen. Res. 2012, 7, 1822. [Google Scholar]
- Guo, Y.; Peng, Y.; Zeng, H.; Chen, G. Progress in mesenchymal stem cell therapy for ischemic stroke. Stem Cells Int. 2021, 2021, 9923566. [Google Scholar] [CrossRef]
- Burns, T.C.; Quinones-Hinojosa, A. Regenerative medicine for neurological diseases—will regenerative neurosurgery deliver? BMJ 2021, 373, 955. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L. Pharmacologic and cell-based therapies for acute spinal cord injury. Neurosurg. Clin. 2021, 32, 389–395. [Google Scholar] [CrossRef]
- Bonilla, C.; Zurita, M. Cell-based therapies for traumatic brain injury: Therapeutic treatments and clinical trials. Biomedicines 2021, 9, 669. [Google Scholar] [CrossRef]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Chiu, A.Y.; Rao, M.S. Cell-based therapy for neural disorders--anticipating challenges. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2011, 8, 744–752. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.F.; Sances, S.; Brill, L.M.; Okamoto, S.; Zaidi, R.; McKercher, S.R.; Akhtar, M.W.; Nakanishi, N.; Lipton, S.A. ATM-dependent phosphorylation of MEF2D promotes neuronal survival after DNA damage. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4640–4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condic, M.L. Totipotency: What it is and what it is not. Stem Cells Dev. 2014, 23, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Belmonte, J.C.I. Stem cells: A renaissance in human biology research. Cell 2016, 165, 1572–1585. [Google Scholar] [CrossRef] [Green Version]
- Sproul, A.A.; Jacob, S.; Pre, D.; Kim, S.H.; Nestor, M.W.; Navarro-Sobrino, M.; Santa-Maria, I.; Zimmer, M.; Aubry, S.; Steele, J.W.; et al. Characterization and molecular profiling of PSEN1 familial Alzheimer’s disease iPSC-derived neural progenitors. PLoS ONE 2014, 9, e84547. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.-N.; Liu, L.-P.; Zheng, Y.-W.; Li, Y.-M. Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach. World J. Stem Cells 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Do, J.T. Neural lineage differentiation from pluripotent stem cells to mimic human brain tissues. Front. Bioeng. Biotechnol. 2019, 7, 400. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Xie, X.; Li, F.; Liu, Y.; Lu, Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem. Biophys. Res. Commun. 2013, 438, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; De Munari, S.; Caro, M.; Caruso, M.; Silini, A.; Delgado, M.; Parolini, O. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J. Tissue Eng. Regen. Med. 2017, 11, 2895–2911. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Wolbank, S.; Peterbauer, A.; Fahrner, M.; Hennerbichler, S.; Van Griensven, M.; Stadler, G.; Redl, H.; Gabriel, C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007, 13, 1173–1183. [Google Scholar] [CrossRef]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef] [Green Version]
- d’Angelo, M.; Cimini, A.; Castelli, V. Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 5241. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, L.; Gao, X.; Chen, B.; Tu, J.; Sun, H.; Liu, X.; He, J.; Liu, J.; Yuan, Q. Neuroprotective effects of bone marrow stem cells overexpressing glial cell line-derived neurotrophic factor on rats with intracerebral hemorrhage and neurons exposed to hypoxia/reoxygenation. Neurosurgery 2011, 68, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Q.; Ding, G.; Zhang, L.; Zhang, Z.G.; Li, Q.; Panda, S.; Lu, M.; Ewing, J.R.; Chopp, M. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J. Cereb. Blood Flow Metab. 2010, 30, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Misra, V.; Lal, A.; El Khoury, R.; Chen, P.R.; Savitz, S.I. Intra-arterial delivery of cell therapies for stroke. Stem Cells Dev. 2012, 21, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ye, R.; Yan, T.; Yu, S.P.; Wei, L.; Xu, G.; Fan, X.; Jiang, Y.; Stetler, R.A.; Liu, G. Cell based therapies for ischemic stroke: From basic science to bedside. Prog. Neurobiol. 2014, 115, 92–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitz, S.I.; Dinsmore, J.; Wu, J.; Henderson, G.V.; Stieg, P.; Caplan, L.R. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: A preliminary safety and feasibility study. Cerebrovasc. Dis. 2005, 20, 101–107. [Google Scholar] [CrossRef]
