Myostatin/Activin-A Signaling in the Vessel Wall and Vascular Calcification
Abstract
1. Introduction
2. Myostatin/Activin-A Signaling: Basic Biology and Functions
2.1. Mstn Biology
2.2. Mstn Signaling and Functions
2.3. Act-A Biology
2.4. Act-A Signaling and Functions
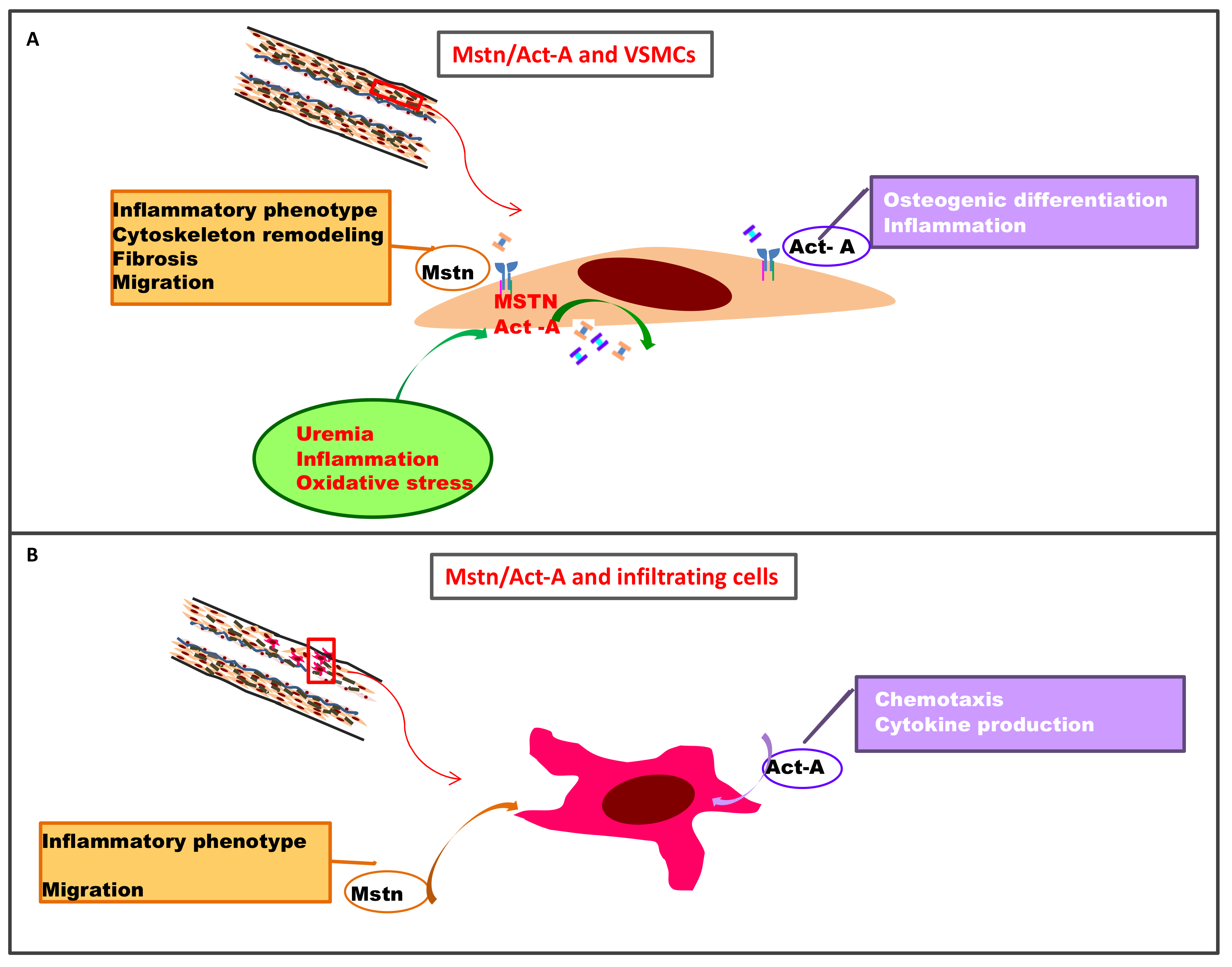
3. Myostatin and Activin-A in the Vessel Wall
3.1. Myostatin: A Role in Arterial Remodeling, VSMC Proliferation, and Accelerated Aging in Atherosclerotic Disease?
3.1.1. Experimental Evidence
3.1.2. Human Specimens
3.1.3. Clinical Studies
3.2. Activin A in the Vessel Wall
3.2.1. Experimental Evidence
3.2.2. Human Specimens
3.2.3. Clinical Studies
4. Myostatin and Activin-A in Vascular Calcification
- -
- calcification in arterial intimal layers in association with macrophages, lipids, and VSMCs, as in classical atherosclerosis;
- -
- calcification in arterial medial layers, as a result of elastin fiber mineralization, VSMC degeneration, and upregulation of osteogenic programs as in CKD or diabetes, e.g., Möckenberg’s medial sclerosis of large arteries. There are some similarities, but also marked differences between intimal atherosclerotic and medial calcification; although inflammation and cytokine production are common to both types, osteogenic differentiation with metaplastic bone formation is only rarely implicated in intimal calcification, whereas it is often seen in medial calcification of peripheral arteries [94]. Hence, it is reasonable to hypothesize that the different types of vascular calcifications present distinct pathogenetic mechanisms. Experimental studies support this idea [95]; so, in a rabbit model, the induction of CKD by subtotal nephrectomy caused media degeneration with calcification in the absence of intimal calcification and lipid deposition [96]. Notably, in CKD, vascular calcification recapitulates many aspects of bone formation, and both processes share several pathogenic mechanisms. Furthermore, vascular calcification is frequently associated with MBD, and its progression is associated with decreased bone mineral density and altered bone turnover.
