Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Calcium Sulphate/Hydroxyapatite Bone Graft Substitute with Gentamicin (HACaS+G)
2.2. Parathormone
2.3. Implants
- Control group (n = 15): K-wire osteosynthesis, 5 weeks later re-osteosynthesis with an angle-stable plate, followed by 6 weeks of once-daily administration of parathormone.
- Intervention group (n = 19): K-wire osteosynthesis, 5 weeks later re-osteosynthesis with an angle-stable plate and introduction of HACaS+G into the defect, followed by 6 weeks of once-daily administration of parathormone.
2.4. Animals, Operative Procedure, and Osteotomy Model
2.5. Follow-Up
2.6. µCT Scan Evaluation
2.7. Quantitative Analysis
2.8. Sacrifice
2.9. Mechanical Testing
2.10. Histology
2.11. Statistical Analysis
3. Results
3.1. Neither PTH Nor PTH+HACaS+G Led to Stable Union of the Bone Defect
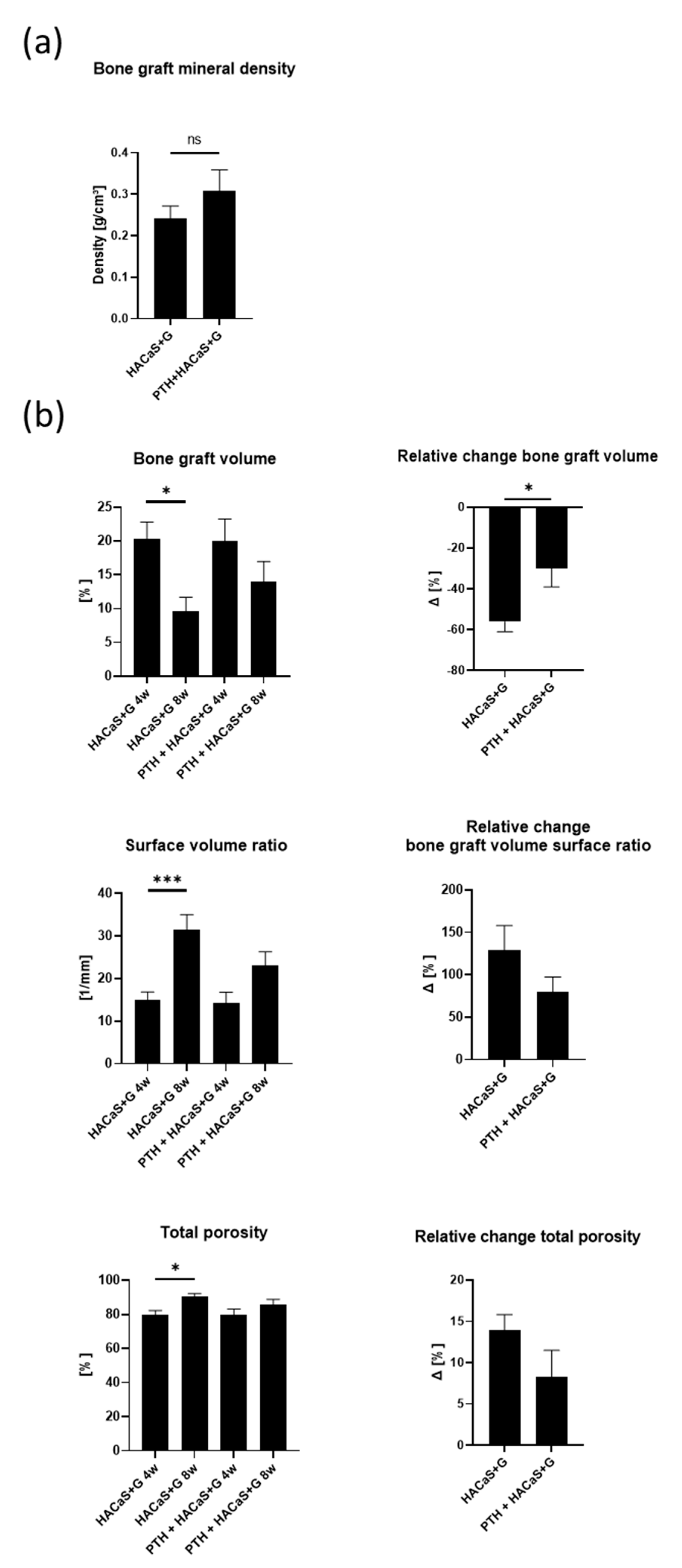
3.2. µCT Analysis of the Bone in the PTH+HACaS+G-Group Showed an Osteoinductive Effect of the Bone Graft Substitute
3.3. µCT Analysis Revealed Biologic Remodeling of the Bone Graft Substitute HACaS+G in Presence of PTH
3.4. PTH Enhanced the Osteoconductive Effect of HACaS+G
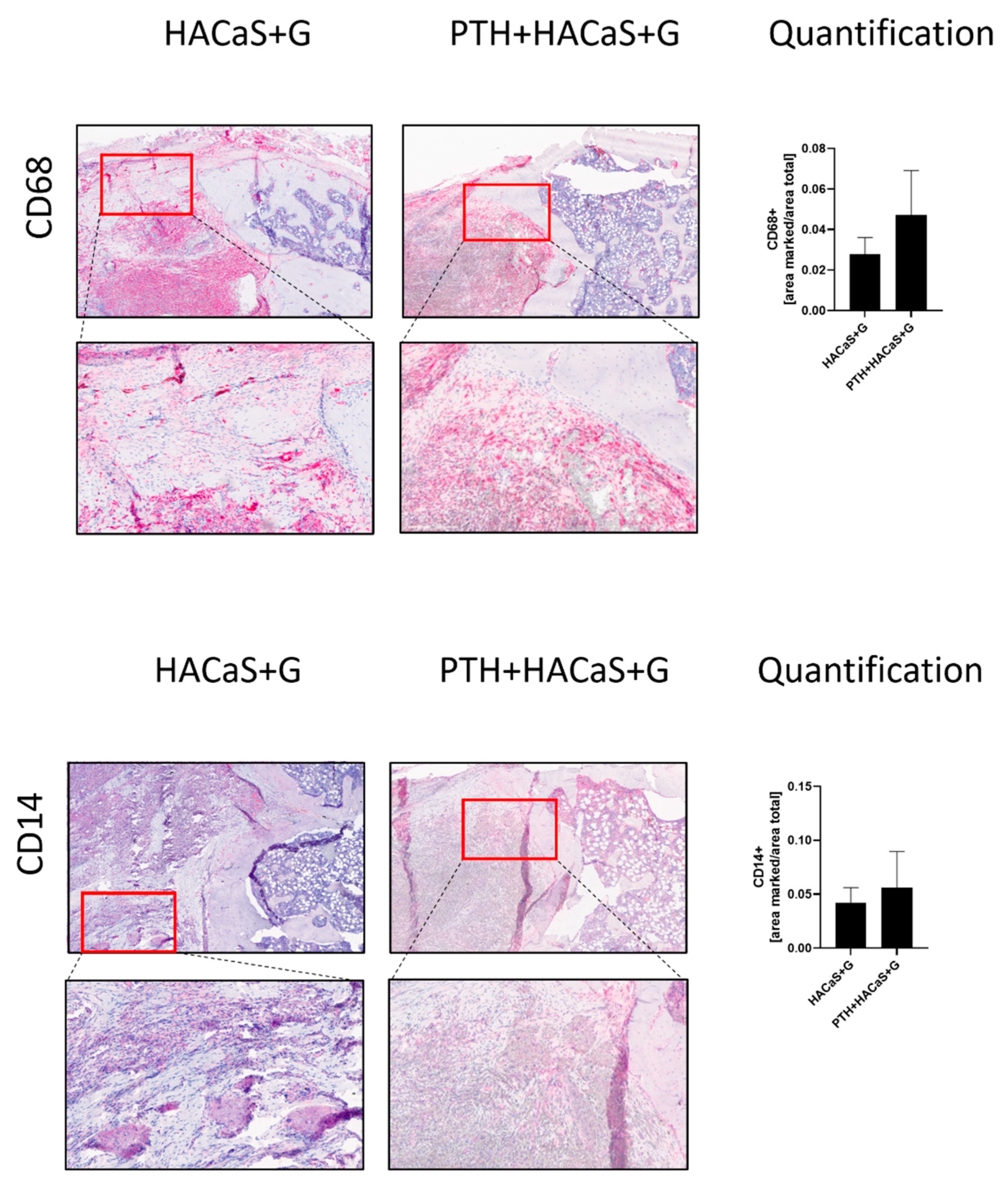
3.4.1. Histological Staining Showed Noticeable Cell Invasion in PTH+HACaS+G
3.4.2. CD14 and CD68 Staining Supported an Osteoconductive Effect of the HACaS+G
3.4.3. PTH Led to Increased Vascularization of the Defect and HACaS+G
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, 7–21. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C.; Tagliabue, L. Treatment of long bone non-unions with polytherapy: Indications and clinical results. Injury 2011, 42, 587–590. [Google Scholar] [CrossRef]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38, S3–S9. [Google Scholar] [CrossRef]
- Jain, A.K.; Sinha, S. Infected nonunion of the long bones. Clin. Orthop. Relat. Res. 2005, 431, 57–65. [Google Scholar] [CrossRef]
- Kanakaris, N.K.; Giannoudis, P.V. The health economics of the treatment of long-bone non-unions. Injury 2007, 38, S77–S84. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice. Bone Jt. J. 2013, 95B, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Abramo, A.; Geijer, M.; Kopylov, P.; Tägil, M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92B, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zalavras, C.G.; Patzakis, M.J.; Holtom, P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin. Orthop. Relat. Res. 2004, 427, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Logoluso, N.; Drago, L.; Gallazzi, E.; George, D.A.; Morelli, I.; Romanò, C.L. Calcium-Based, Antibiotic-Loaded Bone Substitute as an Implant Coating: A Pilot Clinical Study. J. Bone Jt. Infect. 