- Rabinovich, S.; Seledtsov, V.; Banul, N.; Poveshchenko, O.; Senyukov, V.; Astrakov, S.; Samarin, D.; Taraban, V.Y. Cell therapy of brain stroke. Bull. Exp. Biol. Med. 2005, 139, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, J.; Mao, G.; Liu, Y.; Ma, S.; Bao, X.; Gao, J.; Feng, M.; Li, G.; Ma, W. Intra-arterial delivery of human bone marrow mesenchymal stem cells is a safe and effective way to treat cerebral ischemia in rats. Cell Transplant. 2014, 23, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacigaluppi, M.; Pluchino, S.; Martino, G.; Kilic, E.; Hermann, D.M. Neural stem/precursor cells for the treatment of ischemic stroke. J. Neurol. Sci. 2008, 265, 73–77. [Google Scholar] [CrossRef]
- Kamiya, N.; Ueda, M.; Igarashi, H.; Nishiyama, Y.; Suda, S.; Inaba, T.; Katayama, Y. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008, 83, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, A.V.; Chua, J.Y.; Andres, R.H.; Wang, N.; Gaeta, X.; Wang, H.; De, A.; Choi, R.; Chen, S.; Rutt, B.K. Biodistribution of neural stem cells after intravascular therapy for hypoxic–ischemia. Stroke 2010, 41, 2064–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, Y.; Romanko, M.; Kramer, B.C.; Gosiewska, A.; Chopp, M.; Hong, K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012, 1489, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Zhang, J.; Gilad, A.A.; Kedziorek, D.A.; Ruiz-Cabello, J.; Young, R.G.; Pittenger, M.F.; Van Zijl, P.C.; Huang, J.; Bulte, J.W. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008, 39, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Bernhard, F.; Verleysdonk, S.; Buadze, M.; Lourhmati, A.; Klopfer, T.; Schaumann, F.; Schmid, B. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011, 14, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Reitz, M.; Demestre, M.; Sedlacik, J.; Meissner, H.; Fiehler, J.; Kim, S.U.; Westphal, M.; Schmidt, N.O. Intranasal delivery of neural stem/progenitor cells: A noninvasive passage to target intracerebral glioma. Stem Cells Transl. Med. 2012, 1, 866–873. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.; Maas, M.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, N.; Yu, S.P.; Gu, X.; Taylor, T.M.; Song, D.; Liu, X.-f.; Wei, L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013, 22, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Nijboer, C.H.; Kooijman, E.; Van Velthoven, C.T.; Van Tilborg, E.; Tiebosch, I.A.; Eijkelkamp, N.; Dijkhuizen, R.M.; Kesecioglu, J.; Heijnen, C.J. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. 2018, 27, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal stem cell migration and tissue repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Lindsay, S.L.; Barnett, S.C. Therapeutic Potential of Niche-Specific Mesenchymal Stromal Cells for Spinal Cord Injury Repair. Cells 2021, 10, 901. [Google Scholar] [CrossRef]
- Veneruso, V.; Rossi, F.; Villella, A.; Bena, A.; Forloni, G.; Veglianese, P. Stem cell paracrine effect and delivery strategies for spinal cord injury regeneration. J. Control. Release 2019, 300, 141–153. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Camussi, G.; Valadi, H.; Nazarenko, I.; Ekström, K.; Wang, X.; Principe, S.; Shah, N.; Ashraf, N.M.; Fatima, F. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014, 11, 688–701. [Google Scholar] [CrossRef]
- Belair, C.; Sim, S.; Kim, K.-Y.; Tanaka, Y.; Park, I.-H.; Wolin, S.L. The RNA exosome nuclease complex regulates human embryonic stem cell differentiation. J. Cell Biol. 2019, 218, 2564–2582. [Google Scholar] [CrossRef] [Green Version]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The therapeutic potential of mesenchymal stem cell–derived exosomes in treatment of neurodegenerative diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kim, H.S.; Park, J.-M.; Kim, H.W.; Park, M.-K.; Lee, H.-S.; Lim, D.S.; Lee, T.H.; Chopp, M.; Moon, J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol. Aging 2013, 34, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Association, A.S. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Belemkar, S. An update on Alzheimer’s disease: Immunotherapeutic agents, stem cell therapy and gene editing. Life Sci. 2021, 282, 119790. [Google Scholar] [CrossRef]
