Proton-Sensing GPCRs in Health and Disease
Abstract
1. Proton-Sensing GPCRs—Structural Features and Physiology
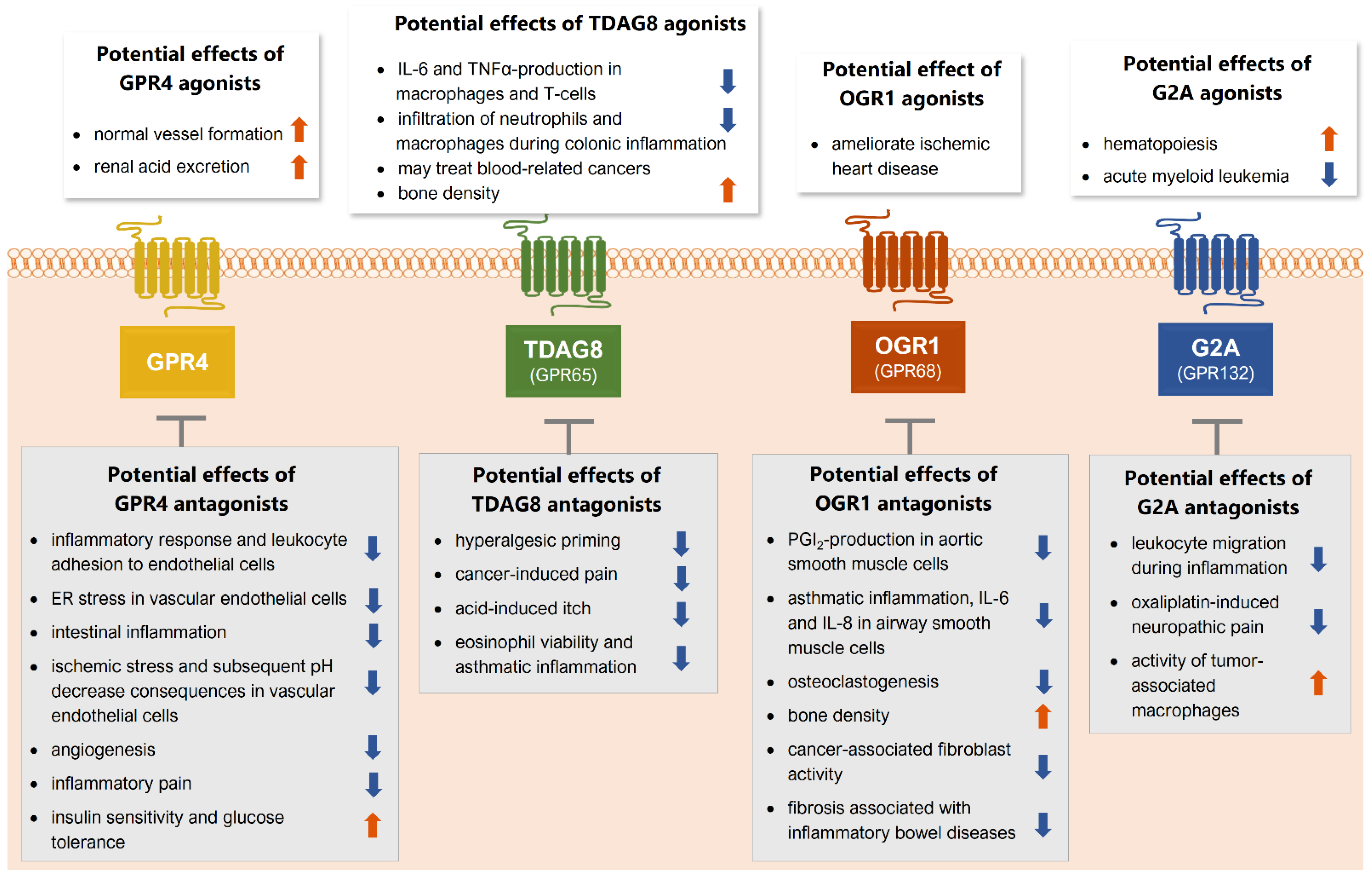
2. GPR4
3. TDAG8 (GPR65)
4. OGR1 (GPR68)
5. G2A (GPR132)
6. Outlook
6.1. Modulating and Targeting the Four GPCRs
6.2. Expanding the Group of Proton-Sensing GPCRs
6.3. Proton-Sensing GPCRs in Persistent Pain States
Funding
Conflicts of Interest
References
- Ludwig, M.G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98. [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Foord, S.M.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.P.; Davenport, A.P.; Spedding, M.; Harmar, A.J. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 42484–42491. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Hirata, K.; Yamashita, T.; Iwai, K.; Kobayashi, S.; Itoh, H.; Ozaki, M.; Ejiri, J.; Shiomi, M.; Inoue, N.; et al. Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arter. Thromb. Vasc. Biol. 2002, 22, 2049–2053. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, S.Y.; Choi, Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 1996, 168, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.G.; Nijagal, A.; McLaughlin, J.; Wang, L.; Witte, O.N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Ishizuka, T.; Yamada, H.; Kamide, Y.; Hisada, T.; Ichimonji, I.; Aoki, H.; Yatomi, M.; Komachi, M.; Tsurumaki, H.; et al. Extracellular acidification induces connective tissue growth factor production through proton-sensing receptor OGR1 in human airway smooth muscle cells. Biochem. Biophys. Res. 2011, 413, 499–503. [Google Scholar] [CrossRef]
- Yang, M.; Mailhot, G.; Birnbaum, M.J.; MacKay, C.A.; Mason-Savas, A.; Odgren, P.R. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J. Biol. Chem. 2006, 281, 23598–23605. [Google Scholar] [CrossRef]
- Huang, X.P.; Karpiak, J.; Kroeze, W.K.; Zhu, H.; Chen, X.; Moy, S.S.; Saddoris, K.A.; Nikolova, V.D.; Farrell, M.S.; Wang, S.; et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 2015, 527, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, A.; Singh, L.S.; Tan, H.; Ding, H.; Liang, Z.; Xu, Y. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim. Biophys. Acta 2006, 1761, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Haack, K.K.V.; McCarty, N.A. Functional Consequences of GPCR Heterodimerization: GPCRs as Allosteric Modulators. Pharmaceuticals 2011, 4, 509–523. [Google Scholar] [CrossRef]
- Wang, J.Q.; Kon, J.; Mogi, C.; Tobo, M.; Damirin, A.; Sato, K.; Komachi, M.; Malchinkhuu, E.; Murata, N.; Kimura, T.; et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004, 279, 45626–45633. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.B.; Kapolka, N.J.; Taghon, G.J.; Morgan, W.M.; Isom, D.G. The evolution and mechanism of GPCR proton sensing. J. Biol. Chem. 2020, 296, 100167. [Google Scholar] [CrossRef]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal. 2013, 25, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.T.; Fante, M.; Kohl, G.; Schreml, J.; Haubner, F.; Kreutz, M.; Haverkampf, S.; Berneburg, M.; Schreml, S. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 2017, 26, 127–132. [Google Scholar] [CrossRef]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Bonetti, L.; Brenner, D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep. 2020, 32, 107848. [Google Scholar] [CrossRef]
- Aoki, H.; Mogi, C.; Okajima, F. Ionotropic and metabotropic proton-sensing receptors involved in airway inflammation in allergic asthma. Mediat. Inflamm. 2014, 2014, 712962. [Google Scholar] [CrossRef]
- Carattino, M.D.; Montalbetti, N. Acid-sensing ion channels in sensory signaling. Am. J. Physiol. Ren. Physiol. 2020, 318, F531–F543. [Google Scholar] [CrossRef]
- Holzer, P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharm. 2009, 283–332. [Google Scholar] [CrossRef]
- Heber, S.; Ciotu, C.I.; Hartner, G.; Gold-Binder, M.; Ninidze, N.; Gleiss, A.; Kress, H.G.; Fischer, M.J.M. TRPV1 antagonist BCTC inhibits pH 6.0-induced pain in human skin. Pain 2020, 161, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S.G. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar]
- Rajagopal, S.; Shenoy, S.K. GPCR desensitization: Acute and prolonged phases. Cell Signal. 2018, 41, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bohm, S.K.; Grady, E.F.; Bunnett, N.W. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem. J. 1997, 322, 1–18. [Google Scholar] [CrossRef]
