Inborn Errors in the LRR Domain of Nod2 and Their Potential Consequences on the Function of the Receptor
Abstract
:1. Introduction
2. NLR Family and Structure
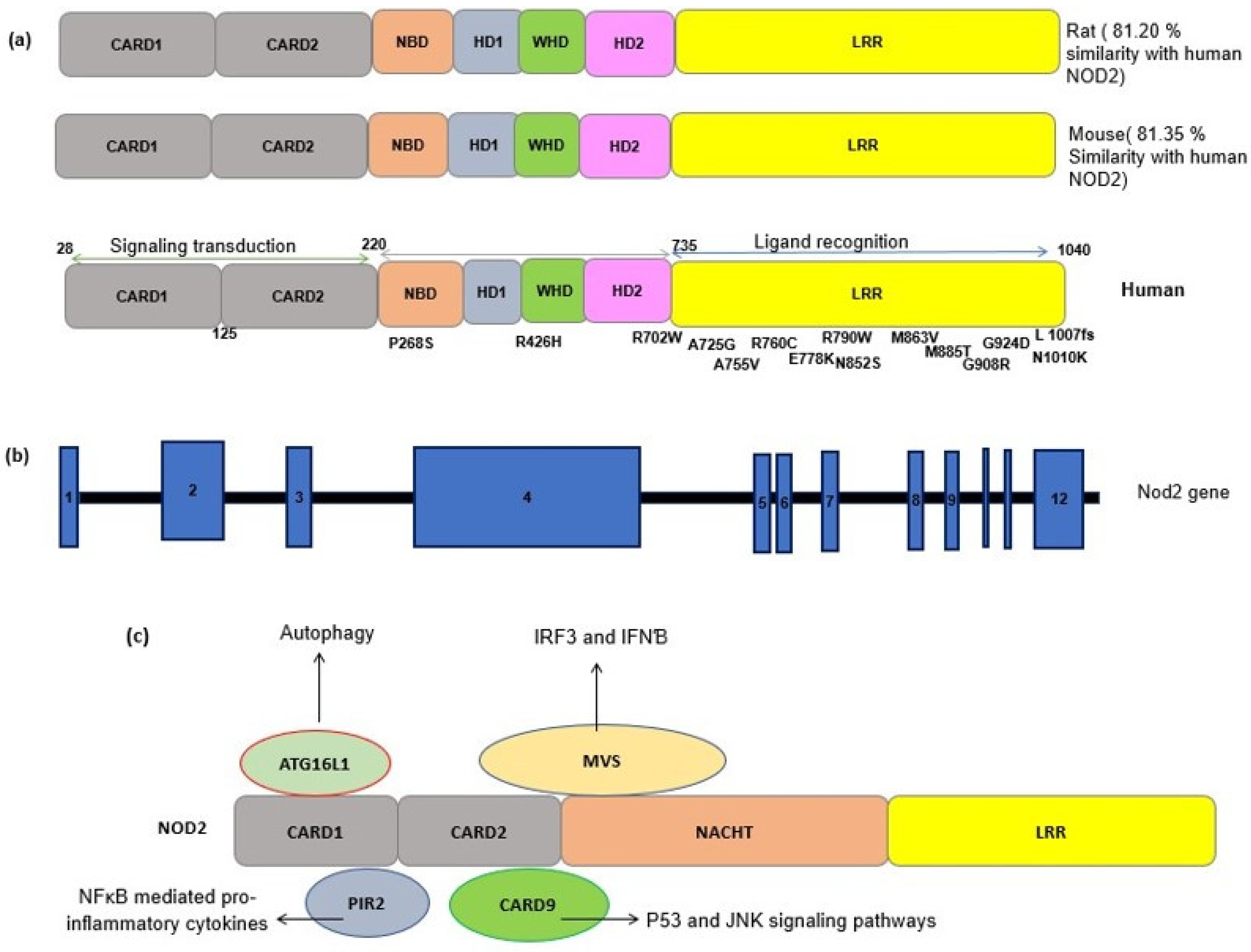
3. NOD2: Cellular and Molecular Mechanisms
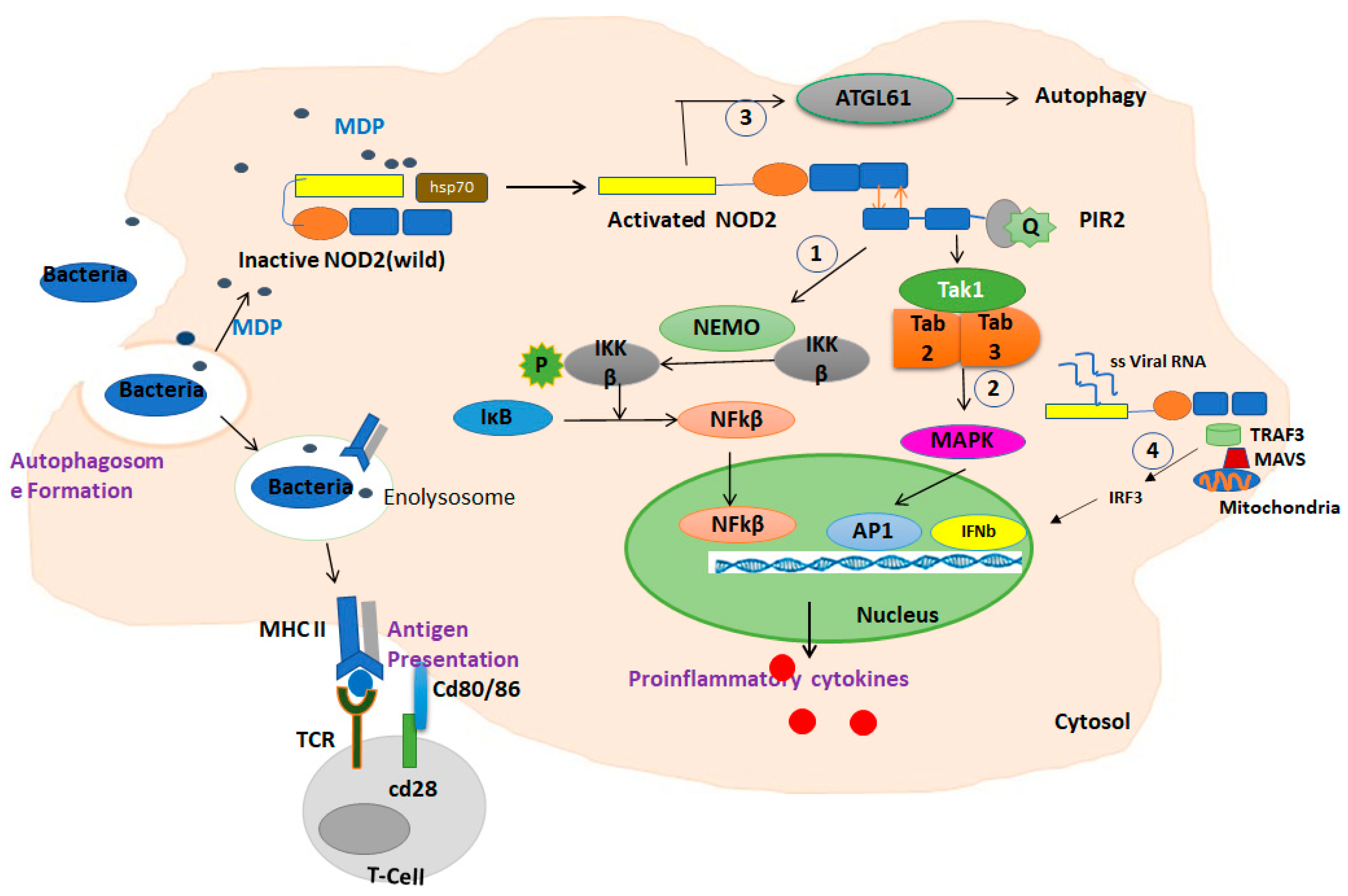
3.1. NOD2 in Immune Pathways
3.2. NOD2 and ER Stress
3.3. NOD2 and Autophagy
3.4. NOD2 and Its Importance in Pulmonary Diseases
4. NOD2 Genetics and Polymorphism
4.1. Characterization and Functionality of Variants
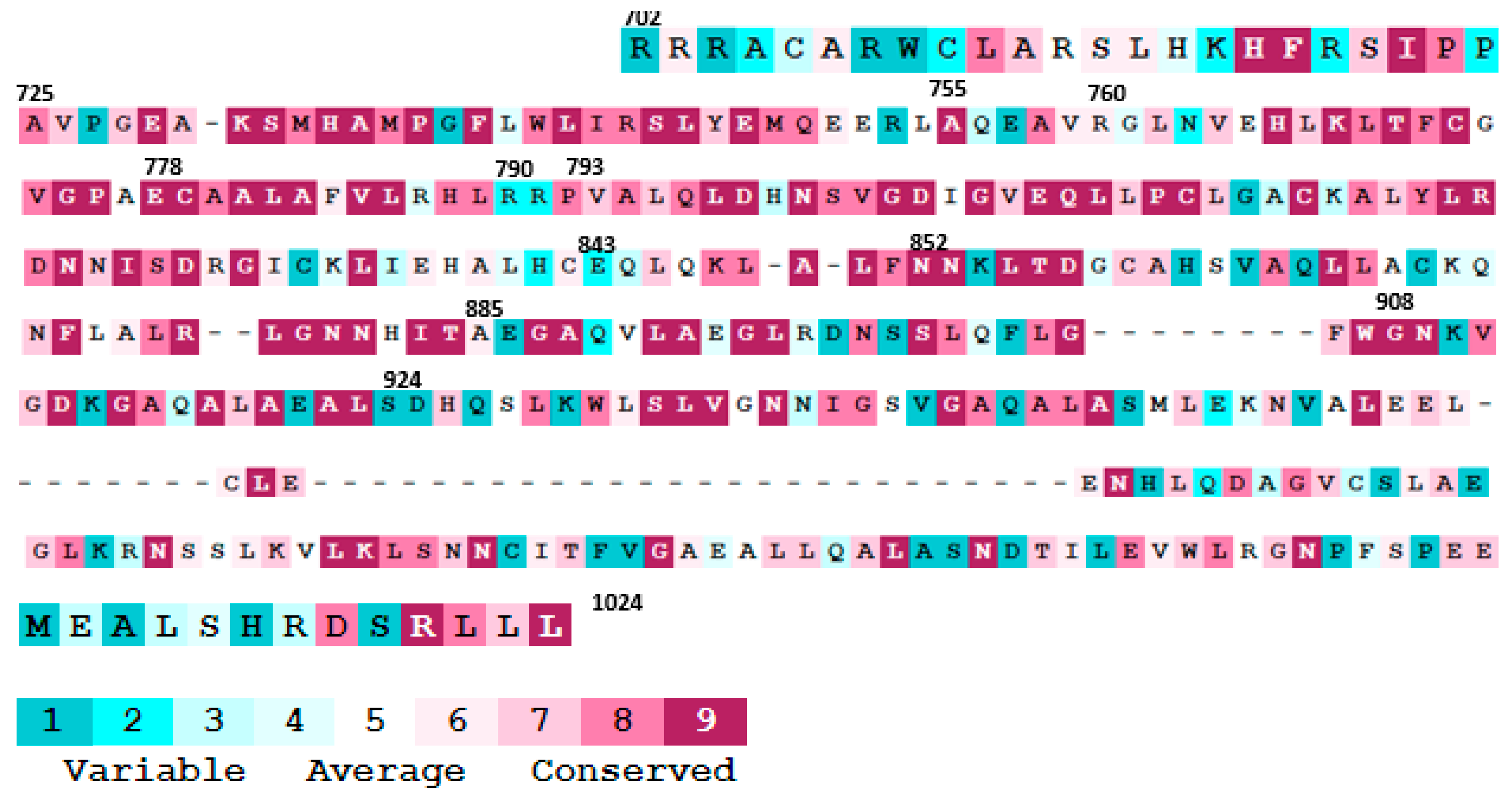
4.2. The Effect of Mutations on the NOD2 Structure and Interatomic Interactions
4.3. Determination of the SNPs’ Effects on Protein Stability and Flexibility
4.4. Prediction of Posttranslational Modification Sites
4.5. Protein–Protein Interaction (PPI) Analysis
5. NOD2: Recent Progress and Future Research Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrillo, J.L.M.; García, F.P.C.; Coronado, O.G.; García, M.A.M.; Cordero, J.F.C. Physiology and Pathology of Innate Immune Response against Pathogens; IntechOpen: London, UK, 2017. [Google Scholar]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Proell, M.; Riedl, S.J.; Fritz, J.H.; Rojas, A.M.; Schwarzenbacher, R. The Nod-like receptor (NLR) family: A tale of similarities and differences. PLoS ONE 2008, 3, e2119. [Google Scholar] [CrossRef]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef]
- Ye, Z.; Ting, J.P. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr. Opin. Immunol. 2008, 20, 3–9. [Google Scholar] [CrossRef]
- King, A.E.; Horne, A.W.; Hombach-Klonisch, S.; Mason, J.; Critchley, H.O. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: A potential role in innate immune protection and menstruation. Mol. Hum. Reprod. 2009, 15, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.A.; Simanski, M.; Rademacher, F.; Schroder, L.; Harder, J. The pattern recognition receptor NOD2 mediates Staphylococcus aureus-induced IL-17C expression in keratinocytes. J. Investig. Dermatol. 2014, 134, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trindade, B.C.; Chen, G.Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol. Rev. 2020, 297, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-like receptors: Versatile cytosolic sentinels. Physiol. Rev. 2015, 95, 149–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersse, K.; Bertrand, M.J.; Lamkanfi, M.; Vandenabeele, P. NOD-like receptors and the innate immune system: Coping with danger, damage and death. Cytokine Growth Factor Rev. 2011, 22, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Park, J.H.; Shaw, M.H.; Marina-Garcia, N.; Chen, G.; Kim, Y.G.; Núñez, G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell. Microbiol. 2008, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strober, W.; Watanabe, T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunol. 2011, 4, 484–495. [Google Scholar] [CrossRef]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Vernaldi, S.; Maekawa, T. Evolution and conservation of plant NLR functions. Front. Immunol. 2013, 4, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, R.; Warner, N.; Inohara, N.; Nunez, G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity 2014, 41, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, M.T.; Carroll, I.M.; Grossniklaus, E.; White, A.; von Furstenberg, R.J.; Barner, R.; Fodor, A.A.; Henning, S.J.; Sartor, R.B.; Gulati, A.S. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut 2014, 63, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Homer, C.R.; Richmond, A.L.; Rebert, N.A.; Achkar, J.P.; McDonald, C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology 2010, 139, 1630–1641. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.N.; Rajaram, M.V.; Azad, A.K.; Amer, A.O.; Valdivia-Arenas, M.A.; Park, J.H.; Nunez, G.; Schlesinger, L.S. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell. Microbiol. 2011, 13, 402–418. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Martinez, D.A.; Nunez-Avellaneda, D.; Castanon-Sanchez, C.A.; Salazar, M.I. NOD2: Activation during bacterial and viral infections, polymorphisms and potential as therapeutic target. Rev. Investig. Clin. 2018, 70, 18–28. [Google Scholar] [CrossRef]