- Krivanek, T.J.; Gale, S.A.; McFeeley, B.M.; Nicastri, C.M.; Daffner, K.R. Promoting Successful Cognitive Aging: A Ten-Year Update. J. Alzheimer's Dis. 2021, 81, 871–920. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Thal, D.R.; Walter, J.; Saido, T.C.; Fändrich, M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015, 129, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhao, C.; Qiu, Y.; Zhou, Z.; Bao, J.; Qian, W. Dendritic/post-synaptic tau and early pathology of Alzheimer’s disease. Front. Mol. Neurosci. 2021, 14, 127. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Burgaletto, C.; Munafò, A.; Di Benedetto, G.; De Francisci, C.; Caraci, F.; Di Mauro, R.; Bucolo, C.; Bernardini, R.; Cantarella, G. The immune system on the TRAIL of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J. Targeting amyloid clearance in Alzheimer’s disease as a therapeutic strategy. Br. J. Pharmacol. 2019, 176, 3447–3463. [Google Scholar] [CrossRef] [PubMed]
- Fillit, H.; Green, A. Aducanumab and the FDA—Where are we now? Nat. Rev. Neurol. 2021, 17, 129–130. [Google Scholar] [CrossRef]
- Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet. Neurol. 2020, 19, 111–112. [Google Scholar] [CrossRef] [Green Version]
- Richard, E.; den Brok, M.G.; van Gool, W.A. Bayes analysis supports null hypothesis of anti-amyloid beta therapy in Alzheimer’s disease. Alzheimers. Dement. 2021, 17, 1051–1055. [Google Scholar] [CrossRef]
- Tan, C.-C.; Yu, J.-T.; Wang, H.-F.; Tan, M.-S.; Meng, X.-F.; Wang, C.; Jiang, T.; Zhu, X.-C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Garcia-Leon, J.A.; Caceres-Palomo, L.; Sanchez-Mejias, E.; Mejias-Ortega, M.; Nuñez-Diaz, C.; Fernandez-Valenzuela, J.J.; Sanchez-Varo, R.; Davila, J.C.; Vitorica, J.; Gutierrez, A. Human Pluripotent Stem Cell-Derived Neural Cells as a Relevant Platform for Drug Screening in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6867. [Google Scholar] [CrossRef]
- Mungenast, A.E.; Siegert, S.; Tsai, L.-H. Modeling Alzheimer’s disease with human induced pluripotent stem (iPS) cells. Mol. Cell. Neurosci. 2016, 73, 13–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Lee, D.; Chang, E.H.; Kim, J.H.; Hwang, J.W.; Kim, J.-Y.; Kyung, J.W.; Kim, S.H.; Oh, J.S.; Shim, S.M. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease model. Stem Cells Dev. 2015, 24, 2378–2390. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef]
- Lilja, A.M.; Malmsten, L.; Röjdner, J.; Voytenko, L.; Verkhratsky, A.; Ögren, S.O.; Nordberg, A.; Marutle, A. Neural stem cell transplant-induced effect on neurogenesis and cognition in Alzheimer Tg2576 mice is inhibited by concomitant treatment with amyloid-lowering or cholinergic 7 nicotinic receptor drugs. Neural Plasticity 2015, 13, 2015. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, T.R.; Blurton-Jones, M.; Morrissette, D.A.; Kitazawa, M.; Oddo, S.; LaFerla, F.M. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J. Neurosci. 2007, 27, 11925–11933. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, H.J.; Kim, H.N.; Oh, S.H.; Bae, J.-S.; Ha, H.-J.; Lee, P.H. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy 2014, 10, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bali, P.; K Lahiri, D.; Banik, A.; Nehru, B.; Anand, A. Potential for stem cells therapy in Alzheimer’s disease: Do neurotrophic factors play critical role? Curr. Alzheimer Res. 2017, 14, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med Sci. 2018, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, L.; Paoletti, F.P.; Bellomo, G.; Mancini, A.; Simoni, S.; Di Filippo, M.; Parnetti, L. CSF and Blood Biomarkers in Neuroinflammatory and Neurodegenerative Diseases: Implications for Treatment. Trends Pharmacol. Sci. 2020, 2020.41, 1023–1037. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. New Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boylan, K. Familial amyotrophic lateral sclerosis. Neurol. Clin. 2015, 33, 807–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.A.; Lally, C.; Kupelian, V.; Flanders, W.D. Estimated Prevalence and Incidence of Amyotrophic Lateral Sclerosis and SOD1 and C9orf72 Genetic Variants. Neuroepidemiology 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Corcia, P.; Beltran, S.; Bakkouche, S.; Couratier, P. Therapeutic news in ALS. Revue. Neurologique 2021. 177, 544–549.