- Kelly, E.; Bailey, C.P.; Henderson, G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharm. 2008, 153, S379–S388. [Google Scholar] [CrossRef] [PubMed]
- Hamm, H.E.; Gilchrist, A. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 1996, 8, 189–196. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Lefkowitz, R.J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465. [Google Scholar] [CrossRef]
- Lu, S.M.; Jang, W.; Inoue, A.; Lambert, N.A. Constitutive G protein coupling profiles of understudied orphan GPCRs. PLoS ONE 2021, 16, e0247743. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; McLaughlin, N.J.D.; Kelher, M.R.; Eckels, P.; Gamboni-Robertson, F.; Banerjee, A.; Silliman, C.C. Lysophosphatidylcholines activate G2A inducing G(alpha i-1)-/G(alpha q/11)- Ca2+ flux, G(beta gamma)-Hck activation and clathrin/beta-arrestin-1/GRK6 recruitment in PMNs. Biochem. J. 2010, 432, 35–45. [Google Scholar] [CrossRef][Green Version]
- Gainetdinov, R.R.; Premont, R.T.; Bohn, L.M.; Lefkowitz, R.J.; Caron, M.G. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004, 27, 107–144. [Google Scholar] [CrossRef]
- Zhang, X.H.; Kim, K.M. Multifactorial Regulation of G Protein-Coupled Receptor Endocytosis. Biomol. Ther. 2017, 25, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Bianchi, F.; Wang, Y.K.; Tang, M.J.; Ye, H.; Glitsch, M.D. Coincidence Detection of Membrane Stretch and Extracellular pH by the Proton-Sensing Receptor OGR1 (GPR68). Curr. Biol. 2018, 28, 3815–3823.e4. [Google Scholar] [CrossRef]
- Xu, J.; Mathur, J.; Vessieres, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J.Y.; et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Sanderlin, E.J.; Marie, M.; Velcicky, J.; Loetscher, P.; Yang, L.V. Pharmacological inhibition of GPR4 remediates intestinal inflammation in a mouse colitis model. Eur. J. Pharm. 2019, 852, 218–230. [Google Scholar] [CrossRef]
- Obinata, H.; Hattori, T.; Nakane, S.; Tatei, K.; Izumi, T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J. Biol. Chem. 2005, 280, 40676–40683. [Google Scholar] [CrossRef]
- Lahvic, J.L.; Ammerman, M.; Li, P.; Blair, M.C.; Stillman, E.R.; Fast, E.M.; Robertson, A.L.; Christodoulou, C.; Perlin, J.R.; Yang, S.; et al. Specific oxylipins enhance vertebrate hematopoiesis via the receptor GPR132. Proc. Natl. Acad. Sci. USA 2018, 115, 9252–9257. [Google Scholar] [CrossRef]
- Dai, S.P.; Huang, Y.H.; Chang, C.J.; Huang, Y.F.; Hsieh, W.S.; Tabata, Y.; Ishii, S.; Sun, W.H. TDAG8 involved in initiating inflammatory hyperalgesia and establishing hyperalgesic priming in mice. Sci. Rep. 2017, 7, 41415. [Google Scholar] [CrossRef]
- Ishii, S.; Kihara, Y.; Shimizu, T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 9083–9087. [Google Scholar] [CrossRef]
- Lin, P.; Ye, R.D. The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J. Biol. Chem. 2003, 278, 14379–14386. [Google Scholar] [CrossRef] [PubMed]
- Tobo, M.; Tomura, H.; Mogi, C.; Wang, J.Q.; Liu, J.P.; Komachi, M.; Damirin, A.; Kimura, T.; Murata, N.; Kurose, H.; et al. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell Signal. 2007, 19, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Nakakura, T.; Tomura, H.; Tobo, M.; Mogi, C.; Wang, J.Q.; He, X.D.; Takano, M.; Damirin, A.; Komachi, M.; et al. Each one of certain histidine residues in G-protein-coupled receptor GPR4 is critical for extracellular proton-induced stimulation of multiple G-protein-signaling pathways. Pharm. Res. 2010, 61, 499–505. [Google Scholar] [CrossRef]
- Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J. Biol. Chem. 2005, 280, 43280. Available online: https://pubmed.ncbi.nlm.nih.gov/16498716/ (accessed on 9 August 2021). [CrossRef]
- Qiao, J.; Huang, F.; Naikawadi, R.P.; Kim, K.S.; Said, T.; Lum, H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am. J. Physiol.-Lung C 2006, 291, L91–L101. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.S.; Dong, L.X.; Leffler, N.R.; Asch, A.S.; Witte, O.N.; Yang, L.V. Activation of GPR4 by Acidosis Increases Endothelial Cell Adhesion through the cAMP/Epac Pathway. PLoS ONE 2011, 6, e27586. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.T.; Yang, L.V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef]