- Negroni, A.; Colantoni, E.; Vitali, R.; Palone, F.; Pierdomenico, M.; Costanzo, M.; Cesi, V.; Cucchiara, S.; Stronati, L. NOD2 induces autophagy to control AIEC bacteria infectiveness in intestinal epithelial cells. Inflamm. Res. 2016, 65, 803–813. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, J.; Li, X.; Blankson, S.; Zhao, S.; Cai, J.; Jiang, Y.; Redmond, H.P.; Wang, J.H. NF-κB activation is critical for bacterial lipoprotein tolerance-enhanced bactericidal activity in macrophages during microbial infection. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Beynon, V.; Cotofana, S.; Brand, S.; Lohse, P.; Mair, A.; Wagner, S.; Mussack, T.; Ochsenkühn, T.; Folwaczny, M.; Folwaczny, C. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm. Bowel Dis. 2008, 14, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.H.; Girardin, S.E.; Fitting, C.; Werts, C.; Mengin-Lecreulx, D.; Caroff, M.; Cavaillon, J.M.; Philpott, D.J.; Adib-Conquy, M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1-and NOD2-activating agonists. Eur. J. Immunol. 2005, 35, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Balaji, K.N. Intracellular pathogen sensor NOD2 programs macrophages to trigger Notch1 activation. J. Biol. Chem. 2011, 286, 5823–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.J.; Kang, M.J.; Lee, S.J.; Kim, C.H.; Kim, J.C.; Kim, T.H.; Kim, D.J.; Kim, D.; Núñez, G.; Park, J.H. Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils. Immunology 2014, 143, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manni, M.; Ding, W.; Stohl, L.L.; Granstein, R.D. Muramyl dipeptide induces Th17 polarization through activation of endothelial cells. J. Immunol. 2011, 186, 3356–3363. [Google Scholar] [CrossRef] [Green Version]
- van Beelen, A.J.; Zelinkova, Z.; Taanman-Kueter, E.W.; Muller, F.J.; Hommes, D.W.; Zaat, S.A.; Kapsenberg, M.L.; de Jong, E.C. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 2007, 27, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Reis e Sousa, C. Dendritic cells in a mature age. Nat. Reviews. Immunol. 2006, 6, 476–483. [Google Scholar] [CrossRef]
- Magalhaes, J.G.; Rubino, S.J.; Travassos, L.H.; Le Bourhis, L.; Duan, W.; Sellge, G.; Geddes, K.; Reardon, C.; Lechmann, M.; Carneiro, L.A. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc. Natl. Acad. Sci. USA 2011, 108, 14896–14901. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Aiba, S.; Shibata, K.-I.; Ohteki, T.; Takada, H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef] [Green Version]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain, O.; Owens, B.M.; Pichulik, T.; Allan, P.; Khatamzas, E.; Leslie, A.; Steevels, T.; Sharma, S.; Mayer, A.; Catuneanu, A.M.; et al. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 2013, 39, 521–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef]
- Dugan, J.W.; Albor, A.; David, L.; Fowlkes, J.; Blackledge, M.T.; Martin, T.M.; Planck, S.R.; Rosenzweig, H.L.; Rosenbaum, J.T.; Davey, M.P. Nucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol. Immunol. 2009, 47, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Wannigama, D.L.; Jacquet, A. NOD2-dependent BCG-induced trained immunity: A way to regulate innate responses to SARS-CoV2? Int. J. Infect. Dis. 2020, 101, 52–55. [Google Scholar] [CrossRef]