- Bucchia, M.; Ramirez, A.; Parente, V.; Simone, C.; Nizzardo, M.; Magri, F.; Dametti, S.; Corti, S. Therapeutic development in amyotrophic lateral sclerosis. Clin. Ther. 2015, 37, 668–680. [Google Scholar] [CrossRef]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef]
- Abati, E.; Bresolin, N.; Comi, G.; Corti, S. Advances, challenges, and perspectives in translational stem cell therapy for amyotrophic lateral sclerosis. Mol. Neurobiol. 2019, 56, 6703–6715. [Google Scholar] [CrossRef]
- Baloh, R.H.; Glass, J.D.; Svendsen, C.N. Stem cell transplantation for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2018, 31, 655–661. [Google Scholar] [CrossRef]
- Guo, Z.; Leong, M.C.-W.; Su, H.; Kwok, K.-W.; Chan, D.T.-M.; Poon, W.-S. Techniques for stereotactic neurosurgery: Beyond the frame, toward the intraoperative magnetic resonance imaging–guided and robot-assisted approaches. World Neurosurg. 2018, 116, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ende, N. The potential for the use of mononuclear cells from human umbilical cord blood in the treatment of amyotrophic lateral sclerosis in SOD1 mice. J. Med. 2000, 31, 21–30. [Google Scholar] [PubMed]
- Garbuzova-Davis, S.; Willing, A.E.; Zigova, T.; Saporta, S.; Justen, E.B.; Lane, J.C.; Hudson, J.E.; Chen, N.; Davis, C.D.; Sanberg, P.R. Intravenous Administration of Human Umbilical Cord Blood Cells in a Mouse Model of Amyotrophic Lateral Sclerosis: Distribution, Migration, and Differentiation. J. Hematotherapy Stem Cell Res. 2003, 12, 255–270. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Sanberg, C.D.; Kuzmin-Nichols, N.; Willing, A.E.; Gemma, C.; Bickford, P.C.; Miller, C.; Rossi, R.; Sanberg, P.R. Human umbilical cord blood treatment in a mouse model of ALS: Optimization of cell dose. PLoS ONE 2008, 3, e2494. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Savelieff, M.G.; Sakowski, S.A.; Feldman, E.L. Stem cell treatments for amyotrophic lateral sclerosis: A critical overview of early phase trials. Expert Opin. Investig. Drugs 2019, 28, 525–543. [Google Scholar] [CrossRef]
- Raore, B.; Federici, T.; Taub, J.; Wu, M.C.; Riley, J.; Franz, C.K.; Kliem, M.A.; Snyder, B.; Feldman, E.L.; Johe, K. Cervical multilevel intraspinal stem cell therapy: Assessment of surgical risks in Gottingen minipigs. Spine 2011, 36, E164–E171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzini, L.; Mareschi, K.; Ferrero, I.; Miglioretti, M.; Stecco, A.; Servo, S.; Carriero, A.; Monaco, F.; Fagioli, F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: A long-term safety study. Cytotherapy 2012, 14, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Sivandzade, F.; Cucullo, L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int. J. Mol. Sci. 2021, 22, 2153. [Google Scholar] [CrossRef] [PubMed]
- Nemade, D.; Thyagarajan Subramanian, V.S. An update on medical and surgical treatments of Parkinson’s disease. Aging Dis. 2021, 12, 1021–1035. [Google Scholar] [CrossRef]
- Tomishima, M.; Kirkeby, A. Bringing Advanced Therapies for Parkinson’s Disease to the Clinic: The Scientist’s Perspective. Parkinson's Dis. 2021, 1–6. [Google Scholar] [CrossRef]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Chan, L.L.; Lim, E.C.; Tan, E.K. Stem Cell Replacement Therapies in Parkinson’s Disease. Ann. Acad Med. Singap. 2019, 48, 112–114. [Google Scholar] [PubMed]
- Guo, X.; Tang, L.; Tang, X. Current Developments in Cell Replacement Therapy for Parkinson’s Disease. Neuroscience 2021, 463, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Arenas, E. Towards stem cell replacement therapies for Parkinson’s disease. Biochem. Biophys. Res. Commun. 2010, 396, 152–156. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Parmar, M. Repairing the Brain: Cell Replacement Using Stem Cell-Based Technologies. Parkinson's Dis. 2018, 8, S131–S137. [Google Scholar] [CrossRef] [Green Version]