4.1. Myostatin and Vascular Calcification
4.2. Activin-A Signaling and Vascular Calcification
4.2.1. Experimental Studies
4.2.2. Human Specimens and Clinical Studies
5. Activin-A Signaling Vascular and Soft Tissue Calcifications: Lessons from Fibrodysplasia Ossificans Progressiva (FOP)
6. Clinical Therapeutic Developments
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weir, M.R. Recognizing the link between chronic kidney disease and cardiovascular disease. Am. J. Manag. Care 2011, 17, 396–402. [Google Scholar]
- Lacolley, P.; Regnault, V.; Avolio, A.P. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovasc. Res. 2018, 114, 513–528. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-Term Prognosis Associated With Coronary Calcification. Observations From a Registry of 25,253 Patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, A.K.; Chhabra, Y.K.; Mahajan, S. Cardiovascular disease in patients with chronic kidney disease: A neglected subgroup. Heart Asia 2016, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Pardali, E.; Sánchez-Duffhues, G.; Ten Dijke, P. BMP signaling in vascular diseases. FEBS Lett. 2012, 586, 1993–2002. [Google Scholar] [CrossRef]
- Yang, P.; Troncone, L.; Augur, Z.M.; Kim, S.S.J.; McNeil, M.E.; Yu, P.B. The role of bone morphogenetic protein signaling in vascular calcification. Bone 2020, 141, 115542. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Esposito, P.; Picciotto, D.; Verzola, D. Activin/myostatin receptor signaling and vascular calcifications in chronic kidney disease: A “liaison dangereuse”? Kidney Res. Clin. Pract. 2019, 38, 407–410. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. The transforming growth factor β superfamily. In Growth Factors and Cytokines in Health and Disease; Leroith, D., Bondy, Eds.; JAI: Stamford, CT, USA, 1996; Volume 1, pp. 357–393. ISBN 1874-5687. [Google Scholar]
- Kusakabe, M.; Cheong, P.-L.; Nikfar, R.; McLennan, I.S.; Koishi, K. The structure of the TGF-beta latency associated peptide region determines the ability of the proprotein convertase furin to cleave TGF-betas. J. Cell Biochem. 2008, 103, 311–320. [Google Scholar] [CrossRef]
- Walker, R.G.; McCoy, J.C.; Czepnik, M.; Mills, M.J.; Hagg, A.; Walton, K.L.; Cotton, T.R.; Hyvönen, M.; Lee, R.T.; Gregorevic, P.; et al. Molecular characterization of latent GDF8 reveals mechanisms of activation. Proc. Natl. Acad. Sci. USA 2018, 115, E866–E875. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Attisano, L.; Wrana, J.L.; Montalvo, E.; Massagué, J. Activation of signalling by the activin receptor complex. Mol. Cell Biol. 1996, 16, 1066–1073. [Google Scholar] [CrossRef]
- Amthor, H.; Nicholas, G.; McKinnell, I.; Kemp, C.F.; Sharma, M.; Kambadur, R.; Patel, K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev. Biol. 2004, 270, 19–30. [Google Scholar] [CrossRef]
- Le, V.Q.; Iacob, R.E.; Tian, Y.; McConaughy, W.; Jackson, J.; Su, Y.; Zhao, B.; Engen, J.R.; Pirruccello-Straub, M.; Springer, T.A. Tolloid cleavage activates latent GDF8 by priming the pro-complex for dissociation. EMBO J. 2018, 37, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Lafuste, P.; Brunelli, S.; Iaconis, S.; Touvier, T.; Mourikis, P.; De Bock, K.; Lonardo, E.; Andolfi, G.; Bouché, A.; et al. Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonizing myostatin. Proc. Natl. Acad. Sci. USA 2012, 109. [Google Scholar] [CrossRef]
- Gray, P.C.; Harrison, C.A.; Vale, W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 5193–5198. [Google Scholar] [CrossRef]
- Kemaladewi, D.U.; Gorter, D.J.J.; Aartsma-Rus, A.; Ommen, G.; ten Dijke, P.; Hoen, P.A.C.t.; Hoogaars, W.M. Cell-type specific regulation of myostatin signaling. FASEB J. 2012, 26, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Topouzis, S.; Liang, L.-F.; Stotish, R.L. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 2004, 26, 262–272. [Google Scholar] [CrossRef]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef]
- Philip, B.; Lu, Z.; Gao, Y. Regulation of GDF-8 signaling by the p38 MAPK. Cell Signal. 2005, 17, 365–375. [Google Scholar] [CrossRef]