- Kim, J.; Yang, Y.L.; Jang, Y.-S. Human β-defensin 2 is involved in CCR2-mediated Nod2 signal transduction, leading to activation of the innate immune response in macrophages. Immunobiology 2019, 224, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zangara, M.T.; Johnston, I.; Johnson, E.E.; McDonald, C. Mediators of metabolism: An unconventional role for NOD1 and NOD2. Int. J. Mol. Sci. 2021, 22, 1156. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chavez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Caruso, R.; Nunez, G. Innate Immunity: ER Stress Recruits NOD1 and NOD2 for Delivery of Inflammation. Curr. Biol. 2016, 26, R508–R511. [Google Scholar] [CrossRef] [Green Version]
- Puleston, D.J.; Simon, A.K. Autophagy in the immune system. Immunology 2014, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K.; Tait, S.W.; Lamkanfi, M.; Amer, A.O.; Nunez, G.; Pagès, G.; Pouysségur, J.; McGargill, M.A.; Green, D.R.; Kanneganti, T.-D. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 2011, 286, 42981–42991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butera, A.; Di Paola, M.; Pavarini, L.; Strati, F.; Pindo, M.; Sanchez, M.; Cavalieri, D.; Boirivant, M.; De Filippo, C. Nod2 deficiency in mice is associated with microbiota variation favouring the expansion of mucosal CD4+ LAP+ regulatory cells. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorbara, M.T.; Ellison, L.K.; Ramjeet, M.; Travassos, L.H.; Jones, N.L.; Girardin, S.E.; Philpott, D.J. The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity 2013, 39, 858–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travassos, L.H.; Carneiro, L.A.; Ramjeet, M.; Hussey, S.; Kim, Y.-G.; Magalhães, J.G.; Yuan, L.; Soares, F.; Chea, E.; Le Bourhis, L. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010, 11, 55–62. [Google Scholar] [CrossRef]
- Cooney, R.; Baker, J.; Brain, O.; Danis, B.; Pichulik, T.; Allan, P.; Ferguson, D.J.; Campbell, B.J.; Jewell, D.; Simmons, A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 2010, 16, 90–97. [Google Scholar] [CrossRef]
- Chaput, C.; Sander, L.E.; Suttorp, N.; Opitz, B. NOD-like receptors in lung diseases. Front. Immunol. 2013, 4, 393. [Google Scholar] [CrossRef] [Green Version]
- Jurez, E.; Carranza, C.; Hernández-Sánchez, F.; León-Contreras, J.C.; Hernández-Pando, R.; Escobedo, D.; Torres, M.; Sada, E. NOD 2 enhances the innate response of alveolar macrophages to M ycobacterium tuberculosis in humans. Eur. J. Immunol. 2012, 42, 880–889. [Google Scholar] [CrossRef]
- Ferwerda, G.; Girardin, S.E.; Kullberg, B.-J.; Le Bourhis, L.; De Jong, D.J.; Langenberg, D.M.; Van Crevel, R.; Adema, G.J.; Ottenhoff, T.H.; Van der Meer, J.W. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005, 1, e34. [Google Scholar] [CrossRef]
- Gonzalez-Mancera, M.S.; Forghani, I.; Mirsaeidi, M. Missense (p. Glu778Lys) and (p. Gly908Arg) variants of NOD2 gene are associated with recurrent pulmonary non-tuberculous mycobacterial infections. Scand. J. Immunol. 2020, 92, e12935. [Google Scholar] [CrossRef]
- Hamzaoui, K.; Abid, H.; Berraies, A.; Ammar, J.; Hamzaoui, A. NOD2 is highly expressed in Behçet disease with pulmonary manifestations. J. Inflamm. 2012, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kinose, D.; Ogawa, E.; Kudo, M.; Marumo, S.; Kiyokawa, H.; Hoshino, Y.; Hirai, T.; Chin, K.; Muro, S.; Mishima, M. Association of COPD exacerbation frequency with gene expression of pattern recognition receptors in inflammatory cells in induced sputum. Clin. Respir. J. 2016, 10, 11–21. [Google Scholar] [CrossRef]