- Sanderlin, E.J.; Leffler, N.R.; Lertpiriyapong, K.; Cai, Q.; Hong, H.; Bakthavatchalu, V.; Fox, J.G.; Oswald, J.Z.; Justus, C.R.; Krewson, E.A.; et al. GPR4 deficiency alleviates intestinal inflammation in a mouse model of acute experimental colitis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; de Valliere, C.; Imenez Silva, P.H.; Leonardi, I.; Gruber, S.; Gerstgrasser, A.; Melhem, H.; Weber, A.; Leucht, K.; Wolfram, L.; et al. The Proton-activated Receptor GPR4 Modulates Intestinal Inflammation. J. Crohns Colitis 2018, 12, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Krewson, E.A.; Yang, L.V. Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 278. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.V.; Tiegs, B.C.; Arend, L.J.; McGraw, D.W.; Penn, R.B.; Petrovic, S. Deletion of the pH sensor GPR4 decreases renal acid excretion. J. Am. Soc. Nephrol. 2010, 21, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, V.; Kuner, R. Pain hypersensitivity mechanisms at a glance. Dis. Model. Mech. 2013, 6, 889–895. [Google Scholar] [CrossRef]
- Huang, C.W.; Tzeng, J.N.; Chen, Y.J.; Tsai, W.F.; Chen, C.C.; Sun, W.H. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol. Cell Neurosci. 2007, 36, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Huang, C.W.; Lin, C.S.; Chang, W.H.; Sun, W.H. Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain. Mol. Pain 2009, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Sin, W.C.; Zhang, Y.; Zhong, W.; Adhikarakunnathu, S.; Powers, S.; Hoey, T.; An, S.; Yang, J. G protein-coupled receptors GPR4 and TDAG8 are oncogenic and overexpressed in human cancers. Oncogene 2004, 23, 6299–6303. [Google Scholar] [CrossRef]
- Yu, M.; Cui, R.; Huang, Y.; Luo, Y.; Qin, S.; Zhong, M. Increased proton-sensing receptor GPR4 signalling promotes colorectal cancer progression by activating the hippo pathway. EBioMedicine 2019, 48, 264–276. [Google Scholar] [CrossRef]
- Insel, P.A.; Sriram, K.; Salmeron, C.; Wiley, S.Z. Proton-sensing G protein-coupled receptors: Detectors of tumor acidosis and candidate drug targets. Future Med. Chem. 2020, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Klatt, W.; Wallner, S.; Brochhausen, C.; Stolwijk, J.A.; Schreml, S. Expression profiles of proton-sensing G-protein coupled receptors in common skin tumors. Sci. Rep. 2020, 10, 15327. [Google Scholar] [CrossRef]
- Wyder, L.; Suply, T.; Ricoux, B.; Billy, E.; Schnell, C.; Baumgarten, B.U.; Maira, S.M.; Koelbing, C.; Ferretti, M.; Kinzel, B.; et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis 2011, 14, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Xu, H.; Chen, X.; Zhong, Q.; Huang, J.; Zhang, Y.; Guo, W.; Yang, Z.; Ding, S.; Chen, P.; et al. The Proton-Sensing G-Protein Coupled Receptor GPR4 Promotes Angiogenesis in Head and Neck Cancer. PLoS ONE 2016, 11, e0152789. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.V.; Radu, C.G.; Roy, M.; Lee, S.; McLaughlin, J.; Teitell, M.A.; Iruela-Arispe, M.L.; Witte, O.N. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol. Cell Biol. 2007, 27, 1334–1347. [Google Scholar] [CrossRef]
- Castellone, R.D.; Leffler, N.R.; Dong, L.; Yang, L.V. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett. 2011, 312, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Justus, C.R.; Jiang, W.; Li, Z.; Lu, J.Q.; Brock, R.S.; McPeek, M.K.; Weidner, D.A.; Yang, L.V.; et al. Comparative study of 3D morphology and functions on genetically engineered mouse melanoma cells. Integr. Biol. (Camb.) 2012, 4, 1428–1436. [Google Scholar] [CrossRef]
- Fukuda, H.; Ito, S.; Watari, K.; Mogi, C.; Arisawa, M.; Okajima, F.; Kurose, H.; Shuto, S. Identification of a Potent and Selective GPR4 Antagonist as a Drug Lead for the Treatment of Myocardial Infarction. ACS Med. Chem. Lett. 2016, 7, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Hosford, P.S.; Mosienko, V.; Kishi, K.; Jurisic, G.; Seuwen, K.; Kinzel, B.; Ludwig, M.G.; Wells, J.A.; Christie, I.N.; Koolen, L.; et al. CNS distribution, signalling properties and central effects of G-protein coupled receptor 4. Neuropharmacology 2018, 138, 381–392. [Google Scholar] [CrossRef]