- Hu, S.-L.; Chang, A.-C.; Huang, C.-C.; Tsai, C.-H.; Lin, C.-C.; Tang, C.-H. Myostatin Promotes Interleukin-1β Expression in Rheumatoid Arthritis Synovial Fibroblasts through Inhibition of miR-21-5p. Front. Immunol. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Steelman, C.A.; Recknor, J.C.; Nettleton, D.; Reecy, J.M. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 580–582. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- McFarlane, C.; Plummer, E.; Thomas, M.; Hennebry, A.; Ashby, M.; Ling, N.; Smith, H.; Sharma, M.; Kambadur, R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J. Cell Physiol. 2006, 209, 501–514. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hopkinson, N.S.; Kemp, P.R. Myostatin induces autophagy in skeletal muscle in vitro. Biochem. Biophys. Res. Commun. 2011, 415, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Demontis, F. GDF11/myostatin and aging. Aging 2014, 6, 351–352. [Google Scholar] [CrossRef]
- Shyh-Chang, N. Metabolic Changes During Cancer Cachexia Pathogenesis. In Translational Research in Breast Cancer: Biomarker Diagnosis, Targeted Therapies and Approaches to Precision Medicine; Song, E., Hu, H., Eds.; Springer: Singapore, 2017; pp. 233–249. ISBN 978-981-10-6020-5. [Google Scholar]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef]
- Hoenig, M.R. Hypothesis: Myostatin is a mediator of cardiac cachexia. Int. J. Cardiol. 2008, 124, 131–133. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Cao, L.; Zhang, Y.; Xia, W.; Zhu, D. Myostatin regulates glucose metabolism via the AMP-activated protein kinase pathway in skeletal muscle cells. Int. J. Biochem. Cell Biol. 2010, 42, 2072–2081. [Google Scholar] [CrossRef]
- Milan, G.; Dalla Nora, E.; Pilon, C.; Pagano, C.; Granzotto, M.; Manco, M.; Mingrone, G.; Vettor, R. Changes in muscle myostatin expression in obese subjects after weight loss. J. Clin. Endocrinol. Metab. 2004, 89, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Milanesi, S.; Viazzi, F.; Ansaldo, F.; Saio, M.; Garibaldi, S.; Carta, A.; Costigliolo, F.; Salvidio, G.; Barisione, C.; et al. Enhanced myostatin expression and signalling promote tubulointerstitial inflammation in diabetic nephropathy. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kellum, E.; Starr, H.; Arounleut, P.; Immel, D.; Fulzele, S.; Wenger, K.; Hamrick, M.W. Myostatin (GDF-8) deficiency increases fracture callus size, Sox-5 expression, and callus bone volume. Bone 2009, 44, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wallner, C.; Jaurich, H.; Wagner, J.M.; Becerikli, M.; Harati, K.; Dadras, M.; Lehnhardt, M.; Behr, B. Inhibition of GDF8 (Myostatin) accelerates bone regeneration in diabetes mellitus type 2. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Vale, W.; Wiater, E.; Gray, P.; Harrison, C.; Bilezikjian, L.; Choe, S. Activins and inhibins and their signaling. Ann. N. Y. Acad. Sci. 2004, 1038, 142–147. [Google Scholar] [CrossRef]
- Mason, A.J.; Niall, H.D.; Seeburg, P.H. Structure of two human ovarian inhibins. Biochem. Biophys. Res. Commun. 1986, 135, 957–964. [Google Scholar] [CrossRef]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the regulation of immunity in health and disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef]
- Kawakami, T.; Ren, S.; Duffield, J.S. Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J. Pathol. 2013, 229, 221–231. [Google Scholar] [CrossRef]
- Harrison, C.A.; Gray, P.C.; Vale, W.W.; Robertson, D.M. Antagonists of activin signaling: Mechanisms and potential biological applications. Trends Endocrinol. Metab. 2005, 16, 73–78. [Google Scholar] [CrossRef]
- Gray, P.C.; Bilezikjian, L.M.; Vale, W. Antagonism of activin by inhibin and inhibin receptors: A functional role for betaglycan. Mol. Cell Endocrinol. 2002, 188, 254–260. [Google Scholar] [CrossRef]
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massagué, J.; Niehrs, C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Ying, S.-Y.; Ueno, N.; Shimasaki, S.; Esch, F.; Hotta, M.; Guillemin, R. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 1986, 321, 779–782. [Google Scholar] [CrossRef]
- Schneider-Kolsky, M.E.; Manuelpillai, U.; Waldron, K.; Dole, A.; Wallace, E.M. The distribution of activin and activin receptors in gestational tissues across human pregnancy and during labour. Placenta 2002, 23, 294–302. [Google Scholar] [CrossRef]
- Bloise, E.; Ciarmela, P.; Dela Cruz, C.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in mammalian physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef] [PubMed]