- Duan, W.; Mehta, A.K.; Magalhaes, J.G.; Ziegler, S.F.; Dong, C.; Philpott, D.J.; Croft, M. Innate signals from Nod2 block respiratory tolerance and program TH2-driven allergic inflammation. J. Allergy Clin. Immunol. 2010, 126, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Xu, Q.; Zhou, C.; Zhou, L.; Dai, W.; Ji, G. The association of nucleotide-binding oligomerization domain 2 gene polymorphisms with the risk of asthma in the Chinese Han population. Mol. Genet. Genom. Med. 2019, 7, e00675. [Google Scholar] [CrossRef] [Green Version]
- Ehtesham, N.; Alani, B.; Mortazavi, D.; Azhdari, S.; Kenarangi, T.; Esmaeilzadeh, E.; Pakzad, B. Association of rs3135500 and rs3135499 Polymorphisms in the MicroRNA-binding Site of Nucleotide-binding Oligomerization Domain 2 (NOD2) Gene with Susceptibility to Rheumatoid Arthritis. Iran. J. Allergy Asthma Immunol. 2021, 1–10. [Google Scholar]
- Icduygu, F.M.; Erdogan, M.O.; Ulasli, S.S.; Yildiz, H.G.; Celik, Z.S.; Unlu, M.; Solak, M. Is There an Association Between NOD2 Gene Polymorphisms and Chronic Obstructive Pulmonary Disease Progression? Int. J. Hum. Genet. 2017, 17, 86–96. [Google Scholar] [CrossRef]
- Ni, G.; Chen, Y.; Wu, F.; Zhu, P.; Song, L. NOD2 promotes cell proliferation and inflammatory response by mediating expression of TSLP in human airway smooth muscle cells. Cell. Immunol. 2017, 312, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-Y.; Hwang, N.; Park, Y.-J.; Perrella, M.A.; Chung, S.W. NOD2 deficiency exacerbates hypoxia-induced pulmonary hypertension and enhances pulmonary vascular smooth muscle cell proliferation. Oncotarget 2018, 9, 12671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polverino, F.; Balestro, E.; Spagnolo, P. Clinical presentations, pathogenesis, and therapy of sarcoidosis: state of the art. J. Clin. Med. 2020, 9, 2363. [Google Scholar] [CrossRef]
- Sato, H.; Williams, H.; Spagnolo, P.; Abdallah, A.; Ahmad, T.; Orchard, T.; Copley, S.; Desai, S.; Wells, A.; Du Bois, R. CARD15/NOD2 polymorphisms are associated with severe pulmonary sarcoidosis. Eur. Respir. J. 2010, 35, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gazouli, M.; Koundourakis, A.; Ikonomopoulos, J.; Gialafos, E.J.; Rapti, A.; Gorgoulis, V.G.; Kittas, C. CARD15/NOD2, CD14, and toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2006, 23, 23–29. [Google Scholar]
- Schürmann, M.; Valentonyte, R.; Hampe, J.; Müller-Quernheim, J.; Schwinger, E.; Schreiber, S. CARD15 gene mutations in sarcoidosis. Eur. Respir. J. 2003, 22, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnard, V.; Calender, A.; Bouvry, D.; Pacheco, Y.; Chapelon-Abric, C.; Jeny, F.; Nunes, H.; Planès, C.; Valeyre, D. G908R NOD2 variant in a family with sarcoidosis. Respir. Res. 2018, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-G.; Ji, X.-Q.; Liu, X.; Li, H.-J.; Zhang, C.-Q. Pulmonary manifestations of Crohn’s disease. World J. Gastroenterol. 2014, 20, 133. [Google Scholar] [CrossRef]
- Storch, I.; Sachar, D.; Katz, S. Pulmonary manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athayde, R.A.B.d.; Costa, F.M.d.; Nascimento, E.C.T.d.; Sales, R.K.B.d.; Costa, A.N. Pulmonary involvement in Crohn’s disease. J. Bras. De Pneumol. 2018, 44, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Massart, A.; Hunt, D.P. Pulmonary manifestations of inflammatory bowel disease. Am. J. Med. 2020, 133, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.M.; Ma, X.; Graviss, E.A. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J. Infect. Dis. 2008, 197, 1713–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaahl, M.; Winter, T.; Warnich, L.; Kotze, M. Analysis of the three common mutations in the CARD15 gene (R702W, G908R and 1007fs) in South African colored patients with inflammatory bowel disease. Mol. Cell. Probes. 2005, 19, 278–281. [Google Scholar] [CrossRef]