2016, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Oezel, L.; Büren, C.; Scholz, A.O.; Windolf, J.; Windolf, C.D. Effect of antibiotic infused calcium sulfate/hydroxyapatite (CAS/HA) insets on implant-associated osteitis in a femur fracture model in mice. PLoS ONE 2019, 14, e0213590. [Google Scholar] [CrossRef]
- Zampelis, V.; Tägil, M.; Lidgren, L.; Isaksson, H.; Atroshi, I.; Wang, J.-S. The effect of a biphasic injectable bone substitute on the interface strength in a rabbit knee prosthesis model. J. Orthop. Surg. Res. 2013, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freischmidt, H.; Armbruster, J.; Reiter, G.; Grützner, P.A.; Helbig, L.; Guehring, T. Individualized Techniques of Implant Coating with an Antibiotic-Loaded, Hydroxyapatite/Calcium Sulphate Bone Graft Substitute. Ther. Clin. Risk Manag. 2020, 16, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. JBJS 2020, 102, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojda, S.J.; Donahue, S.W. Parathyroid hormone for bone regeneration. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 2586–2594. [Google Scholar] [CrossRef] [Green Version]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.-Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of Parathyroid Hormone (1–34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Mori, S.; Mashiba, T.; Kaji, Y.; Iwata, K.; Komatsubara, S.; Seki, A.; Sun, Y.-X.; Yamamoto, T. Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone 2007, 40, 1475–1482. [Google Scholar] [CrossRef]
- Leiblein, M.; Henrich, D.; Fervers, F.; Kontradowitz, K.; Marzi, I.; Seebach, C. Do antiosteoporotic drugs improve bone regeneration in vivo? Eur. J. Trauma Emerg. Surg. 2020, 46, 287–299. [Google Scholar] [CrossRef]
- Kakar, S.; Einhorn, T.A.; Vora, S.; Miara, L.J.; Hon, G.; Wigner, N.A.; Toben, D.; Jacobsen, K.A.; Al-Sebaei, M.O.; Song, M.; et al. Enhanced Chondrogenesis and Wnt Signaling in PTH-Treated Fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 1903–1912. [Google Scholar] [CrossRef]
