IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Adoptive T Cell Therapy Model and IL7-Fc Treatment
2.4. Flow Cytometry
2.5. Calculation of the Absolute Cell Numbers
2.6. Monitoring the Repopulation of Leukocytes in Peripheral Blood
2.7. CFSE Dilution Assay of pmel-1 CD8+ T Cells in WT and RAG 1−/− Mice
3. Results
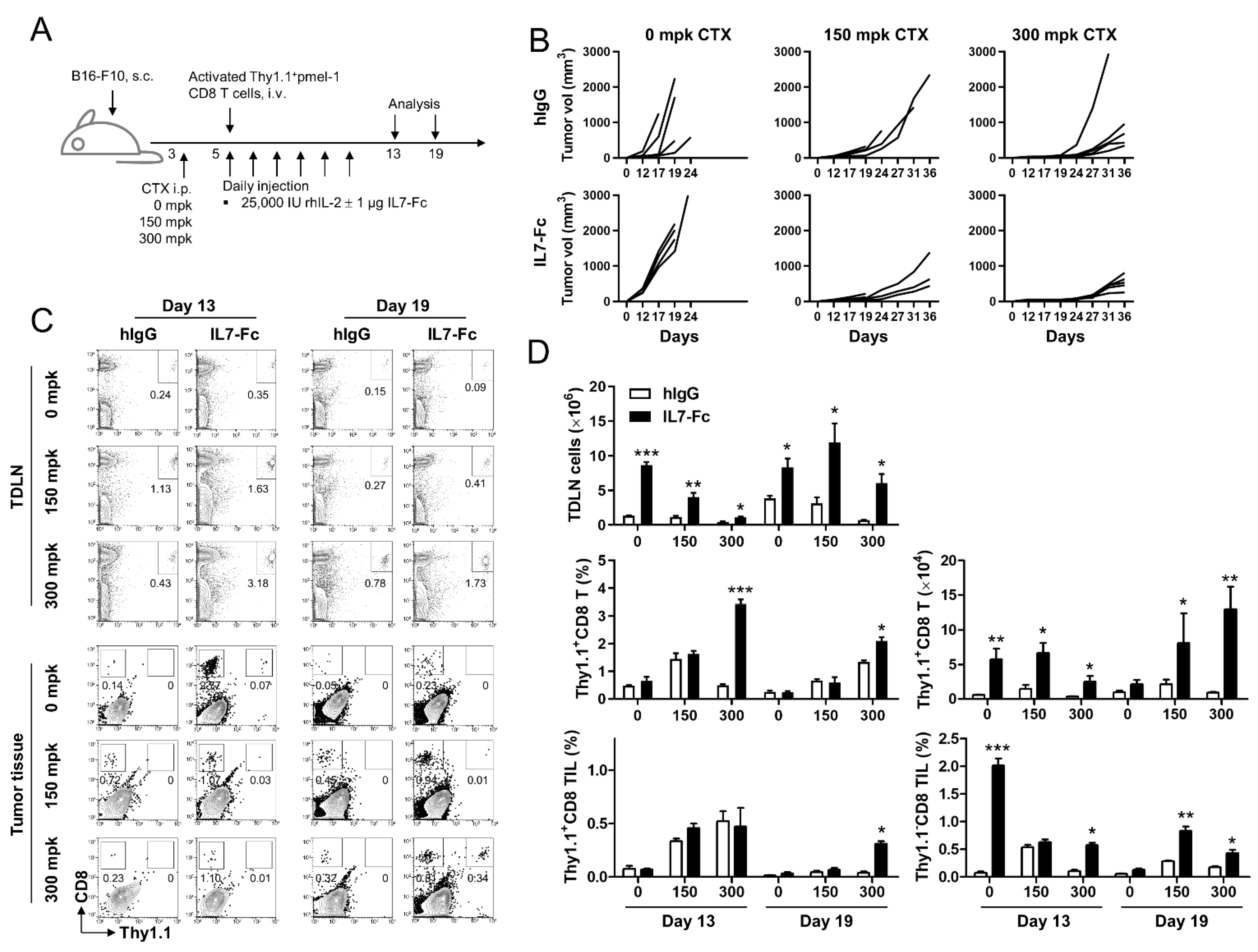
3.1. The Fc Fusion Protein of IL-7 Enhances the Anti-Tumor Effect of Activated pmel-1 CD8+ T Cells Only with Prior Lymphodepletion
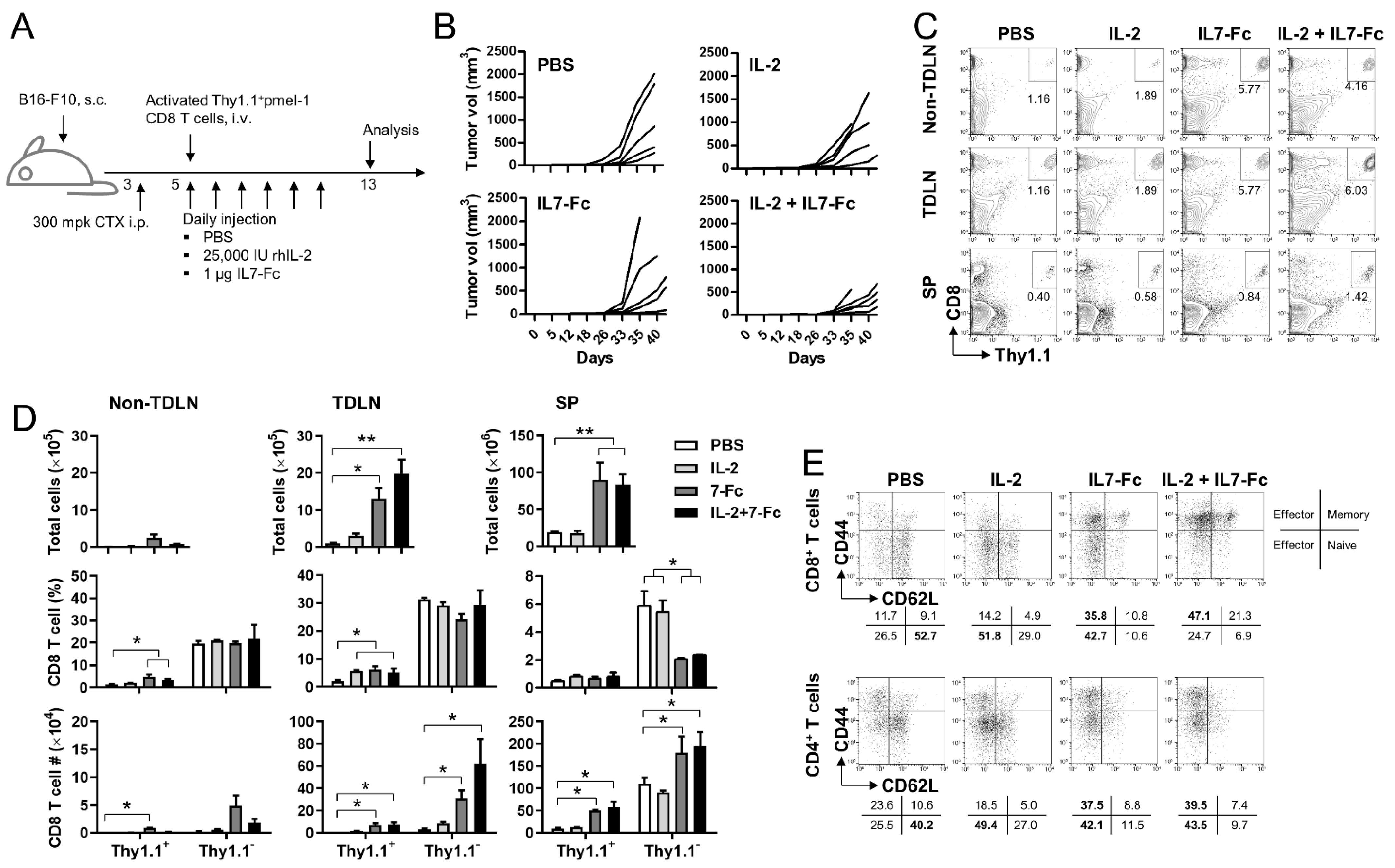
3.2. IL7-Fc in Combination with rhIL-2 Enhance the Anti-Tumor Responses of Activated pmel-1 CD8+ T Cells under Lymphopenic Condition
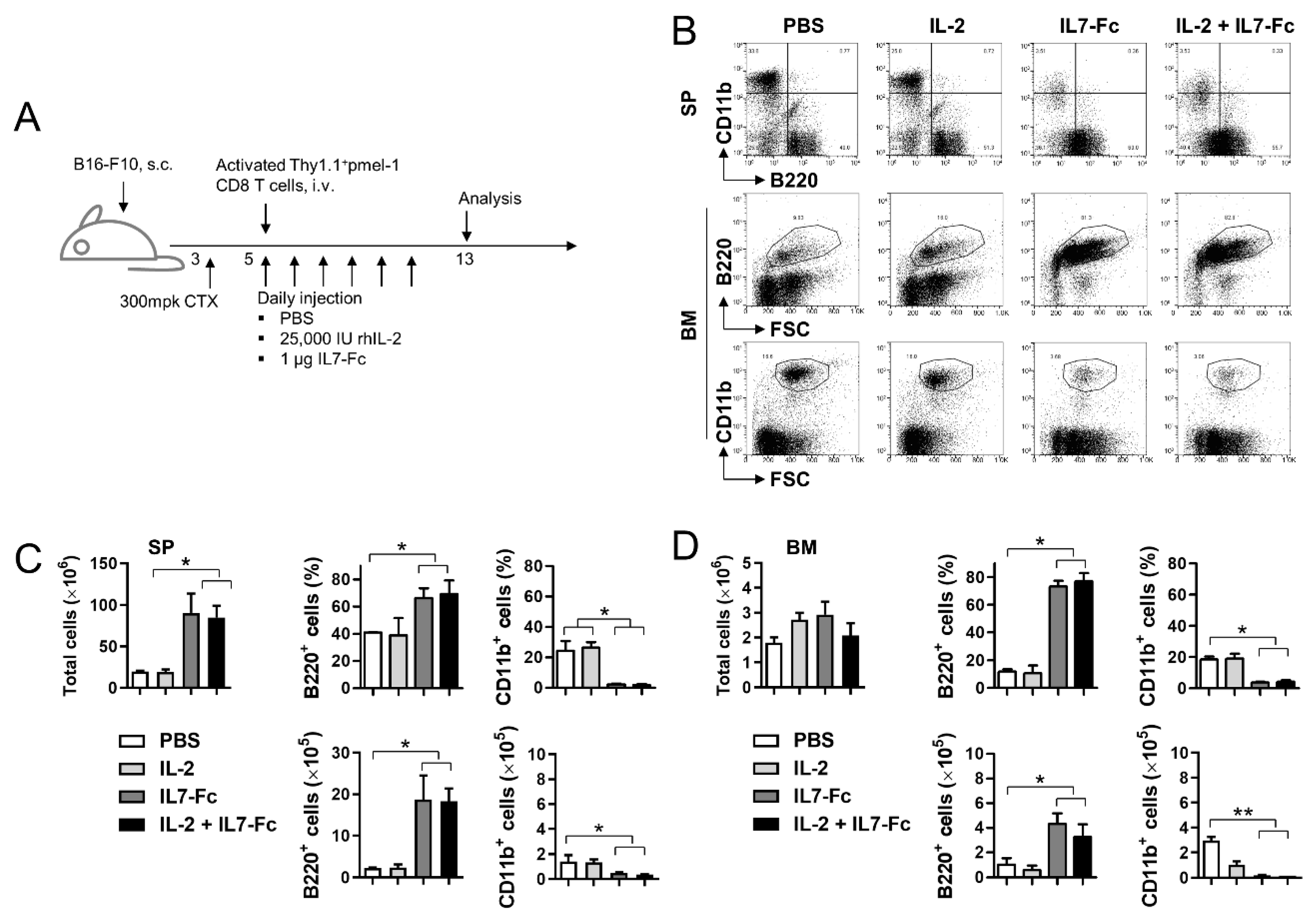
3.3. IL7-Fc Promotes the Repopulation of B Cells
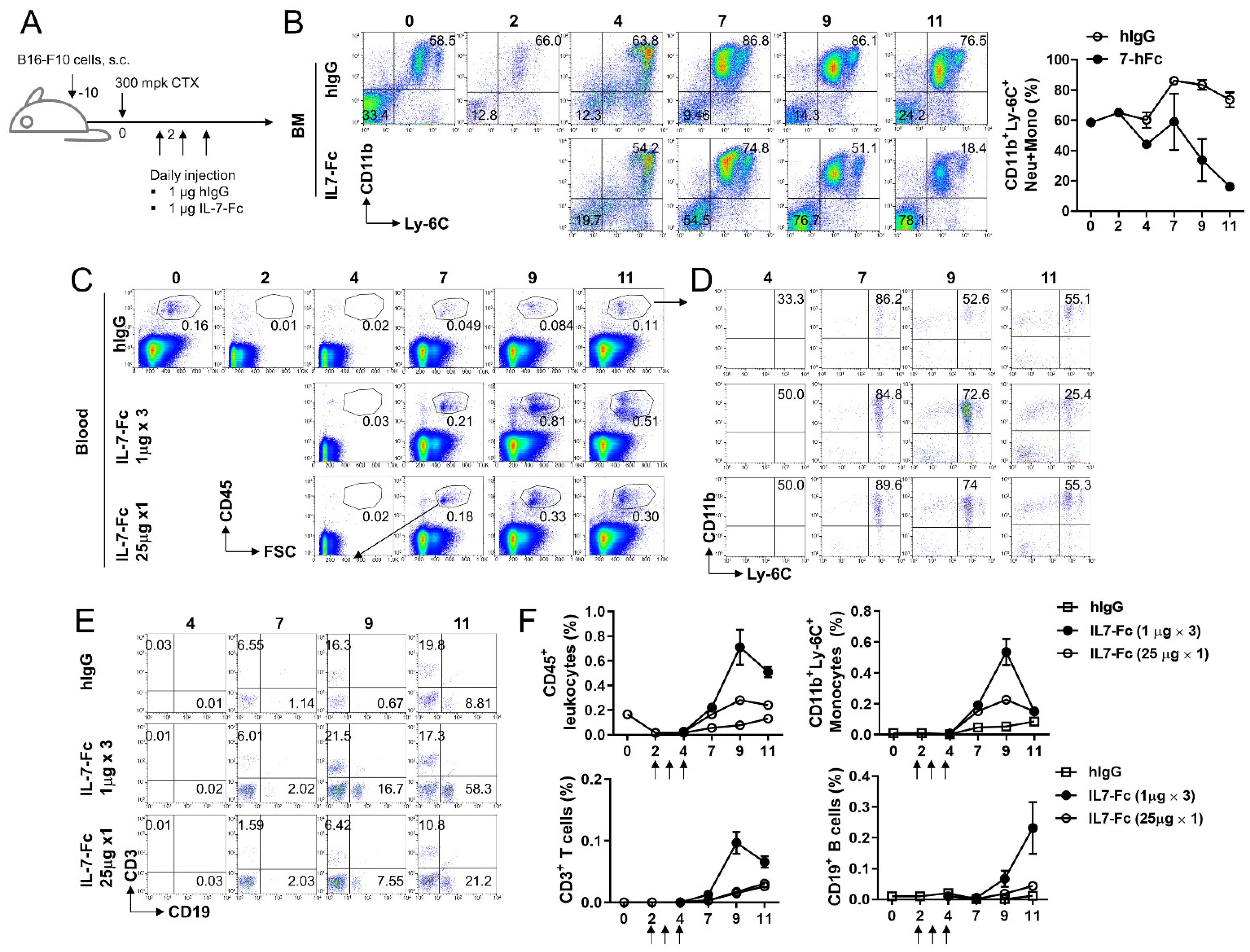
3.4. Repeated Injection of IL7-Fc Causes the Accumulation of Monocytic Cells in Peripheral Blood by Potentially Promoting the Differentiation of Myeloid Cells in Bone Marrow
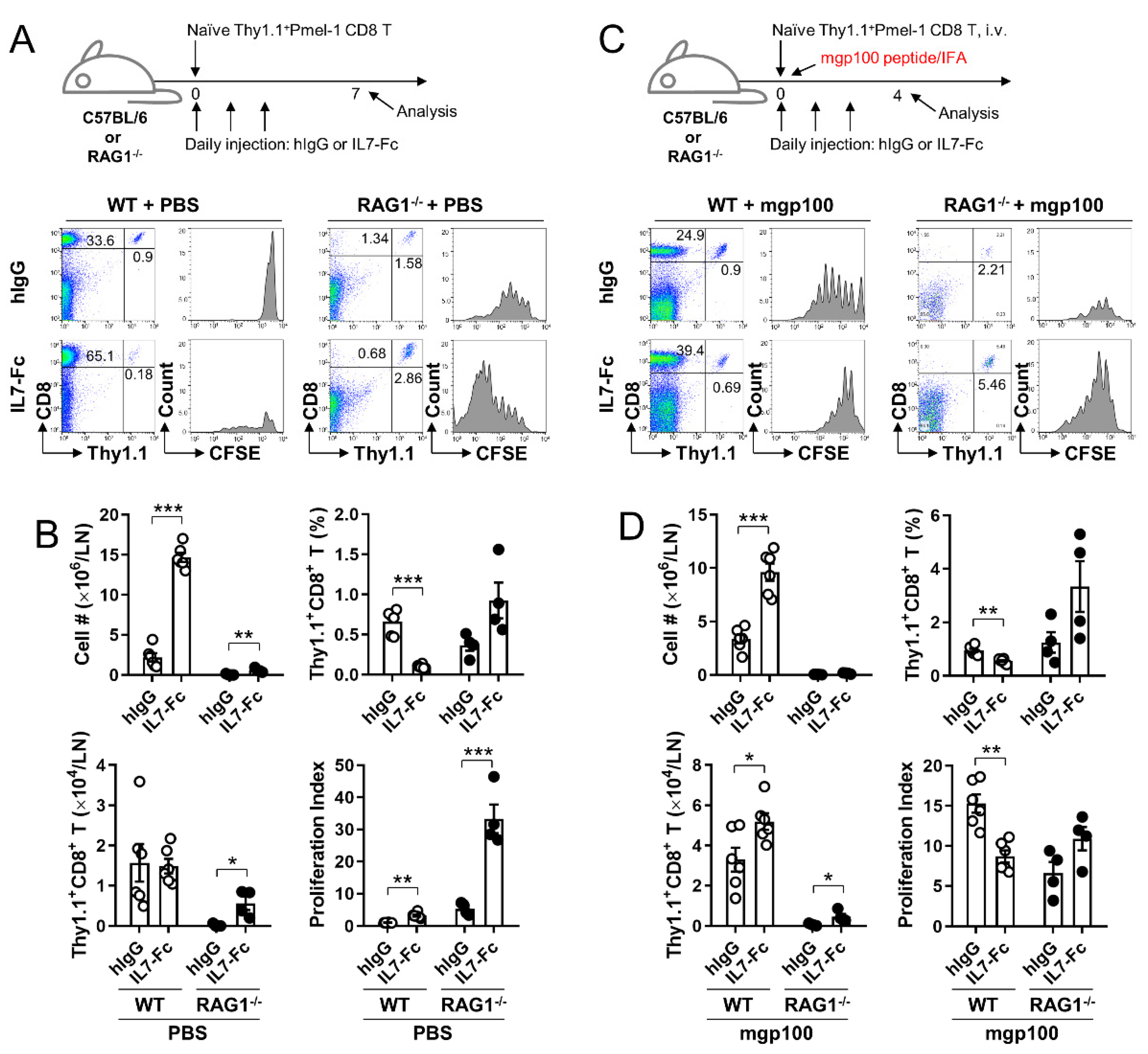