- Pan, H.; Dai, Y.; Tang, S.; Wang, J. Polymorphisms of NOD2 and the risk of tuberculosis: A validation study in the Chinese population. Int. J. Immunogenet. 2012, 39, 233–240. [Google Scholar] [CrossRef]
- Sechi, L.A.; Gazouli, M.; Ikonomopoulos, J.; Lukas, J.C.; Scanu, A.M.; Ahmed, N.; Fadda, G.; Zanetti, S. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn’s disease, and Sardinians: The way ahead. J. Clin. Microbiol. 2005, 43, 5275–5277. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Gaur, R.; Mittal, M.; Biswas, S.; Das, R.; Girdhar, B.; Bajaj, B.; Katoch, V.; Kumar, A.; Mohanty, K. Absence of nucleotide-binding oligomerization domain-containing protein 2 variants in patients with leprosy and tuberculosis. Int. J. Immunogenet. 2012, 39, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Nebel, A.; Kwiatkowski, R.; van Helden, P.D.; Hoal, E.G.; Schreiber, S. Host susceptibility to tuberculosis: CARD15 polymorphisms in a South African population. Mol. Cell. Probes 2007, 21, 148–151. [Google Scholar] [CrossRef]
- Diler, S.B.; Polat, F.; Yaraş, S. The P268S and M863V Polymorphisms of the NOD2/CARD15 gene in Crohn’s disease and ulcerative colitis. Cytol. Genet. 2019, 53, 424–429. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Zhang, Y.; Cheng, X.; Guo, X.; Yang, X. NOD2/CARD15 gene polymorphisms and sarcoidosis susceptibility: Review and meta-analysis. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 115. [Google Scholar]
- Cubillos-Angulo, J.M.; Fernandes, C.D.; Araújo, D.N.; Carmo, C.A.; Arriaga, M.B.; Andrade, B.B. The influence of single nucleotide polymorphisms of NOD2 or CD14 on susceptibility to tuberculosis: A systematic review. Syst. Rev. 2021, 10, 174. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, S.; Wei, W.; Zheng, Q.; Wang, Y.; Ying, X.; Lu, D. Correlation between MBL2/CD14/TNF-α gene polymorphisms and susceptibility to spinal tuberculosis in Chinese population. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhao, Z.; Chen, F.; Zhang, L.; Li, G.; Ma, K.; Bai, X.; Zuo, Y. Polymorphisms in the promoter of the CD14 gene and their associations with susceptibility to pulmonary tuberculosis. Tissue Antigens 2012, 80, 437–443. [Google Scholar] [CrossRef]
- Zhao, M.; Xue, Y.; Zhao, Z.; Li, F.; Fan, D.; Wei, L.; Sun, X.; Zhang, X.; Wang, X.; Zhang, Y. Association of CD14 G(-1145)A and C(-159)T polymorphisms with reduced risk for tuberculosis in a Chinese Han population. Genet. Mol. Res. 2012, 11, 3425–3431. [Google Scholar] [CrossRef]
- Alavi-Naini, R.; Salimi, S.; Sharifi-Mood, B.; Davoodikia, A.; Moody, B.; Naghavi, A. Association between the CD14 gene C-159T polymorphism and serum soluble CD14 with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 1383–1387. [Google Scholar] [CrossRef]
- Rosas-Taraco, A.G.; Revol, A.; Salinas-Carmona, M.C.; Rendon, A.; Caballero-Olin, G.; Arce-Mendoza, A.Y. CD14 C(-159)T polymorphism is a risk factor for development of pulmonary tuberculosis. J. Infect. Dis. 2007, 196, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Ge, H.; Xu, L.; Xu, F. CD14–159C/T polymorphism contributes to the susceptibility to tuberculosis: Evidence from pooled 1700 cases and 1816 controls. Mol. Biol. Rep. 2014, 41, 3481–3486. [Google Scholar] [CrossRef]