- Barker, R.A. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 2019, 25, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, N.K.; Kumar, S.K.; Balaraju, S.; Radhakrishnan, R.C.; Bansal, A.; Dixit, A.; Rao, D.K.; Das, M.; Jan, M.; Gupta, P.K.; et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. J. Lab. Clin. Med. 2010, 155, 62–70. [Google Scholar] [CrossRef]
- Osborn, T.M.; Hallett, P.J.; Schumacher, J.M.; Isacson, O. Advantages and Recent Developments of Autologous Cell Therapy for Parkinson’s Disease Patients. Front. Cell. Neurosci. 2020, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Survival and engraftment of dopaminergic neurons manufactured by a Good Manufacturing Practice-compatible process. Cytotherapy 2014, 16, 1305–1312. [Google Scholar] [CrossRef]
- Takahashi, J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen. Ther. 2020, 13, 18–22. [Google Scholar] [CrossRef]
- Kim, T.W.; Koo, S.Y.; Studer, L. Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front. Cell Dev. Biol 2020, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Ten Ham, R.M.; Nievaart, J.C.; Hoekman, J.; Cooper, R.S.; Frederix, G.W.; Leufkens, H.G.; Klungel, O.H.; Ovelgönne, H.; Hoefnagel, M.H.; Turner, M.L. Estimation of manufacturing development costs of cell-based therapies: A feasibility study. Cytotherapy 2021, 23, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, A. Stem cell treatments for neurodegenerative diseases: Challenges from a science, business and healthcare perspective. Neurodegener. Dis. Manag. 2015, 5, 85–87. [Google Scholar] [CrossRef]




| Title | Brief Summary | Intervention in Experimental Arm | Primary Outcome | Status | NCT Number |
|---|---|---|---|---|---|
| Phase 2b, Randomized, Double-Blind, Active-Controlled Study to Assess the Efficacy and Safety of AstroStem, Autologous Adipose Tissue Derived Mesenchymal Stem Cells, in Patients with Alzheimer’s Disease | This is a phase 2b randomized, double-blind, active-controlled study with 2 treatment arms, to compare the efficacy and safety of AstroStem versus donepezil treatment in patients with mild AD. Eligible patients diagnosed with AD within one year of the start of treatment will be enrolled. Patients who are randomized into the treatment group will be administered via intravenously AstroStem and donepezil placebo every 4 weeks from Week 0 to Week 16. | Autologous adipose tissue derived mesenchymal stem cells (AdMSCs) administered intravenously and donepezil placebo. | Change from baseline to Week 28 in the ADAS-Cog score. | Not Yet Recruiting | NCT04482413 |
| Open-Label, Single-Center, Phase I/II Clinical Trial to Evaluate the Safety and the Efficacy of Exosomes Derived from Allogenic Adipose Mesenchymal Stem Cells in Patients with Mild to Moderate Dementia Due to Alzheimer’s Disease | The purpose of this study is to evaluate the safety and efficacy of Exosomes Derived from Allogenic Adipose Mesenchymal Stem Cells(MSCs-Exos)in subjects with mild to moderate dementia due to Alzheimer’s Disease. | Low dosage (5 μg) MSCs-Exos administrated for nasal drip twice a week for 12 weeks. Mild dosage (10 μg) MSCs-Exos administrated for nasal drip twice a week for 12 weeks. High dosage (20 μg) MSCs-Exos administrated for nasal drip twice a week for 12 weeks. | Number of participants with treatment-related abnormal laboratory values of liver or kidney function. Number of participants with treatment-related adverse events as assessed by Common Terminology Criteria for Adverse Events (CTCAE) v4.0. | Recruiting | NCT04388982 |
| Phase I, Prospective, Open-label Trial to Evaluate the Safety, Tolerability and Exploratory Outcomes of Multiple Allogeneic Human Mesenchymal Stem Cells (HMSC) Infusions in Patients with Mild to Moderate Alzheimer’s Disease | The purpose of this interventional research study is to test the safety, possible side effects, and possible effectiveness of human mesenchymal stem cell (HMSC) infusions when given to people with a diagnosis of mild to moderate Alzheimer’s disease. | Participants in the hMSC treatment group will receive a total of 4 doses administered intravenously, once a week, every 13 weeks within a year period. | Incidence of any Treatment-Emergent Serious Adverse Events (TE-SAEs). All adverse events will be evaluated for relationship with the study intervention. | Recruiting | NCT04040348 |