- Tretter, Y.P.; Hertel, M.; Munz, B.; ten Bruggencate, G.; Werner, S.; Alzheimer, C. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat. Med. 2000, 6, 812–815. [Google Scholar] [CrossRef]
- Feijen, A.; Goumans, M.J.; van den Eijnden-van Raaij, A.J. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development 1994, 120, 3621–3637. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Ouchi, N.; Shimano, M.; Pimentel, D.R.; Papanicolaou, K.N.; Panse, K.D.; Tsuchida, K.; Lara-Pezzi, E.; Lee, S.-J.; Walsh, K. Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation 2009, 120, 1606–1615. [Google Scholar] [CrossRef]
- Maeshima, A.; Zhang, Y.Q.; Nojima, Y.; Naruse, T.; Kojima, I. Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J. Am. Soc. Nephrol. 2001, 12, 1685–1695. [Google Scholar] [CrossRef]
- Yamashita, S.; Maeshima, A.; Kojima, I.; Nojima, Y. Activin A Is a Potent Activator of Renal Interstitial Fibroblasts. J. Am. Soc. Nephrol. 2004, 15, 91–101. [Google Scholar] [CrossRef]
- Chen, J.L.; Walton, K.L.; Winbanks, C.E.; Murphy, K.T.; Thomson, R.E.; Makanji, Y.; Qian, H.; Lynch, G.S.; Harrison, C.A.; Gregorevic, P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014, 28, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Centrella, M.; McCarthy, T.L.; Canalis, E. Activin-A binding and biochemical effects in osteoblast-enriched cultures from fetal-rat parietal bone. Mol. Cell. Biol. 1991, 11, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Funaba, M.; Ogawa, K.; Abe, M. Expression and localization of activin receptors during endochondral bone development. Eur. J. Endocrinol. 2001, 144, 63–71. [Google Scholar] [CrossRef][Green Version]
- Solagna, F.; Patel, K.; Huber, T.B.; Solagna, F.; Tezze, C.; Lindenmeyer, M.T.; Lu, S.; Wu, G.; Liu, S.; Zhao, Y.; et al. Pro-cachectic factors link experimental and human chronic kidney disease to skeletal muscle wasting programs. J. Clin. Invesitig. 2021, 131, e135821. [Google Scholar] [CrossRef]
- Lotinun, S.; Pearsall, R.S.; Davies, M.V.; Marvell, T.H.; Monnell, T.E.; Ucran, J.; Fajardo, R.J.; Kumar, R.; Underwood, K.W.; Seehra, J.; et al. A soluble activin receptor Type IIA fusion protein (ACE-011) increases bone mass via a dual anabolic-antiresorptive effect in Cynomolgus monkeys. Bone 2010, 46, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ten Dijke, P. Immunoregulation by members of the TGFβ superfamily. Nat. Rev. Immunol. 2016, 16, 723–740. [Google Scholar] [CrossRef]
- Arai, K.Y.; Ono, M.; Kudo, C.; Fujioka, A.; Okamura, R.; Nomura, Y.; Nishiyama, T. IL-1beta stimulates activin betaA mRNA expression in human skin fibroblasts through the MAPK pathways, the nuclear factor-kappaB pathway, and prostaglandin E2. Endocrinology 2011, 152, 3779–3790. [Google Scholar] [CrossRef]
- Hully, J.R.; Chang, L.; Schwall, R.H.; Widmer, H.R.; Terrell, T.G.; Gillett, N.A. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology 1994, 20, 854–862. [Google Scholar] [CrossRef]
- Maeshima, A.; Nojima, Y.; Kojima, I. Activin A: An autocrine regulator of cell growth and differentiation in renal proximal tubular cells. Kidney Int. 2002, 62, 446–454. [Google Scholar] [CrossRef][Green Version]
- Ishisaki, A.; Yamato, K.; Nakao, A.; Nonaka, K.; Ohguchi, M.; ten Dijke, P.; Nishihara, T. Smad7 is an activin-inducible inhibitor of activin-induced growth arrest and apoptosis in mouse B cells. J. Biol. Chem. 1998, 273, 24293–24296. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, Y.; Kizaki, M.; Yamato, K.; Kawamura, C.; Umezawa, A.; Ji, H.; Nishihara, T.; Ikeda, Y. Mcl-1, an early-induction molecule, modulates activin A-induced apoptosis and differentiation of CML cells. Oncogene 2001, 20, 704–713. [Google Scholar] [CrossRef]
- Liu, M.; Mao, C.; Li, J.; Han, F.; Yang, P. Effects of the Activin A-Follistatin System on Myocardial Cell Apoptosis through the Endoplasmic Reticulum Stress Pathway in Heart Failure. Int. J. Mol. Sci. 2017, 18, 374. [Google Scholar] [CrossRef]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef]