- Wenzel, J.; Hansen, C.E.; Bettoni, C.; Vogt, M.A.; Lembrich, B.; Natsagdorj, R.; Huber, G.; Brands, J.; Schmidt, K.; Assmann, J.C.; et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 1753–1761. [Google Scholar] [CrossRef]
- Velcicky, J.; Miltz, W.; Oberhauser, B.; Orain, D.; Vaupel, A.; Weigand, K.; Dawson King, J.; Littlewood-Evans, A.; Nash, M.; Feifel, R.; et al. Development of Selective, Orally Active GPR4 Antagonists with Modulatory Effects on Nociception, Inflammation, and Angiogenesis. J. Med. Chem. 2017, 60, 3672–3683. [Google Scholar] [CrossRef]
- Miltz, W.; Velcicky, J.; Dawson, J.; Littlewood-Evans, A.; Ludwig, M.G.; Seuwen, K.; Feifel, R.; Oberhauser, B.; Meyer, A.; Gabriel, D.; et al. Design and synthesis of potent and orally active GPR4 antagonists with modulatory effects on nociception, inflammation, and angiogenesis. Bioorg. Med. Chem. 2017, 25, 4512–4525. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.G.; Cheng, D.; Nijagal, A.; Riedinger, M.; McLaughlin, J.; Yang, L.V.; Johnson, J.; Witte, O.N. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol. Cell Biol. 2006, 26, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S.; Heise, C.E.; Nguyen, T.; O’Dowd, B.F.; Lynch, K.R. Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 2001, 153, 429–434. [Google Scholar] [CrossRef]
- Mogi, C.; Tobo, M.; Tomura, H.; Murata, N.; He, X.D.; Sato, K.; Kimura, T.; Ishizuka, T.; Sasaki, T.; Sato, T.; et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009, 182, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, Y.; Fujita, Y.; Kuwabara, H.; Nagasaki, M.; Komai, T.; Oda, T. Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur. J. Pharm. 2012, 683, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tcymbarevich, I.; Richards, S.M.; Russo, G.; Kuhn-Georgijevic, J.; Cosin-Roger, J.; Baebler, K.; Lang, S.; Bengs, S.; Atrott, K.; Bettoni, C.; et al. Lack of the pH-sensing Receptor TDAG8 [GPR65] in Macrophages Plays a Detrimental Role in Murine Models of Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.C.; Dai, S.P.; Chiang, H.; Huang, H.S.; Sun, W.H. Temporal expression patterns of distinct cytokines and M1/M2 macrophage polarization regulate rheumatoid arthritis progression. Mol. Biol. Rep. 2020. [Google Scholar] [CrossRef]
- Hsieh, W.S.; Kung, C.C.; Huang, S.L.; Lin, S.C.; Sun, W.H. TDAG8, TRPV1, and ASIC3 involved in establishing hyperalgesic priming in experimental rheumatoid arthritis. Sci. Rep. 2017, 7, 8870. [Google Scholar] [CrossRef]
- Hang, L.H.; Yang, J.P.; Yin, W.; Wang, L.N.; Guo, F.; Ji, F.H.; Shao, D.H.; Xu, Q.N.; Wang, X.Y.; Zuo, J.L. Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur. J. Neurosci. 2012, 36, 2107–2117. [Google Scholar] [CrossRef]
- Lin, S.H.; Steinhoff, M.; Ikoma, A.; Chang, Y.C.; Cheng, Y.R.; Chandra Kopparaju, R.; Ishii, S.; Sun, W.H.; Chen, C.C. Involvement of TRPV1 and TDAG8 in Pruriception Associated with Noxious Acidosis. J. Investig. Dermatol. 2017, 137, 170–178. [Google Scholar] [CrossRef][Green Version]
- Ihara, Y.; Kihara, Y.; Hamano, F.; Yanagida, K.; Morishita, Y.; Kunita, A.; Yamori, T.; Fukayama, M.; Aburatani, H.; Shimizu, T.; et al. The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc. Natl. Acad. Sci. USA 2010, 107, 17309–17314. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Dean, E.; Yang, L.V. Acidosis decreases c-Myc oncogene expression in human lymphoma cells: A role for the proton-sensing G protein-coupled receptor TDAG8. Int. J. Mol. Sci. 2013, 14, 20236–20255. [Google Scholar] [CrossRef]
- Justus, C.R.; Sanderlin, E.J.; Dong, L.; Sun, T.; Chi, J.T.; Lertpiriyapong, K.; Yang, L.V. Contextual tumor suppressor function of T cell death-associated gene 8 (TDAG8) in hematological malignancies. J. Transl. Med. 2017, 15, 204. [Google Scholar] [CrossRef]
- Kottyan, L.C.; Collier, A.R.; Cao, K.H.; Niese, K.A.; Hedgebeth, M.; Radu, C.G.; Witte, O.N.; Hershey, G.K.K.; Rothenberg, M.E.; Zimmermann, N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 2009, 114, 2774–2782. [Google Scholar] [CrossRef]
- Sato, K.; Tobo, A.; Mogi, C.; Tobo, M.; Yamane, N.; Tosaka, M.; Tomura, H.; Im, D.S.; Okajima, F. The protective role of proton-sensing TDAG8 in the brain injury in a mouse ischemia reperfusion model. Sci. Rep. 2020, 10, 17193. [Google Scholar] [CrossRef] [PubMed]