| Alzheimer’s Autism and Cognitive Impairment Stem Cell Treatment Study | The purpose of the study is to evaluate the use of autologous Bone Marrow Derived Stem Cells (BMSC) as a mean to improve cognitive impairment as occurs in Alzheimer’s Disease and other dementias and to improve behavior and socialization issues which occur in adult with autism spectrum disorder. The use of Near Infrared Light, in conjunction with the use of BMSC, will also be assessed. | Intravenous administration of BMSC Fraction (14cc). Intravenous administration of BMSC Fraction (14cc) combined with Near Infrared Light exposure. Intravenous administration of BMSC Fraction (14cc) combined with Intranasal BMSC Fraction. | Mini-Mental Status Exam (MMSE). The change from pretreatment baseline to each time point (1, 3, 6, and 12 months post-treatment) will be assessed. Autism Spectrum Quotient Exam. The change in scoring from pretreatment baseline to each time point (1, 3, 6, and 12 months post-treatment) will be assessed. | Recruiting | NCT03724136 |
| Randomized, Double-blind, Placebo-controlled, Phase I / IIa Clinical Trial for Evaluation of Safety and Potential Therapeutic Effect After Transplantation of CB-AC-02 in Patients with Alzheimer’s Disease | The objective of the study is to evaluate the safety and the potential therapeutic effects of intravenous transplantation of placenta-derived mesenchymal stem cells (CB-AC-02) in patients with Alzheimer’ disease in two treatment groups. | Group1: CB-AC-02 administration on day 0. Group2: CB-AC-02 administration on day 0 and on week 4. | The safety and tolerability of treatment with CB-AC-02 will be assessed by analysis of adverse events, abnormal findings, and standard laboratory tests. | Recruiting | NCT02899091 |
| Phase IIa Study of Allogeneic Human Mesenchymal Stem Cells in Subjects with Mild to Moderate Dementia Due to Alzheimer’s Disease | The purpose of the study is to assess the safety and tolerability of ischemia-tolerant allogeneic human mesenchymal stem cells (hMSCs) administered intravenously versus placebo to subjects with mild to moderate dementia due to Alzheimer’s disease. Secondarily, to assess the preliminary efficacy of hMSCs versus placebo in subjects with Alzheimer’s-related dementia. | Intravenous administration of allogeneic hMSCs and Lactated Riunger’s Solution. | Safety of allogeneic hMSCs administration by assessment of adverse events. | Recruiting | NCT02833792 |
| Title | Brief Summary | Intervention in Experimental Arm | Primary Outcome | Status | NCT Number |
|---|---|---|---|---|---|
| Clinical Trial in Phase II of Intramuscular Infusion of Autologous Bone Marrow Stem Cells in Patients with Amyotrophic Lateral Sclerosis | The purpose of this study is to assess the positive effects of autologous bone marrow mononuclear cells (BMNC) injection on the natural loss of motor units and on the increase in the size of the motor unit that occurs in patients with ALS during the evolution of the disease. | Intramuscular infusion of autologous BMNC into the transverse abdominal (TA) muscle of one of the lower limbs versus intramuscular infusion of saline solution (placebo) in the TA muscle of the contralateral side. | Rate of serious and non-serious adverse events related to the use of bone marrow mononuclear cells in patients with Amyotrophic Lateral Sclerosis. D50 index obtained from stimulus intensity curves. | Not yet recruiting | NCT04849065 |
| The Evaluation of the Effect of Wharton’s Jelly Mesenchymal Stem Cells (WJMSCs) on the Immune System of Patients with Amyotrophic Lateral Sclerosis | The objective of this study is to evaluate the safety of intrathecal administration of WJMSCs and the impact on the immune system of patients with Amyotrophic Lateral Sclerosis. | Three intrathecal administration of mesenchymal stem cells isolated from Wharton’s jelly. | Number of Serious Adverse Event of Special Interest (S)AESI, including meningitis, toxic encephalopathy encephalitis, high fever, and epileptic seizures not connected to conditions above. | Recruiting | NCT04651855 |