- Guo, W.; Wong, S.; Bhasin, S. AAV-mediated administration of myostatin pro-peptide mutant in adult Ldlr null mice reduces diet-induced hepatosteatosis and arteriosclerosis. PLoS ONE 2013, 8, e71017. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Milanesi, S.; Bertolotto, M.; Garibaldi, S.; Villaggio, B.; Brunelli, C.; Balbi, M.; Ameri, P.; Montecucco, F.; Palombo, D.; et al. Myostatin mediates abdominal aortic atherosclerosis progression by inducing vascular smooth muscle cell dysfunction and monocyte recruitment. Sci. Rep. 2017, 7, 46362. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Tu, P.; Bhasin, S.; Hruz, P.W.; Herbst, K.L.; Castellani, L.W.; Hua, N.; Hamilton, J.A.; Guo, W. Genetic disruption of myostatin reduces the development of proatherogenic dyslipidemia and atherogenic lesions in Ldlr null mice. Diabetes 2009, 58, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Welten, S.M.J.; de Jong, R.C.M.; Wezel, A.; de Vries, M.R.; Boonstra, M.C.; Parma, L.; Jukema, J.W.; van der Sluis, T.C.; Arens, R.; Bot, I.; et al. Inhibition of 14q32 microRNA miR-495 reduces lesion formation, intimal hyperplasia and plasma cholesterol levels in experimental restenosis. Atherosclerosis 2017, 261, 26–36. [Google Scholar] [CrossRef]
- Goossens, E.A.C.; de Vries, M.R.; Jukema, J.W.; Quax, P.H.A.; Nossent, A.Y. Myostatin Inhibits Vascular Smooth Muscle Cell Proliferation and Local 14q32 microRNA Expression, But Not Systemic Inflammation or Restenosis. Int. J. Mol. Sci. 2020, 21, 3508. [Google Scholar] [CrossRef]
- Qiu, S.; Mintz, J.D.; Salet, C.D.; Han, W.; Giannis, A.; Chen, F.; Yu, Y.; Su, Y.; Fulton, D.J.; Stepp, D.W. Increasing muscle mass improves vascular function in obese (db/db) mice. J. Am. Heart Assoc. 2014, 3, e000854. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Ali, M.I.; Ma, M.W.; McCarthy, C.G.; Islam, B.N.; Fox, L.G.; Mintz, J.D.; Larion, S.; Fulton, D.J.; Stepp, D.W. Effect of myostatin deletion on cardiac and microvascular function. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Esposito, P.; Verzola, D.; La Porta, E.; Milanesi, S.; Grignano, M.A.; Avella, A.; Gregorini, M.; Abelli, M.; Ticozzelli, E.; Rampino, T.; et al. Myostatin in the arterial wall of patients with end-stage renal disease. J. Atheroscler. Thromb. 2020, 27, 1039–1052. [Google Scholar] [CrossRef]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, L.; Hua, Y.; Huang, K.; Wang, I.; Huang, L.; Fu, Q.; Chen, A.; Chan, P.; Fan, H.; et al. Vasculopathy in the setting of cardiorenal syndrome: Roles of protein-bound uremic toxins. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1–H13. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Pucci, G.; Ministrini, S.; Nulli Migliola, E.; Nunziangeli, L.; Battista, F.; D’Abbondanza, M.; Anastasio, F.; Crapa, M.E.; Sanesi, L.; Carbone, F.; et al. Relationship between serum myostatin levels and carotid-femoral pulse wave velocity in healthy young male adolescents: The MACISTE study. J. Appl. Physiol. 2021, 130, 987–992. [Google Scholar] [CrossRef]
- McCarthy, S.A.; Bicknell, R. Inhibition of vascular endothelial cell growth by activin-A. J. Biol. Chem. 1993, 268, 23066–23071. [Google Scholar] [CrossRef]
- Kojima, I.; Mogami, H.; Kawamura, N.; Yasuda, H.; Shibata, H. Modulation of growth of vascular smooth muscle cells by activin A. Exp. Cell Res. 1993, 206, 152–156. [Google Scholar] [CrossRef]
- Kozaki, K.; Akishita, M.; Eto, M.; Yoshizumi, M.; Toba, K.; Inoue, S.; Ishikawa, M.; Hashimoto, M.; Kodama, T.; Yamada, N.; et al. Role of activin-A and follistatin in foam cell formation of THP-1 macrophages. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2389–2394. [Google Scholar] [CrossRef]
- Engelse, M.A.; Lardenoye, J.H.P.; Neele, J.M.; Grimbergen, J.M.; De Vries, M.R.; Lamfers, M.L.M.; Pannekoek, H.; Quax, P.H.A.; De Vries, C.J.M. Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. Circ. Res. 2002, 90, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, G.T.L.; Grauls, G.E.; Bruggeman, C.A.; Stassen, F.R. Adenoviral activin A expression prevents vein graft intimal hyperplasia in a rat model. Interact Cardiovasc. Thorac. Surg. 2009, 8, 31–34. [Google Scholar] [CrossRef]
- Ryanto, G.R.T.; Ikeda, K.; Miyagawa, K.; Tu, L.; Guignabert, C.; Humbert, M.; Fujiyama, T.; Yanagisawa, M.; Hirata, K.-I.; Emoto, N. An endothelial activin A-bone morphogenetic protein receptor type 2 link is overdriven in pulmonary hypertension. Nat. Commun. 2021, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Engelse, M.A.; Neele, J.M.; van Achterberg, T.A.; van Aken, B.E.; van Schaik, R.H.; Pannekoek, H.; de Vries, C.J. Human activin-A is expressed in the atherosclerotic lesion and promotes the contractile phenotype of smooth muscle cells. Circ. Res. 1999, 85, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Yndestad, A.; Halvorsen, B.; Ueland, T.; Waehre, T.; Otterdal, K.; Scholz, H.; Endresen, K.; Gullestad, L.; Frøland, S.S.; et al. Potential anti-inflammatory role of activin A in acute coronary syndromes. J. Am. Coll. Cardiol. 2004, 44, 369–375. [Google Scholar] [CrossRef]