- Hikiji, H.; Endo, D.; Horie, K.; Harayama, T.; Akahoshi, N.; Igarashi, H.; Kihara, Y.; Yanagida, K.; Takeda, J.; Koji, T.; et al. TDAG8 activation inhibits osteoclastic bone resorption. FASEB J. 2014, 28, 871–879. [Google Scholar] [CrossRef]
- Ma, X.D.; Hang, L.H.; Shao, D.H.; Shu, W.W.; Hu, X.L.; Luo, H. TDAG8 activation attenuates cerebral ischaemia-reperfusion injury via Akt signalling in rats. Exp. Neurol. 2017, 293, 115–123. [Google Scholar] [CrossRef]
- Xu, Y.; Casey, G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics 1996, 35, 397–402. [Google Scholar] [CrossRef]
- Retraction Note to: Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat. Cell Biol. 2006, 8, 299. [CrossRef] [PubMed]
- Wiley, S.Z.; Sriram, K.; Salmeron, C.; Insel, P.A. GPR68: An Emerging Drug Target in Cancer. Int. J. Mol. Sci. 2019, 20, 559. [Google Scholar] [CrossRef]
- Russell, J.L.; Goetsch, S.C.; Aguilar, H.R.; Coe, H.; Luo, X.; Liu, N.; van Rooij, E.; Frantz, D.E.; Schneider, J.W. Regulated Expression of pH Sensing G Protein-Coupled Receptor-68 Identified through Chemical Biology Defines a New Drug Target for Ischemic Heart Disease. ACS Chem. Biol. 2012, 7, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Tomura, H.; Wang, J.Q.; Komachi, M.; Damirin, A.; Mogi, C.; Tobo, M.; Kon, J.; Misawa, N.; Sato, K.; Okajima, F. Prostaglandin I-2 production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J. Biol. Chem. 2005, 280, 34458–34464. [Google Scholar] [CrossRef] [PubMed]
- Ichimonji, I.; Tomura, H.; Mogi, C.; Sato, K.; Aoki, H.; Hisada, T.; Dobashi, K.; Ishizuka, T.; Mori, M.; Okajima, F. Extracellular acidification stimulates IL-6 production and Ca2+ mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am. J. Physiol.-Lung C 2010, 299, L567–L577. [Google Scholar] [CrossRef]
- Aoki, H.; Mogi, C.; Hisada, T.; Nakakura, T.; Kamide, Y.; Ichimonji, I.; Tomura, H.; Tobo, M.; Sato, K.; Tsurumaki, H.; et al. Proton-Sensing Ovarian Cancer G Protein-Coupled Receptor 1 on Dendritic Cells Is Required for Airway Responses in a Murine Asthma Model. PLoS ONE 2013, 8, e79985. [Google Scholar] [CrossRef]
- Singh, L.S.; Berk, M.; Oates, R.; Zhao, Z.; Tan, H.; Jiang, Y.; Zhou, A.; Kirmani, K.; Steinmetz, R.; Lindner, D.; et al. Ovarian cancer G protein-coupled receptor 1, a new metastasis suppressor gene in prostate cancer. J. Natl. Cancer Inst. 2007, 99, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, L. Effects of ovarian cancer G protein coupled receptor 1 on the proliferation, migration, and adhesion of human ovarian cancer cells. Chin. Med. J. (Engl.) 2011, 124, 1327–1332. [Google Scholar] [PubMed]
- Horman, S.R.; To, J.; Lamb, J.; Zoll, J.H.; Leonetti, N.; Tu, B.; Moran, R.; Newlin, R.; Walker, J.R.; Orth, A.P. Functional profiling of microtumors to identify cancer associated fibroblast-derived drug targets. Oncotarget 2017, 8, 99913–99930. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Harimoto, N.; Yokobori, T.; Muranushi, R.; Hoshino, K.; Hagiwara, K.; Gantumur, D.; Handa, T.; Ishii, N.; Tsukagoshi, M.; et al. Conophylline Inhibits Hepatocellular Carcinoma by Inhibiting Activated Cancer-associated Fibroblasts Through Suppression of G Protein-coupled Receptor 68. Mol. Cancer Ther. 2021, 20, 1019–1028. [Google Scholar] [CrossRef]
- Wiley, S.Z.; Sriram, K.; Liang, W.; Chang, S.E.; French, R.; McCann, T.; Sicklick, J.; Nishihara, H.; Lowy, A.M.; Insel, P.A. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J. 2018, 32, 1170–1183. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, S.; Zhang, Y.; Yin, J.; Yin, W.; Tao, S.; Wang, Y.; Zhang, C. Proton-sensing GPCR-YAP Signalling Promotes Cancer-associated Fibroblast Activation of Mesenchymal Stem Cells. Int. J. Biol. Sci. 2016, 12, 389–396. [Google Scholar] [CrossRef]
- Yan, L.; Singh, L.S.; Zhang, L.; Xu, Y. Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 2014, 33, 157–164. [Google Scholar] [CrossRef]
- Komarova, S.V.; Pereverzev, A.; Shum, J.W.; Sims, S.M.; Dixon, S.J. Convergent signaling by acidosis and receptor activator of NF-kappaB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc. Natl. Acad. Sci. USA 2005, 102, 2643–2648. [Google Scholar] [CrossRef]
- Nakakura, T.; Mogi, C.; Tobo, M.; Tomura, H.; Sato, K.; Kobayashi, M.; Ohnishi, H.; Tanaka, S.; Wayama, M.; Sugiyama, T.; et al. Deficiency of proton-sensing ovarian cancer G protein-coupled receptor 1 attenuates glucose-stimulated insulin secretion. Endocrinology 2012, 153, 4171–4180. [Google Scholar] [CrossRef]