| A Phase II Study of Intrathecal Autologous Adipose-derived Mesenchymal Stromal Cells for Amyotrophic Lateral Sclerosis | The purpose of this open label, Phase II multi-site clinical study is to determine the safety and efficacy of intrathecal treatment delivered to the cerebrospinal fluid (CSF) of mesenchymal stem cells in ALS patients every 3 months for a total of 4 injections over 12 months. | Autologous adipose-derived Mesenchymal Stromal Cells (aaMSCs) will be administered intrathecally at a single dose suspended in 5-10 mL Lactated Ringer’s. Reduced dose treatments will be allowed based on specific adverse events. | Number of adverse events recorded from the time of enrollment until the end of the follow-up period or, in the case of early withdrawal, to the time of study withdrawal. | Recruiting | NCT03268603 |
| A Phase 1/2a Open-Label Study to Investigate the Safety of the Transplantation (by Injection) of Human Glial Restricted Progenitor Cells (hGRPs; Q-Cells®) Into Subjects with Amyotrophic Lateral Sclerosis (ALS): Assessment of Localized Therapeutic Activity by Blinded Observation and Lateral Transplantation (ALTA-BOLT) | This study is a non-randomized, open-label, partially blinded, sequential cohort, dose-escalation study designed to obtain preliminary data on the safety, tolerability, and early efficacy of hGRPs transplantation in subjects with ALS. Following an initial cohort receiving cell transplants unilaterally in the lumbar spinal cord, subsequent cohorts will receive escalating doses transplanted unilaterally in cervical spinal cord. Subjects and outcome measure assessors will be blinded to side of treatment. | Unilateral lumbar surgical transplantation of human cells of the glial lineage. | Safety measured by the number of therapy-related adverse events. | Not yet recruiting | NCT02478450 |
| A Multicenter Phase I/II Clinical Trial, Randomized, Controlled with Placebo, Triple Blind to Evaluate Safety, and Indications of Efficiency of the Intravenous Administration of the Therapy With 3 Doses of MSC in Patients with ASL Moderated to Severe | This is a multicenter phase I/II randomized, controlled with placebo, triple blind clinical trial aimed to evaluate the safety of intravenous administration of 3 doses of autologous mesenchymal stem cells from adipose tissue in patients with Amyotrophic Lateral Sclerosis. Forty patients will be enrolled and randomized into one of four arms and the follow-up phase, from the cell infusion/placebo, will be 6 months. | Intravenous administration of MSCs at different doses. | Number of adverse serious unexpected reactions or not, attributable to the treatment. Complications in the place of the infusion. Appearance of new neurological effect not attributable to the natural progression of pathology. | Active, not recruiting | NCT02290886 |
| Title | Brief Summary | Intervention in Experimental Arm | Primary Outcome | Status | NCT Number |
|---|---|---|---|---|---|
| Clinical Investigation of Transplantation of Neural Stem Cell-derived Neurons for the Treatment of Parkinson’s Disease | This is a prospective study to demonstrate the safety and efficacy of differentiated neurons-derived from adult CNS progenitor cells transplanted in selected patients with Parkinson’s disease. | Intracerebral stereotactic microinjections of cell suspension into basal ganglia structures | Evaluation of various aspects of Parkinson’s disease, including non-motor and motor experiences, by Unified Parkinson’s Disease Rating Scale (UPDRS) Motor scale | Not yet recruiting | NCT03309514 |