- Miyoshi, T.; Hirohata, S.; Uesugi, T.; Hirota, M.; Ohnishi, H.; Nogami, K.; Hatanaka, K.; Ogawa, H.; Usui, S.; Kusachi, S. Relationship between activin A level and infarct size in patients with acute myocardial infarction undergoing successful primary coronary intervention. Clin. Chim. Acta 2009, 401, 3–7. [Google Scholar] [CrossRef]
- Lin, J.-F.; Hsu, S.-Y.; Teng, M.-S.; Wu, S.; Hsieh, C.-A.; Jang, S.-J.; Liu, C.-J.; Huang, H.-L.; Ko, Y.-L. Activin A Predicts Left Ventricular Remodeling and Mortality in Patients with ST-Elevation Myocardial Infarction. Acta Cardiol. Sin. 2016, 32, 420–427. [Google Scholar] [CrossRef]
- Chen, N.X.; Moe, S.M. Vascular calcification: Pathophysiology and risk factors. Curr. Hypertens. Rep. 2012, 14, 228–237. [Google Scholar] [CrossRef]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef]
- Amann, K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1599–1605. [Google Scholar] [CrossRef]
- Drüeke, T.B. Arterial intima and media calcification: Distinct entities with different pathogenesis or all the same? Clin. J. Am. Soc Nephrol. 2008, 3, 1583–1584. [Google Scholar] [CrossRef]
- Shobeiri, N.; Adams, M.A.; Holden, R.M. Vascular calcification in animal models of CKD: A review. Am. J. Nephrol. 2010, 31, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Tvedegaard, E. Arterial disease in chronic renal failure--an experimental study in the rabbit. Acta Pathol. Microbiol. Immunol. Scand. A 1987, 290, 1–28. [Google Scholar] [PubMed]
- Lee, S.M.; Kim, S.E.; Lee, J.Y.; Jeong, H.J.; Son, Y.K.; An, W.S. Serum myostatin levels are associated with abdominal aortic calcification in dialysis patients. Kidney Res. Clin. Pract. 2019, 38, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Hofbauer, L.C.; Rauner, M.; Goettsch, C.; Chapurlat, R.; Schoppet, M. Serum myostatin levels are negatively associated with abdominal aortic calcification in older men: The STRAMBO study. Eur. J. Endocrinol. 2012, 167, 873–880. [Google Scholar] [CrossRef]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Sugatani, T.; Agapova, O.A.; Fang, Y.; Gaut, J.P.; Faugere, M.C.; Malluche, H.H.; Hruska, K.A. The activin receptor is stimulated in the skeleton, vasculature, heart, and kidney during chronic kidney disease. Kidney Int. 2018, 93, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Agapova, O.A.; Fang, Y.; Sugatani, T.; Seifert, M.E.; Hruska, K.A. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int. 2016, 89, 1231–1243. [Google Scholar] [CrossRef]
- Sugatani, T. Systemic Activation of Activin A Signaling Causes Chronic Kidney Disease-Mineral Bone Disorder. Int. J. Mol. Sci. 2018, 19, 2490. [Google Scholar] [CrossRef]
- Sugatani, T.; Alvarez, U.M.; Hruska, K.A. Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J. Cell Biochem. 2003, 90, 59–67. [Google Scholar] [CrossRef]
- Sugatani, T.; Agapova, O.A.; Fang, Y.; Berman, A.G.; Wallace, J.M.; Malluche, H.H.; Faugere, M.-C.; Smith, W.; Sung, V.; Hruska, K.A. Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int. 2017, 91, 86–95. [Google Scholar] [CrossRef]
- Verhulst, A.; Evenepoel, P.; D’Haese, P.C. Ligand trap for the activin type IIA receptor. The long-sought drug to overcome the calcification paradox in CKD? Kidney Int. 2017, 91, 11–13. [Google Scholar] [CrossRef]
- Shiozaki, M.; Sakai, R.; Tabuchi, M.; Nakamura, T.; Sugino, K.; Sugino, H.; Eto, Y. Evidence for the participation of endogenous activin A/erythroid differentiation factor in the regulation of erythropoiesis. Proc. Natl. Acad. Sci. USA 1992, 89, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Carrancio, S.; Markovics, J.; Wong, P.; Leisten, J.; Castiglioni, P.; Groza, M.C.; Raymon, H.K.; Heise, C.; Daniel, T.; Chopra, R.; et al. An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br. J. Haematol. 2014, 165, 870–882. [Google Scholar] [CrossRef]