- Girardelli, M.; Loganes, C.; Pin, A.; Stacul, E.; Decleva, E.; Vozzi, D.; Baj, G.; De Giacomo, C.; Tommasini, A.; Bianco, A.M. Novel NOD2 mutation in early-onset inflammatory bowel phenotype. Inflamm. Bowel Dis. 2018, 24, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Frade-Proud’Hon-Clerc, S.; Smol, T.; Frenois, F.; Sand, O.; Vaillant, E.; Dhennin, V.; Bonnefond, A.; Froguel, P.; Fumery, M.; Guillon-Dellac, N. A novel rare missense variation of the NOD2 gene: Evidences of implication in Crohn’s disease. Int. J. Mol. Sci. 2019, 20, 835. [Google Scholar] [CrossRef] [Green Version]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipoor, S.D.; Mirsaeidi, M. Inborn errors E778K and G908R in NOD2 gene increase risk of nontuberculous mycobacterial infection: A computational study. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tanabe, T.; Chamaillard, M.; Ogura, Y.; Zhu, L.; Qiu, S.; Masumoto, J.; Ghosh, P.; Moran, A.; Predergast, M.M.; Tromp, G. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004, 23, 1587–1597. [Google Scholar] [CrossRef]
- Maekawa, S.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Crystal structure of NOD2 and its implications in human disease. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hugot, J.-P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.-P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Da Ros, S.; Nelson, J.; Zhu, X.; Jiang, T.; Okram, B.; Jiang, S.; Michellys, P.-Y.; Iskandar, M.; Espinola, S. Identification of potent and selective RIPK2 inhibitors for the treatment of inflammatory diseases. ACS Med. Chem. Lett. 2017, 8, 1048–1053. [Google Scholar] [CrossRef]
- Honjo, H.; Watanabe, T.; Kamata, K.; Minaga, K.; Kudo, M. RIPK2 as a new therapeutic target in inflammatory bowel diseases. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nachbur, U.; Stafford, C.A.; Bankovacki, A.; Zhan, Y.; Lindqvist, L.M.; Fiil, B.K.; Khakham, Y.; Ko, H.-J.; Sandow, J.J.; Falk, H. A RIPK2 inhibitor delays NOD signalling events yet prevents inflammatory cytokine production. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Aguayo-Ortiz, R.; Danmaliki, G.I.; Zare, A.; Said, A.; Moore, J.; Pandya, V.; Manaloor, R.; Fong, S.; Blankstein, A.R. Identification and characterization of novel receptor-interacting serine/threonine-protein kinase 2 inhibitors using structural similarity analysis. J. Pharmacol. Exp. Ther. 2018, 365, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Hrdinka, M.; Schlicher, L.; Dai, B.; Pinkas, D.M.; Bufton, J.C.; Picaud, S.; Ward, J.A.; Rogers, C.; Suebsuwong, C.; Nikhar, S. Small molecule inhibitors reveal an indispensable scaffolding role of RIPK 2 in NOD 2 signaling. EMBO J. 2018, 37, e99372. [Google Scholar] [CrossRef] [PubMed]
- Topal, Y.; Gyrd-Hansen, M. RIPK2 NODs to XIAP and IBD. Semin. Cell Dev. Biol. 2021, 109, 144–150. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Marrack, P. Old and new adjuvants. Curr. Opin. Immunol. 2017, 47, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Okada, H.; Kalinski, P.; Ueda, R.; Hoji, A.; Kohanbash, G.; Donegan, T.E.; Mintz, A.H.; Engh, J.A.; Bartlett, D.L.; Brown, C.K. Induction of CD8+ T-cell responses against novel glioma–associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011, 29, 330. [Google Scholar]
- Hung, I.F.; Zhang, A.J.; To, K.K.; Chan, J.F.; Li, C.; Zhu, H.-S.; Li, P.; Li, C.; Chan, T.-C.; Cheng, V.C. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: A double blind randomized controlled trial. Clin. Infect. Dis. 2014, 59, 1246–1255. [Google Scholar] [CrossRef]