3.5. In the Lymphopenic Environment, IL7-Fc Efficiently Enhances the Proliferation of Activated pmel-1 CD8+ T Cells, Which Is Impeded in the Non-Lymphopenic Environment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinrichs, C.S.; Rosenberg, S.A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014, 257, 56–71. [Google Scholar] [CrossRef] [Green Version]
- Croci, D.O.; Fluck, M.F.Z.; Rico, M.J.; Matar, P.; Rabinovich, G.A.; Scharovsky, O.G. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol. Immunother. 2007, 56, 1687–1700. [Google Scholar] [CrossRef]
- Caswell, D.R.; Swanton, C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer Regression and Autoimmunity in Patients After Clonal Repopulation with Antitumor Lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, A.K.K.J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Busch, D.H.; Fräßle, S.P.; Sommermeyer, D.; Buchholz, V.R.; Riddell, S.R. Role of memory T cell subsets for adoptive immunotherapy. Semin. Immunol. 2016, 28, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.; Lowery, F.J.; Copeland, A.R.; Bahadiroglu, E.; Mukherjee, R.; Jia, L.; Anibal, J.T.; Sachs, A.; Adebola, S.O.; Gurusamy, D.; et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 2020, 370, 1328–1334. [Google Scholar] [CrossRef]
- Gattinoni, L.; Finkelstein, S.E.; Klebanoff, C.; Antony, P.A.; Palmer, D.; Spiess, P.J.; Hwang, L.N.; Yu, Z.; Wrzesinski, C.; Heimann, D.M.; et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005, 202, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-C.; Habtetsion, T.; Cao, Y.; Zhonglin, H.; Liu, C.; Kuczma, M.; Chen, T.; Hao, Z.; Bryan, L.; Munn, D.; et al. Adjuvant IL-7 potentiates adoptive T cell therapy by amplifying and sustaining polyfunctional antitumor CD4+ T cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Pilipow, K.; Roberto, A.; Roederer, M.; Waldmann, T.A.; Mavilio, D.; Lugli, E. IL15 and T-cell Stemness in T-cell–Based Cancer Immunotherapy. Cancer Res. 2015, 75, 5187–5193. [Google Scholar] [CrossRef] [Green Version]
- Fry, T.J.; Mackall, C.L. The Many Faces of IL-7: From Lymphopoiesis to Peripheral T Cell Maintenance. J. Immunol. 2005, 174, 6571–6576. [Google Scholar] [CrossRef]