- Andreassen, T.T.; Ejersted, C.; Oxlund, H. Intermittent Parathyroid Hormone (1–34) Treatment Increases Callus Formation and Mechanical Strength of Healing Rat Fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 960–968. [Google Scholar] [CrossRef]
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of Experimental Fracture-Healing by Systemic Administration of Recombinant Human Parathyroid Hormone (PTH 1–34). JBJS 2005, 87, 731–741. [Google Scholar]
- Oteo-Alvaro, A.; Moreno, E. Atrophic humeral shaft nonunion treated with teriparatide (rh PTH 1–34): A case report. J. Shoulder Elb. Surg. 2010, 19, e22–e28. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Ha, Y.-C.; Koo, K.-H. Teriparatide, a nonsurgical solution for femoral nonunion? A report of three cases. Osteoporos. Int. 2012, 23, 2897–2900. [Google Scholar] [CrossRef] [PubMed]
- Helbig, L.; Omlor, G.W.; Ivanova, A.; Guehring, T.; Sonntag, R.; Kretzer, J.P.; Minkwitz, S.; Wildemann, B.; Schmidmaier, G. Bone morphogenetic proteins −7 and −2 in the treatment of delayed osseous union secondary to bacterial osteitis in a rat model. BMC Musculoskelet. Disord. 2018, 19, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helbig, L.; Guehring, T.; Titze, N.; Nurjadi, D.; Sonntag, R.; Armbruster, J.; Wildemann, B.; Schmidmaier, G.; Gruetzner, A.; Freischmidt, H. A new sequential animal model for infection-related non-unions with segmental bone defect. BMC Musculoskelet. Disord. 2020, 21, 329. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nakajima, A.; Shiomi, K.; Moriya, H.; Einhorn, T.A.; Yamazaki, M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone 2005, 37, 711–719. [Google Scholar] [CrossRef]
- Komatsu, D.E.; Brune, K.A.; Liu, H.; Schmidt, A.L.; Han, B.; Zeng, Q.Q.; Yang, X.; Nunes, J.S.; Lu, Y.; Geiser, A.G.; et al. Longitudinal in Vivo Analysis of the Region-Specific Efficacy of Parathyroid Hormone in a Rat Cortical Defect Model. Endocrinology 2009, 150, 1570–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Shimoji, N.; Shiomi, K.; Shimizu, S.; Moriya, H.; Einhorn, T.A.; Yamazaki, M. Mechanisms for the Enhancement of Fracture Healing in Rats Treated With Intermittent Low-Dose Human Parathyroid Hormone (1–34). J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 2038–2047. [Google Scholar] [CrossRef]