- Eng, N.F.; Bhardwaj, N.; Mulligan, R.; Diaz-Mitoma, F. The potential of 1018 ISS adjuvant in hepatitis B vaccines: HEPLISAV™ review. Hum. Vaccines Immunother. 2013, 9, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, A.; Papagno, L.; Vernejoul, F.; Lioux, T.; Jospin, F.; Chanut, B.; Perouzel, E.; Rochereau, N.; Appay, V.; Verrier, B. New chimeric TLR7/NOD2 agonist is a potent adjuvant to induce mucosal immune responses. EBioMedicine 2020, 58, 102922. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Tono, Y.; Miyahara, Y.; Muraoka, D.; Harada, N.; Kageyama, S.; Sasaki, T.; Hori, Y.; Soga, N.; Uchida, K. First-in-human phase I clinical trial of the NY-ESO-1 protein cancer vaccine with NOD2 and TLR9 stimulants in patients with NY-ESO-1-expressing refractory solid tumors. Cancer Immunol. Immunother. 2020, 69, 663–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wex, T.; Ebert, M.P.; Kropf, S.; Dierkes, J.; Schuettler, K.; Roecken, C.; Hoecker, M.; Malfertheiner, P. Gene polymorphisms of the NOD-2/CARD-15 gene and the risk of gastric cancer in Germany. Anticancer Res. 2008, 28, 757–762. [Google Scholar] [PubMed]
- Hnatyszyn, A.; Szalata, M.; Stanczyk, J.; Cichy, W.; Slomski, R. Association of c. 802C> T polymorphism of NOD2/CARD15 gene with the chronic gastritis and predisposition to cancer in H. pylori infected patients. Exp. Mol. Pathol. 2010, 88, 388–393. [Google Scholar] [CrossRef]
- Angeletti, S.; Galluzzo, S.; Santini, D.; Ruzzo, A.; Vincenzi, B.; Ferraro, E.; Spoto, C.; Lorino, G.; Graziano, N.; Calvieri, A. NOD2/CARD15 polymorphisms impair innate immunity and increase susceptibility to gastric cancer in an Italian population. Hum. Immunol. 2009, 70, 729–732. [Google Scholar] [CrossRef]
| Number | SNPs | Mutation | Location | Population | Result | Infection (Disease) | Method | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | P268S | CCC > TCC | NBD domain | African Americans | Minor allele T is associated with a decreased risk of TB (Protective) | Tuberculosis | Sequencing of the coding regions of the NOD2 gene | [70] |
| R702W | CGG > TGG [14]4 | HD2 Exon 4 | Minor allele T is associated with a decreased risk of TB(Protective) | |||||
| A725G | GCT > GGT | HD2 Exon 4 | the minor allele G increased the risk of TB | |||||
| 2 | R702W | CGG > TGG | South African | No association | Inflammatory bowel disease (CD & UC) | PCR of the Exons 4, 8 and 11- HEX-SSCP &RFLP | [71] | |
| A725G | GCT > GGT | Increased risk of TB | ||||||
| G908R | Rs2066845 | No association | ||||||
| 1007fs(insC3020) | L1007P rs5743293 | No association | ||||||
| 3 | rs3135499 | Promoter | Han Chinese from Jiangsu Province | T genotype protective | Tuberculosis | TaqMan-based allelic discrimination system | [72] | |
| rs7194886 | Promoter | Increased risk for T allele carriers | ||||||
| rs8057341 | Promoter | |||||||
| rs9302752 | Promoter | T genotype protective | ||||||
| 4 | insC3020 | rs5743293 | Sardinian population. | Significant Association (Increased the susceptibility) | CD & Mycobacterium avium subsp. paratuberculosis | PCR & sequencing | [73] | |
| R702W | Rs2066844 | |||||||
| G908R | Rs2066845 | |||||||
| 5 | insC3020 | 1007fs | northern Indian states | No mutation was observed in the patients and controls | TB and leprosy | PCR-RFLP confirmed by gene sequencing | [74] | |
| R702W | Rs2066844 | |||||||
| G908R | rs2066845 | |||||||
| 6 | R702W | South African | No association | Tuberculosis | Tag Man platform genotyping | [75] | ||
| G908R | ||||||||
| insC3020 | ||||||||
| 7 | P268S | C > T rs2066842 | Exon 4 | Caucasian patients | No association | Sarcoidosis | Tag Man platform genotyping | [61] |
| R587R | T > G rs1861759 | Exon 4 | ||||||
| R702W | C > T rs2066844 | Exon 4 | ||||||
| G908R | G > C rs2066845 | Exon 8 | ||||||
| insC3020 | rs2066847 | Exon 11 | ||||||
| 8 | P268S | Turkish population | Association with CD | Crohn’s Disease and Ulcerative Colitis | PCR-RFLP | [76] | ||
| M863V | No mutant was found | |||||||
| 9 | R702W | rs2066844 CGG > TGG | Meta analysis | C allele is a risk factor | sarcoidosis | Meta-analysis | [77] | |