- Li, J.; Fredericks, M.; Cannell, M.; Wang, K.; Sako, D.; Maguire, M.C.; Grenha, R.; Liharska, K.; Krishnan, L.; Bloom, T.; et al. ActRIIB:ALK4-Fc alleviates muscle dysfunction and comorbidities in murine models of neuromuscular disorders. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Griffin, T.P.; Zhu, X.; Islam, M.N.; Conley, S.M.; Eirin, A.; Tang, H.; O’Shea, P.M.; Palmer, A.K.; McCoy, R.G.; et al. Senescence marker activin A is increased in human diabetic kidney disease: Association with kidney function and potential implications for therapy. BMJ Open Diabetes Res. Care 2019, 7, e000720. [Google Scholar] [CrossRef]
- Yano, S.; Nagai, A.; Isomura, M.; Yamasaki, M.; Kijima, T.; Takeda, M.; Hamano, T.; Nabika, T. Relationship between Blood Myostatin Levels and Kidney Function:Shimane CoHRE Study. PLoS ONE 2015, 10, e0141035. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-S.; Lu, Y.-W.; Hsu, C.-Y.; Chang, C.-C.; Chou, R.-H.; Liu, L.-K.; Chen, L.-K.; Huang, P.-H.; Chen, J.-W.; Lin, S.-J. Increased activin A levels in prediabetes and association with carotid intima-media thickness: A cross-sectional analysis from I-Lan Longitudinal Aging Study. Sci. Rep. 2018, 8, 9957. [Google Scholar] [CrossRef]
- Yonata, A.; Ali, Z.; Indrajaya, T.; Bahar, E.; Effendi, I.; Suhaimi, N.; Suprapti, S. The Association between the Activin A Serum Level and Carotid Intima-Media Thickness in Chronic Kidney Disease Patients. Int. J. Nephrol. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Hüning, I.; Gillessen-Kaesbach, G. Fibrodysplasia ossificans progressiva: Clinical course, genetic mutations and genotype-phenotype correlation. Mol. Syndromol. 2014, 5, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum. Mutat. 2009, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef]
- Cappato, S.; Giacopelli, F.; Ravazzolo, R.; Bocciardi, R. The Horizon of a Therapy for Rare Genetic Diseases: A “Druggable” Future for Fibrodysplasia Ossificans Progressiva. Int. J. Mol. Sci. 2018, 19, 989. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.H.; Jaspers, R.T. Past, Present, and Future Perspective of Targeting Myostatin and Related Signaling Pathways to Counteract Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 153–206. [Google Scholar] [CrossRef]
- Campbell, C.; McMillan, H.J.; Mah, J.K.; Tarnopolsky, M.; Selby, K.; McClure, T.; Wilson, D.M.; Sherman, M.L.; Escolar, D.; Attie, K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 2017, 55, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.O.; Chico, T.J.A.; Arthur, H.M. Hereditary Haemorrhagic Telangiectasia, an Inherited Vascular Disorder in Need of Improved Evidence-Based Pharmaceutical Interventions. Genes 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.-M.; Yang, P.; Joshi, S.; Augur, Z.M.; Kim, S.S.J.; Bocobo, G.A.; Dinter, T.; Troncone, L.; Chen, P.-S.; McNeil, M.E.; et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Platzbecker, U.; Germing, U.; Götze, K.S.; Kiewe, P.; Mayer, K.; Chromik, J.; Radsak, M.; Wolff, T.; Zhang, X.; Laadem, A.; et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): A multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017, 18, 1338–1347. [Google Scholar] [CrossRef]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Porter, J.; Origa, R.; Forni, G.L.; Voskaridou, E.; Galactéros, F.; Taher, A.T.; Arlet, J.-B.; Ribeil, J.-A.; Garbowski, M.; et al. Sotatercept, a novel transforming growth factor β ligand trap, improves anemia in β-thalassemia: A phase II, open-label, dose-finding study. Haematologica 2019, 104, 477–484. [Google Scholar] [CrossRef]
- Coyne, D.W.; Singh, H.N.; Smith, W.T.; Giuseppi, A.C.; Connarn, J.N.; Sherman, M.L.; Dellanna, F.; Malluche, H.H.; Hruska, K.A. Sotatercept Safety and Effects on Hemoglobin, Bone, and Vascular Calcification. Kidney Int. Rep. 2019, 4, 1585–1597. [Google Scholar] [CrossRef]
- Sherman, M.L.; Borgstein, N.G.; Mook, L.; Wilson, D.; Yang, Y.; Chen, N.; Kumar, R.; Kim, K.; Laadem, A. Multiple-dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA-IgG1), a Novel erythropoietic agent, in healthy postmenopausal women. J. Clin. Pharmacol. 2013, 53, 1121–1130. [Google Scholar] [CrossRef]
- Komrokji, R.; Garcia-Manero, G.; Ades, L.; Prebet, T.; Steensma, D.P.; Jurcic, J.G.; Sekeres, M.A.; Berdeja, J.; Savona, M.R.; Beyne-Rauzy, O.; et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: A phase 2, dose-ranging trial. Lancet Haematol. 2018, 5, e63–e72. [Google Scholar] [CrossRef]