- Bradley, L.M.; Haynes, L.; Swain, S.L. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005, 26, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 receptor expression: Intelligent design. Nat. Rev. Immunol. 2007, 7, 144–154. [Google Scholar] [CrossRef]
- Nam, H.J.; Song, M.-Y.; Choi, D.-H.; Yang, S.-H.; Jin, H.-T.; Sung, Y.-C. Marked enhancement of antigen-specific T-cell responses by IL-7-fused nonlytic, but not lytic, Fc as a genetic adjuvant. Eur. J. Immunol. 2010, 40, 351–358. [Google Scholar] [CrossRef]
- Kim, S.; Choi, D.H.; Heo, M.; Lee, B.H.; Yang, S.H.; Sung, Y.C.; Lee, H.; Shin, E.C.; Park, S.H. A Single Administration of Fc-Fused Recombinant Human IL-7 Induces Expansions of T Lymphocytes in Healthy Adult Volunteers. J Immunol. 2019, 202, 189-15. [Google Scholar]
- Kim, J.; Kim, Y.; Choi, D.; Jo, S.; Park, H.W.; Hong, S.; Park, S.; Kim, S.; Moon, S.; You, G.; et al. Hybrid Fc-fused interleukin-7 induces an inflamed tumor microenvironment and improves the efficacy of cancer immunotherapy. Clin. Transl. Immunol. 