| G908R | rs2066845 | no associated | ||||||
| insC3020 | rs2066847 | no associated | ||||||
| R587R | rs1861759 | no associated | ||||||
| 10 | C-159 T | rs2569190 | Meta analysis | GG is common in TB | Tuberculosis | Meta-analysis | [78] | |
| A-1145G | rs2569191 | T allele is a risk factor in TB | ||||||
| IV | rs1861759 | TG genotype is higher in TB | ||||||
| rs7194886 | T allele is a risk factor of TB | |||||||
| R702W | rs2066844 | CC genotype is a risk factor for TB | ||||||
| P 507 T/S | rs2066842 C > A/T | CC genotype is a risk factor for TB | ||||||
| 11 | -159C > T | -159C > T | promoter of CD14 | Chinese | Higher risk increased promoter activity/increased sNOD2 | spinal TB | Seq. | [79] |
| 12 | G-1619A | rs2915863 | promoter of CD14 | Han Chinese | Increased susceptibly/ increased sNOD2 | tuberculosis | PCR and seq | [80] |
| T-1359G | rs3138078 | |||||||
| A-1145G | rs2569191 | |||||||
| C-159T | rs2569190 | |||||||
| 13 | C(-159)T | promoter of CD14 | Han Chinese | T allele is a RF | tuberculosis | PCR-DNA sequencing | [81] | |
| G(-1145)A | G allele is a RF | |||||||
| 14 | C(-159)T | promoter of CD14 | increased level of serum soluble CD14 | tuberculosis | [82] | |||
| 15 | C(-159)T | promoter of CD14 | Mexico | increased Tb susceptibility/ increased level of serum soluble CD14 | PCR-RFLP | [83] | ||
| 16 | C(-159)T | Promoter | Meta analysis | increased risk of TB | Meta-analysis | [84] | ||
| 17 | R426H | rs562225614 G > A | Exon 4 | Case report | Early Onset Inflammatory Bowel Phenotype | IBD-Increased expression of inflammatory cytokines | Sequencing | [85] |
| 18 | N1010K | 3030A > C | LRR domain Exon 12 | CD | Sequencing | [86] |
| LRR Domain SNPs | Provean Score | Role | Polyphen-2 Score | Role | PANTHER Score | Role |
|---|---|---|---|---|---|---|
| A725G | −1.275 | Neutral | 0.04 | benign | 455 | probably Damaging |
| A755V | −0.942 | Neutral | 1 | probably Damaging | 456 | probably Damaging |
| R760C | −3.651 | Deleterious | 0.22 | benign | 176 | probably benign |
| E778K | −2.579 | Deleterious | 0.998 | probably Damaging | 455 | probably Damaging |
| R790W | −4.021 | Deleterious | 0.998 | probably Damaging | 176 | probably benign |
| V793M | −0.804 | Neutral | 0.85 | probably Damaging | 455 | probably Damaging |
| E843K | 0.482 | Neutral | 0.783 | probably Damaging | 176 | probably benign |
| N852S | −3.049 | Deleterious | 0.998 | probably Damaging | 455 | probably Damaging |
| M863V | −0.07 | Neutral | 0 | benign | 176 | probably benign |
| A885T | −1.407 | Neutral | 0.835 | probably Damaging | 455 | probably Damaging |
| G908R | −5.822 | Deleterious | 1 | probably Damaging | 457 | probably Damaging |
| G924D | 0.149 | Neutral | 0.411 | benign | 176 | probably benign |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alipoor, S.D.; Mirsaeidi, M. Inborn Errors in the LRR Domain of Nod2 and Their Potential Consequences on the Function of the Receptor. Cells 2021, 10, 2031. https://doi.org/10.3390/cells10082031
Alipoor SD, Mirsaeidi M. Inborn Errors in the LRR Domain of Nod2 and Their Potential Consequences on the Function of the Receptor. Cells. 2021; 10(8):2031. https://doi.org/10.3390/cells10082031
Chicago/Turabian StyleAlipoor, Shamila D., and Mehdi Mirsaeidi. 2021. "Inborn Errors in the LRR Domain of Nod2 and Their Potential Consequences on the Function of the Receptor" Cells 10, no. 8: 2031. https://doi.org/10.3390/cells10082031
APA StyleAlipoor, S. D., & Mirsaeidi, M. (2021). Inborn Errors in the LRR Domain of Nod2 and Their Potential Consequences on the Function of the Receptor. Cells, 10(8), 2031. https://doi.org/10.3390/cells10082031