- Foster, S.R.; Hauser, A.S.; Vedel, L.; Strachan, R.T.; Huang, X.P.; Gavin, A.C.; Shah, S.D.; Nayak, A.P.; Haugaard-Kedstrom, L.M.; Penn, R.B.; et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 2019, 179, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, X.P.; Kenakin, T.P.; Slocum, S.T.; Chen, X.; Martini, M.L.; Liu, J.; Jin, J. Design, Synthesis, and Characterization of Ogerin-Based Positive Allosteric Modulators for G Protein-Coupled Receptor 68 (GPR68). J. Med. Chem. 2019, 62, 7557–7574. [Google Scholar] [CrossRef] [PubMed]
- Maeyashiki, C.; Melhem, H.; Hering, L.; Baebler, K.; Cosin-Roger, J.; Schefer, F.; Weder, B.; Hausmann, M.; Scharl, M.; Rogler, G.; et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1alpha/JNK Pathway in an Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 1438. [Google Scholar] [CrossRef]
- Weng, Z.; Fluckiger, A.C.; Nisitani, S.; Wahl, M.I.; Le, L.Q.; Hunter, C.A.; Fernal, A.A.; Le Beau, M.M.; Witte, O.N. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 1998, 95, 12334–12339. [Google Scholar] [CrossRef] [PubMed]
- Kern, K.; Schafer, S.M.G.; Cohnen, J.; Pierre, S.; Osthues, T.; Tarighi, N.; Hohmann, S.; Ferreiros, N.; Brune, B.; Weigert, A.; et al. The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue. Front. Immunol. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Le, L.Q.; Kabarowski, J.H.; Weng, Z.; Satterthwaite, A.B.; Harvill, E.T.; Jensen, E.R.; Miller, J.F.; Witte, O.N. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 2001, 14, 561–571. [Google Scholar] [CrossRef]
- Hohmann, S.W.; Angioni, C.; Tunaru, S.; Lee, S.; Woolf, C.J.; Offermanns, S.; Geisslinger, G.; Scholich, K.; Sisignano, M. The G2A receptor (GPR132) contributes to oxaliplatin-induced mechanical pain hypersensitivity. Sci. Rep. 2017, 7, 446. [Google Scholar] [CrossRef]
- Frasch, S.C.; Zemski-Berry, K.; Murphy, R.C.; Borregaard, N.; Henson, P.M.; Bratton, D.L. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J. Immunol. 2007, 178, 6540–6548. [Google Scholar] [CrossRef]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Waibel, M.; Radu, C.G.; Yang, L.V.; Witte, O.N.; Schulze-Osthoff, K.; Wesselborg, S.; Lauber, K. Migration to apoptotic “Find-me” signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 2008, 283, 5296–5305. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Ueno, S.; Chen, M.X.; Harvey, J.; Dowell, S.J.; Irving, A.J.; Brown, A.J. N-Palmitoylglycine and other N-acylamides activate the lipid receptor G2A/GPR132. Pharm. Res. Perspect. 2019, 7, e00542. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Huynh, H.; Chen, P.; Pena-Llopis, S.; Wan, Y. Macrophage PPARgamma inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef]
- Kabarowski, J.H.S.; Feramisco, J.D.; Le, L.Q.; Gu, J.L.; Luoh, S.W.; Simon, M.I.; Witte, O.N. Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc. Natl. Acad. Sci. USA 2000, 97, 12109–12114. [Google Scholar] [CrossRef]
- Nii, T.; Prabhu, V.V.; Ruvolo, V.; Madhukar, N.; Zhao, R.; Mu, H.; Heese, L.; Nishida, Y.; Kojima, K.; Garnett, M.J.; et al. Imipridone ONC212 activates orphan G protein-coupled receptor GPR132 and integrated stress response in acute myeloid leukemia. Leukemia 2019, 33, 2805–2816. [Google Scholar] [CrossRef]
- Yin, H.; Chu, A.; Li, W.; Wang, B.; Shelton, F.; Otero, F.; Nguyen, D.G.; Caldwell, J.S.; Chen, Y.A. Lipid G Protein-coupled Receptor Ligand Identification Using beta-Arrestin PathHunter (TM) Assay. J. Biol. Chem. 2009, 284, 12328–12338. [Google Scholar] [CrossRef]
- Ufret-Vincenty, C.A.; Klein, R.M.; Hua, L.; Angueyra, J.; Gordon, S.E. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 2011, 286, 9688–9698. [Google Scholar] [CrossRef]
- Hammond, G.R.; Fischer, M.J.; Anderson, K.E.; Holdich, J.; Koteci, A.; Balla, T.; Irvine, R.F. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 2012, 337, 727–730. [Google Scholar] [CrossRef]
- Honore, E. The neuronal background K2P channels: Focus on TREK1. Nat. Rev. Neurosci. 2007, 8, 251–261. [Google Scholar] [CrossRef]