- Komatsubara, S.; Mori, S.; Mashiba, T.; Nonaka, K.; Seki, A.; Akiyama, T.; Miyamoto, K.; Cao, Y.; Manabe, T.; Norimatsu, H. Human parathyroid hormone (1–34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone 2005, 36, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.; McCauley, L.K. Effects of Intermittent Administration of Parathyroid Hormone and Parathyroid Hormone-Related Protein on Fracture Healing: A Narrative Review of Animal and Human Studies. JBMR Plus 2019, 3, e10250. [Google Scholar] [CrossRef]
- Dobnig, H.; Turner, R.T. The Effects of Programmed Administration of Human Parathyroid Hormone Fragment (1–34) on Bone Histomorphometry and Serum Chemistry in Rats. Endocrinology 1997, 138, 4607–4612. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Zhou, H.; Pan, B.; Lu, L.; Liu, J.; Kang, Y.; Yao, X.; Feng, S. Effectiveness of Teriparatide on Fracture Healing: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0168691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Potty, A.; Vyas, P.; Lane, J. The Role of Recombinant PTH in Human Fracture Healing: A Systematic Review. J. Orthop. Trauma 2014, 28, 57–62. [Google Scholar] [CrossRef]
- Hong, H.; Song, T.; Liu, Y.; Li, J.; Jiang, Q.; Song, Q.; Deng, Z. The effectiveness and safety of parathyroid hormone in fracture healing: A meta-analysis. Clinics 2019, 74, e800. [Google Scholar] [CrossRef]
- Tägil, M.; McDonald, M.M.; Morse, A.; Peacock, L.; Mikulec, K.; Amanat, N.; Godfrey, C.; Little, D.G. Intermittent PTH(1–34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone 2010, 46, 852–859. [Google Scholar] [CrossRef]
- Chen, H.; Frankenburg, E.P.; Goldstein, S.A.; McCauley, L.K. Combination of Local and Systemic Parathyroid Hormone Enhances Bone Regeneration. Clin. Orthop. Relat. Res. 2003, 416, 291–302. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, H.-S.; Lee, D.-W.; Lee, J.-W. Is Parathyroid Hormone A Viable Solution For Nonunion? A Systematic Review And Pooled Analysis. Acta Orthopædica Belg. 2017, 83, 527–535. [Google Scholar]
- Canintika, A.F.; Dilogo, I.H. Teriparatide for treating delayed union and nonunion: A systematic review. J. Clin. Orthop. Trauma 2020, 11, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Kastirr, I.; Reichardt, M.; Andresen, R.; Radmer, S.; Schröder, G.; Westphal, T.; Mittlmeier, T.; Schober, H.C. Therapy of aseptic nonunions with parathyroid hormone. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 169–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular endothelial growth factor: An essential component of angiogenesis and fracture healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausman, M.R.; Schaffler, M.B.; Majeska, R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001, 29, 560–564. [Google Scholar] [CrossRef]