- Ruckle, J.; Jacobs, M.; Kramer, W.; Pearsall, A.E.; Kumar, R.; Underwood, K.W.; Seehra, J.; Yang, Y.; Condon, C.H.; Sherman, M.L. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J. Bone Miner Res. Off. J. Am. Soc. Bone Miner Res. 2009, 24, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Abdulkadyrov, K.M.; Salogub, G.N.; Khuazheva, N.K.; Sherman, M.L.; Laadem, A.; Barger, R.; Knight, R.; Srinivasan, S.; Terpos, E. Sotatercept in patients with osteolytic lesions of multiple myeloma. Br. J. Haematol. 2014, 165, 814–823. [Google Scholar] [CrossRef]
- Raftopoulos, H.; Laadem, A.; Hesketh, P.J.; Goldschmidt, J.; Gabrail, N.; Osborne, C.; Ali, M.; Sherman, M.L.; Wang, D.; Glaspy, J.A.; et al. Sotatercept (ACE-011) for the treatment of chemotherapy-induced anemia in patients with metastatic breast cancer or advanced or metastatic solid tumors treated with platinum-based chemotherapeutic regimens: Results from two phase 2 studies. Support. Care Cancer 2016, 24, 1517–1525. [Google Scholar] [CrossRef]
- Piga, A.; Perrotta, S.; Gamberini, M.R.; Voskaridou, E.; Melpignano, A.; Filosa, A.; Caruso, V.; Pietrangelo, A.; Longo, F.; Tartaglione, I.; et al. Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with b-thalassemia. Blood 2019, 133, 1279–1289. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Viprakasit, V.; Taher, A.T.; Georgiev, P.; Kuo, K.H.M.; Coates, T.; Voskaridou, E.; Liew, H.-K.; Pazgal-Kobrowski, I.; Forni, G.L.; et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2020, 382, 1219–1231. [Google Scholar] [CrossRef] [PubMed]

| Name | Structure | Target | Indication | Phase Status [Ref] | Type | Patients n | Main Results |
|---|---|---|---|---|---|---|---|
| Sotatercept(ACE-011 ACVR2A-Fc) | Extracellular domain ActRIIA + human IgG1 Fc domain | MSTN GDF11 ActivinsBMPs | Anemia | Phase 1 [125] Phase 2 [126] Phase 2 [124] Phase 2 [124] | RCT Open-label RCT Open-label | 31 74 43 50 | Dose-dependent increase in Hb levels, hematocrit, and RBC count Increased Hb levels in ESA-refractory MDS Target Hb levels achieved in higher proportion of treated patients than placebo group, with dose-dependent results |
| Vascular calcification and bone mineral density disorders | Phase 1 [125] Phase 2 [124] | RCT RCT | 31 43 | Increased bone mineral density and biomarkers of bone formation Abdominal aortic vascular calcification slowed in a dose-related manner, less consistent data on BMD improvement | |||
| Osteoporosis | Phase 2 [127] | RCT | 48 | Increased bone-specific ALP, decreased CTX | |||
| Β-thalassemia | Phase 2 [123] | Open-label | 46 | Increased Hb levels, reduction of transfusion burden | |||
| PAH | Phase 2 [128] | RCT | 106 | Reduction of pulmonary vascular resistance, improvement of 6-min walking test distance, lower pro-BNP levels | |||
| Multiple myeloma | Phase 2 [129] | RCT | 30 | Anabolic improvement in BMD and bone formation | |||
| Anemia CT-induced | Phase 2 [130] | RCT | 55 | Terminated early for slow recruitment | |||
| Luspatercept(ACE-536 ACVR2B-Fc) | Extracellular domain ActRIIB + human IgG1 Fc domain | MSTN GDF11 Activins BMPs | Anemia, MDS | Phase 2 [121] Phase 3 [122] | Open-label RCT | 58 229 | Higher Hb levels, lower transfusion burden Trasfusion independence in 38% of the patients |
| Β-thalassemia | Phase 2 [131] Phase 3 [132] | Open-label RCT | 64 336 | Reduction >20% in transfusion burden in 81% of cases Reduction >33% in transfusion burden in 70.5% of the patients, >50% in 40.2% of the patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, P.; Verzola, D.; Picciotto, D.; Cipriani, L.; Viazzi, F.; Garibotto, G. Myostatin/Activin-A Signaling in the Vessel Wall and Vascular Calcification. Cells 2021, 10, 2070. https://doi.org/10.3390/cells10082070
Esposito P, Verzola D, Picciotto D, Cipriani L, Viazzi F, Garibotto G. Myostatin/Activin-A Signaling in the Vessel Wall and Vascular Calcification. Cells. 2021; 10(8):2070. https://doi.org/10.3390/cells10082070
Chicago/Turabian StyleEsposito, Pasquale, Daniela Verzola, Daniela Picciotto, Leda Cipriani, Francesca Viazzi, and Giacomo Garibotto. 2021. "Myostatin/Activin-A Signaling in the Vessel Wall and Vascular Calcification" Cells 10, no. 8: 2070. https://doi.org/10.3390/cells10082070
APA StyleEsposito, P., Verzola, D., Picciotto, D., Cipriani, L., Viazzi, F., & Garibotto, G. (2021). Myostatin/Activin-A Signaling in the Vessel Wall and Vascular Calcification. Cells, 10(8), 2070. https://doi.org/10.3390/cells10082070