- Afrasiabi, E.; Blom, T.; Ekokoski, E.; Tuominen, R.K.; Tornquist, K. Sphingosylphosphorylcholine enhances calcium entry in thyroid FRO cells by a mechanism dependent on protein kinase C. Cell Signal. 2006, 18, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Lassen, K.G.; McKenzie, C.I.; Mari, M.; Murano, T.; Begun, J.; Baxt, L.A.; Goel, G.; Villablanca, E.J.; Kuo, S.Y.; Huang, H.; et al. Genetic Coding Variant in GPR65 Alters Lysosomal pH and Links Lysosomal Dysfunction with Colitis Risk. Immunity 2016, 44, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Tcymbarevich, I.V.; Eloranta, J.J.; Rossel, J.B.; Obialo, N.; Spalinger, M.; Cosin-Roger, J.; Lang, S.; Kullak-Ublick, G.A.; Wagner, C.A.; Scharl, M.; et al. The impact of the rs8005161 polymorphism on G protein-coupled receptor GPR65 (TDAG8) pH-associated activation in intestinal inflammation. BMC Gastroenterol. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; Smith, C.E.; El-Sayed, W.; Poulter, J.A.; Shore, R.C.; Logan, C.V.; Mogi, C.; Sato, K.; Okajima, F.; Harada, A.; et al. Mutations in the pH-Sensing G-protein-Coupled Receptor GPR68 Cause Amelogenesis Imperfecta. Am. J. Hum. Genet. 2016, 99, 984–990. [Google Scholar] [CrossRef]
- Ogawa, A.; Obinata, H.; Hattori, T.; Kishi, M.; Tatei, K.; Ishikawa, O.; Izumi, T. Identification and analysis of two splice variants of human G2A generated by alternative splicing. J. Pharm. Exp. 2010, 332, 469–478. [Google Scholar] [CrossRef]
- Mashiko, M.; Kurosawa, A.; Tani, Y.; Tsuji, T.; Takeda, S. GPR31 and GPR151 are activated under acidic conditions. J. Biochem. 2019. [Google Scholar] [CrossRef]
- Guo, Y.D.; Zhang, W.L.; Giroux, C.; Cai, Y.L.; Ekambaram, P.; Dilly, A.K.; Hsu, A.; Zhou, S.L.; Maddipati, K.R.; Liu, J.J.; et al. Identification of the Orphan G Protein-coupled Receptor GPR31 as a Receptor for 12-(S)-Hydroxyeicosatetraenoic Acid. J. Biol. Chem. 2011, 286, 33832–33840. [Google Scholar] [CrossRef]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1(+) cells by bacterial metabolites. Nature 2019, 566, 110–114. [Google Scholar] [CrossRef]
- Zhang, X.J.; Cheng, X.; Yan, Z.Z.; Fang, J.; Wang, X.Z.; Wang, W.J.; Liu, Z.Y.; Shen, L.J.; Zhang, P.; Wang, P.X.; et al. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat. Med. 2018, 24, 73–83. [Google Scholar] [CrossRef]
- Van Doren, L.; Nguyen, N.; Garzia, C.; Fletcher, E.K.; Stevenson, R.; Jaramillo, D.; Kuliopulos, A.; Covic, L. Lipid Receptor GPR31 (G-Protein-Coupled Receptor 31) Regulates Platelet Reactivity and Thrombosis Without Affecting Hemostasis. Arter. Throm. Vas 2021, 41, E33–E45. [Google Scholar]
- Fehrenbacher, N.; Tojal da Silva, I.; Ramirez, C.; Zhou, Y.; Cho, K.J.; Kuchay, S.; Shi, J.; Thomas, S.; Pagano, M.; Hancock, J.F.; et al. The G protein-coupled receptor GPR31 promotes membrane association of KRAS. J. Cell Biol. 2017, 216, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Price, M.P.; Welsh, M.J. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 2006, 29, 578–586. [Google Scholar] [CrossRef]
- Kweon, H.J.; Suh, B.C. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013, 46, 295–304. [Google Scholar] [CrossRef]
- Osthues, T.; Zimmer, B.; Rimola, V.; Klann, K.; Schilling, K.; Mathoor, P.; Angioni, C.; Weigert, A.; Geisslinger, G.; Munch, C.; et al. The Lipid Receptor G2A (GPR132) Mediates Macrophage Migration in Nerve Injury-Induced Neuropathic Pain. Cells 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Zhang, J.; Wu, B.; Jiang, M.; Cao, H.Z.; Wu, H.; Gao, Y.J. G protein-coupled receptor GPR151 is involved in trigeminal neuropathic pain through the induction of G beta gamma/extracellular signal-regulated kinase-mediated neuroinflammation in the trigeminal ganglion. Pain 2021, 162, 1434–1448. [Google Scholar] [CrossRef]
- Xia, L.P.; Luo, H.; Ma, Q.; Xie, Y.K.; Li, W.; Hu, H.; Xu, Z.Z. GPR151 in nociceptors modulates neuropathic pain via regulating P2X3 function and microglial activation. Brain 2021. [Google Scholar] [CrossRef]


| Agonist | EC50 or Active Concentration | Test System and/or Consequence | Refs |
| Protons | pH 7.6–5.6 | pH-dependent cAMP assay in various transfected cell lines | [1,62,63] |
| Antagonist | IC50 or Active Concentration | Test System and Consequence | Refs |
| Compound 3b (dibenzazepine derivative) | IC50: 67 nM | HEK-293-cell-based Luciferase assay, mouse myocardial infarction model | [66] |