| A Safety and Efficacy Study of the Effects of Mesenchymal Stem Cells (MSCs) Differentiated into Neural Stem Cells (NSCs) on the Motor and Non-motor Symptoms in People with Parkinson’s Disease | This study is predicted to confirm the short term and long-term safety outcomes of the treatment of PD patients with umbilical cord derived stem cells. | Allogenic Umbilical Cord derived stem cells injected intravenously to enrolled PD patients. Allogenic Umbilical Cord derived stem cells (MSCs) differentiated into neural stem cells (NSCs) injected intrathecally and intravenously to enrolled PD patients. | Safety and tolerability assessment by report of Treatment-Emergent Adverse Events (TEAEs) because of the injection | Recruiting | NCT03684122 |
| Clinical Study of Stereotactic Transplantation of Human Amniotic Epithelial Stem Cells (hAESCs) in the Treatment of Parkinson’s Disease (PD) | The purpose of this study is to evaluate the safety and efficacy of stereotactic transplantation of hAESCs for Parkinson’s disease. These cells are derived from placental amnion donated after cesarean section in healthy women. | Stereotactic transplantation of hAESCs into lateral ventricles to Parkinson’s disease participants. | Safety and tolerability assessment by report of adverse events | Not yet recruiting | NCT04414813 |
| Allogeneic Bone Marrow-derived Mesenchymal Stem Cells as a Disease-modifying Therapy for Idiopathic Parkinson’s Disease: Phase IIa Double-blind Randomized Placebo Controlled Trial | The purpose of this study is to select the safest and most effective number of repeat doses of allogeneic bone marrow-derived mesenchymal stem cell (MSC) infusions to slow the progression of Parkinson’s disease | Two treatment infusions of MSC cells and 1 placebo every 3 months Three treatment infusions of MSC cells and 1 placebo every 3 months | Safest number of effective doses of MSC as measured by the Part III of the Movement Disorder Society Unified Parkinson’s disease Rating Scale (MDS-UPDRS) scale at different times. | Recruiting | NCT04506073 |
| A Randomized, Double-Blind, Single Center, Phase 2, Efficacy and Safety Study of Autologous HB-adMSCs vs Placebo for the Treatment of Patients with Parkinson’s Disease | This is a randomized, double-blind, single center, phase 2 study aimed to assess efficacy and safety of multiple Hope Biosciences adipose derived mesenchymal stem cells (HB-adMSCs) versus placebo for the treatment of Parkinson’s disease. The trial includes a screening period of up to 4 weeks, a 32-week treatment period, and a safety follow-up period of 20 weeks after the last investigational product administration. | HB-adMSCs will be administered intravenously to study participants | Evaluation of changes in MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Incidence of treatment-emergent Adverse Event (TEAEs). Incidence of special interest AE, including thromboembolic events, infections, and hypersensitivities. Laboratory values: CMP, CBC, and coagulation panel. Report of vital signs and physical examination. | Recruiting | NCT04928287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaventura, G.; Munafò, A.; Bellanca, C.M.; La Cognata, V.; Iemmolo, R.; Attaguile, G.A.; Di Mauro, R.; Di Benedetto, G.; Cantarella, G.; Barcellona, M.L.; et al. Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells 2021, 10, 1992. https://doi.org/10.3390/cells10081992
Bonaventura G, Munafò A, Bellanca CM, La Cognata V, Iemmolo R, Attaguile GA, Di Mauro R, Di Benedetto G, Cantarella G, Barcellona ML, et al. Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells. 2021; 10(8):1992. https://doi.org/10.3390/cells10081992
Chicago/Turabian StyleBonaventura, Gabriele, Antonio Munafò, Carlo Maria Bellanca, Valentina La Cognata, Rosario Iemmolo, Giuseppe Antonino Attaguile, Rosaria Di Mauro, Giulia Di Benedetto, Giuseppina Cantarella, Maria Luisa Barcellona, and et al. 2021. "Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases?" Cells 10, no. 8: 1992. https://doi.org/10.3390/cells10081992
APA StyleBonaventura, G., Munafò, A., Bellanca, C. M., La Cognata, V., Iemmolo, R., Attaguile, G. A., Di Mauro, R., Di Benedetto, G., Cantarella, G., Barcellona, M. L., Cavallaro, S., & Bernardini, R. (2021). Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells, 10(8), 1992. https://doi.org/10.3390/cells10081992