2020, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Jr, H.S.; Cumano, A.; Vieira, P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J. Exp. Med. 2005, 201, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Freeden-Jeffry, U.; Vieira, P.; Lucian, L.A.; McNeil, T.; Burdach, S.E.G.; Murray, R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995, 181, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Peschon, J.J.; Morrissey, P.J.; Grabstein, K.H.; Ramsdell, F.J.; Maraskovsky, E.; Gliniak, B.C.; Park, L.S.; Ziegler, S.F.; Williams, D.E.; Ware, C.B.; et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994, 180, 1955–1960. [Google Scholar] [CrossRef] [Green Version]
- Aiello, F.B.; Keller, J.R.; Klarmann, K.D.; Dranoff, G.; Mazzucchelli, R.; Durum, S.K. IL-7 Induces Myelopoiesis and Erythropoiesis. J. Immunol. 2007, 178, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yu, Q.; Erman, B.; Appelbaum, J.; Montoya-Durango, D.; Grimes, H.L.; Singer, A. Suppression of IL7Rα Transcription by IL-7 and Other Prosurvival Cytokines: A Novel Mechanism for Maximizing IL-7-Dependent T Cell Survival. Immunity 2004, 21, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Vranjkovic, A.; Crawley, A.M.; Gee, K.; Kumar, A.; Angel, J.B. IL-7 decreases IL-7 receptor (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int. Immunol. 2007, 19, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Schietinger, A.; Delrow, J.J.; Basom, R.S.; Blattman, J.N.; Greenberg, P.D. Rescued Tolerant CD8 T Cells Are Preprogrammed to Reestablish the Tolerant State. Science 2012, 335, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Wardemann, H.; Yurasov, S.; Schaefer, A.; Young, J.W.; Meffre, E.; Nussenzweig, M.C. Predominant Autoantibody Production by Early Human B Cell Precursors. Science 2003, 301, 1374–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Jiang, N.; Ebert, P.J.; Kidd, B.A.; Müller, S.; Lund, P.J.; Juang, J.; Adachi, K.; Tse, T.; Birnbaum, M.; et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific αβ CD8+ T Lymphocytes. Immunity 2015, 42, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Guilliams, M.; Haskó, G. Quorum sensing in the immune system. Nat. Rev. Immunol. 2018, 18, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Thaventhiran, J.; Hoffmann, A.; Magiera, L.; de la Roche, M.; Lingel, H.; Brunner-Weinzierl, M.; Fearon, D.T. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc. Natl. Acad. Sci. USA 2012, 109, E2223–E2229. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, E.M.; Cho, E.; Singh, R.; Kim, S.-H.; Han, C.; Han, S.; Lee, D.G.; Kim, Y.H.; Kwon, B.S.; Choi, B.K. IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model. Cells 2021, 10, 2018. https://doi.org/10.3390/cells10082018
Yu EM, Cho E, Singh R, Kim S-H, Han C, Han S, Lee DG, Kim YH, Kwon BS, Choi BK. IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model. Cells. 2021; 10(8):2018. https://doi.org/10.3390/cells10082018
Chicago/Turabian StyleYu, Eun M., Eunjung Cho, Rohit Singh, Seon-Hee Kim, Chungyong Han, Seongeun Han, Don G. Lee, Young H. Kim, Byoung S. Kwon, and Beom K. Choi. 2021. "IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model" Cells 10, no. 8: 2018. https://doi.org/10.3390/cells10082018