- Langer, M.; Prisby, R.; Peter, Z.; Guignandon, A.; Lafage-Proust, M.; Peyrin, F. Simultaneous 3D Imaging of Bone and Vessel Microstructure in a Rat Model. IEEE Trans. Nucl. Sci. 2011, 58, 139–145. [Google Scholar] [CrossRef]
- Lee, S.; Prisby, R.D. Short-term intermittent parathyroid hormone (1–34) administration increased angiogenesis and matrix metalloproteinase 9 in femora of mature and middle-aged C57BL/6 mice. Exp. Physiol. 2020, 105, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, C.; Shi, H.; Cheng, Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS ONE 2019, 14, e0226163. [Google Scholar] [CrossRef] [PubMed]






| Scan | Rotation Step | Frame Averaging |
|---|---|---|
| Pre-op scan | 1° | 2 |
| 4- and 8-week in vivo scan | 0.6° | 4 |
| Ex vivo scan | 0.4° | 6 |
| BMD | TMD | ||
|---|---|---|---|
| In Vivo | VOI_control | No threshold | 90–255 |
| VOI_bone | No threshold | 90–255 | |
| VOI_cer | 1–255 | 90–255 | |
| Ex Vivo | VOI_control | No threshold | 80–255 |
| VOI_bone | No threshold | 80–255 | |
| VOI_cer | 1–255 | 80–255 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freischmidt, H.; Armbruster, J.; Bonner, E.; Guehring, T.; Nurjadi, D.; Bechberger, M.; Sonntag, R.; Schmidmaier, G.; Grützner, P.A.; Helbig, L. Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute. Cells 2021, 10, 2058. https://doi.org/10.3390/cells10082058
Freischmidt H, Armbruster J, Bonner E, Guehring T, Nurjadi D, Bechberger M, Sonntag R, Schmidmaier G, Grützner PA, Helbig L. Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute. Cells. 2021; 10(8):2058. https://doi.org/10.3390/cells10082058
Chicago/Turabian StyleFreischmidt, Holger, Jonas Armbruster, Emma Bonner, Thorsten Guehring, Dennis Nurjadi, Maren Bechberger, Robert Sonntag, Gerhard Schmidmaier, Paul Alfred Grützner, and Lars Helbig. 2021. "Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute" Cells 10, no. 8: 2058. https://doi.org/10.3390/cells10082058
APA StyleFreischmidt, H., Armbruster, J., Bonner, E., Guehring, T., Nurjadi, D., Bechberger, M., Sonntag, R., Schmidmaier, G., Grützner, P. A., & Helbig, L. (2021). Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute. Cells, 10(8), 2058. https://doi.org/10.3390/cells10082058