| NE 52-QQ57 (compound 13 in reference) | IC50: 70 nM | pH-dependent cAMP assay in HeLa and HEK-293 cells, angiogenesis growth factor model, CFA-model for inflammatory pain | [69] |
| Compound 39c (imidazopyridine derivative) | IC50: 110 nM | pH-dependent cAMP assay in HeLa and HEK-293 cells, mouse VEGF-angiogenesis model, rat antigen-induced arthritis model, CFA-induced inflammatory pain | [70] |
| Agonist | EC50 or Active Concentration | Test System and/or Consequence | Refs |
| Protons | pH 7.2–5.7 | pH-dependent cAMP assay in stably TDAG8-transfected CHO cells | [42] |
| Psychosine | EC50: 3.4 µM (cAMP assay) | cAMP assay, calcium-mobilization in TDAG8-transfected HEK-293 cells | [72] |
| BTB09089 | active concentration > 5 µM | cAMP assay in splenocytes | [74] |
| Positive Allosteric Modulators (PAMs) | IC50 or Active Concentration | Test System and Consequence | Refs |
| ZINC13684400 | micromolar range | library of drugs and compounds tested in a yeast TDAG8 expressing system, using cAMP production as readout | [11] |
| Negative Allosteric Modulators (NAMs) | IC50 or Active Concentration | Test System and Consequence | Refs |
| ZINC62678696 | micromolar range | library of drugs and compounds tested in a yeast TDAG8 expressing system, using cAMP production as readout | [11] |
| Agonist | EC50 or Active Concentration | Test System and/or Consequence | Refs |
| Protons | pH 7.8–5.6, maximum activity at pH 6.8 | pH-dependent cAMP assay in various cells | [1,105] |
| 3,5-disubstituted isoxazoles | micromolar range | Calcium transients in transfected Notch-activated epicardium-derived cells (NECs) | [90] |
| CART(42–89)(9–28) shorter variant of cocaine- and amphetamine-regulated transcript | EC50: 1 µM | Identified via:
| [103] |
| steocrin-derived peptide (115–133) | EC50: 380 nM | [103] | |
| pro-opiomelanocortin-derived peptide (141–162) | EC50: 1.3 µM | [103] | |
| Positive Allosteric Modulators (PAMs) | IC50 or Active Concentration | Test System and Consequence | Refs |
| ogerin | Kb: ~10 µM, requires the presence of protons | library of drugs and compounds tested in a yeast OGR1 expressing system, using cAMP production as readout | [11] |
| lorazepam | non-selective, micromolar range | [48] | |
| MS48107 | Kb: ~1–10 µM | cAMP assay in transfected HEK-293 cells. | [104] |
| Agonist | EC50 or Active Concentration | Test System and/or Consequence | Refs |
| Protons | pH 8.2–6.6 | Gq-activation, generation of IP3 in NIH-3T3 fibroblasts | [5] |
| 9S-HODE | EC50: ~0.5 µM [118] | [Ca2+]i-increase in stably G2A-transfected CHO cells | [39,118] |
| 11-HETE | EC50: ~1 µM [118] | [Ca2+]i-increase in stably G2A-transfected CHO cells | [39,118] |
| N-palmitoylglycine | EC50: ~800 nM, similar for human- rat- and mouse-G2A | yeast assay and β-arrestin association assay (in HEK-293 cells) | [113] |
| N-linoleoylglycine | EC50: ~800 nM, similar for human- rat- and mouse-G2A | yeast assay and β-arrestin association assay (in HEK-293 cells) | [113] |
| ONC212 (second-generation imipridone) | ~400 nM | PathHunter β-arrestin association assay in HEK-293 cells | [117] |
| 11,12-EET | ~10 µM | PathHunter β-arrestin association assay in HEK-293 cells | [40] |
| 9,10-EpOME | ~10 µM | PathHunter β-arrestin association assay in HEK-293 cells | [40] |
| Antagonist | IC50 or Active Concentration | Test System and Consequence | Refs |
| Lysophosphatidylcholine (LPC) | ~10 µM | inhibits Gq-dependent, generation of IP3 in NIH-3T3 fibroblasts | [5] |
| Telmisartan | ~10 µM | β-arrestin association assay in HEK-293 cells | [113] |
| GSK1820795A | ~1 µM | β-arrestin association assay in HEK-293 cells | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisignano, M.; Fischer, M.J.M.; Geisslinger, G. Proton-Sensing GPCRs in Health and Disease. Cells 2021, 10, 2050. https://doi.org/10.3390/cells10082050
Sisignano M, Fischer MJM, Geisslinger G. Proton-Sensing GPCRs in Health and Disease. Cells. 2021; 10(8):2050. https://doi.org/10.3390/cells10082050
Chicago/Turabian StyleSisignano, Marco, Michael J. M. Fischer, and Gerd Geisslinger. 2021. "Proton-Sensing GPCRs in Health and Disease" Cells 10, no. 8: 2050. https://doi.org/10.3390/cells10082050
APA StyleSisignano, M., Fischer, M. J. M., & Geisslinger, G. (2021). Proton-Sensing GPCRs in Health and Disease. Cells, 10(8), 2050. https://doi.org/10.3390/cells10082050






