Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Primary Human Corneal Fibroblasts
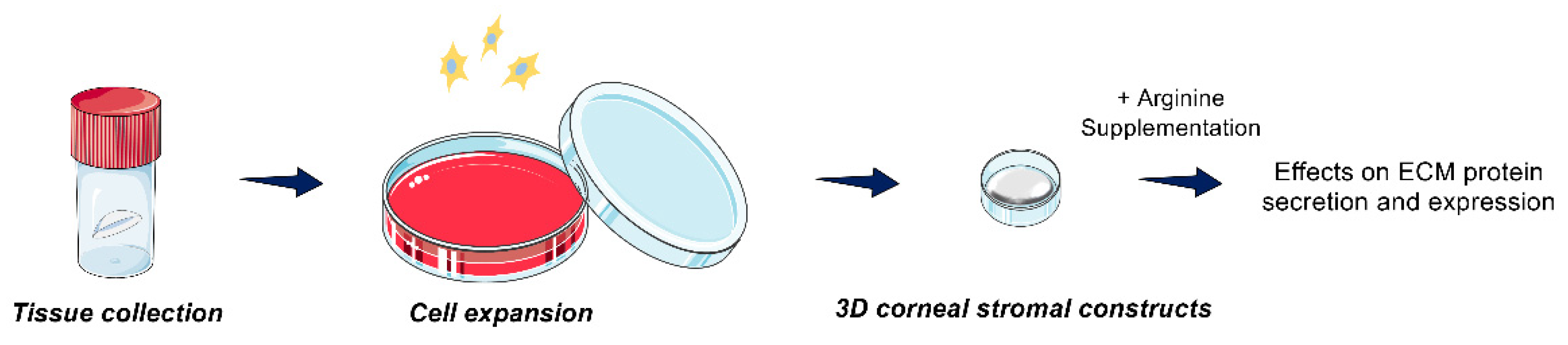
2.2. 3D In Vitro Model
2.3. Metabolite Extraction
2.4. Targeted Mass Spectrometry
2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot
2.7. Statistical Analysis
3. Results
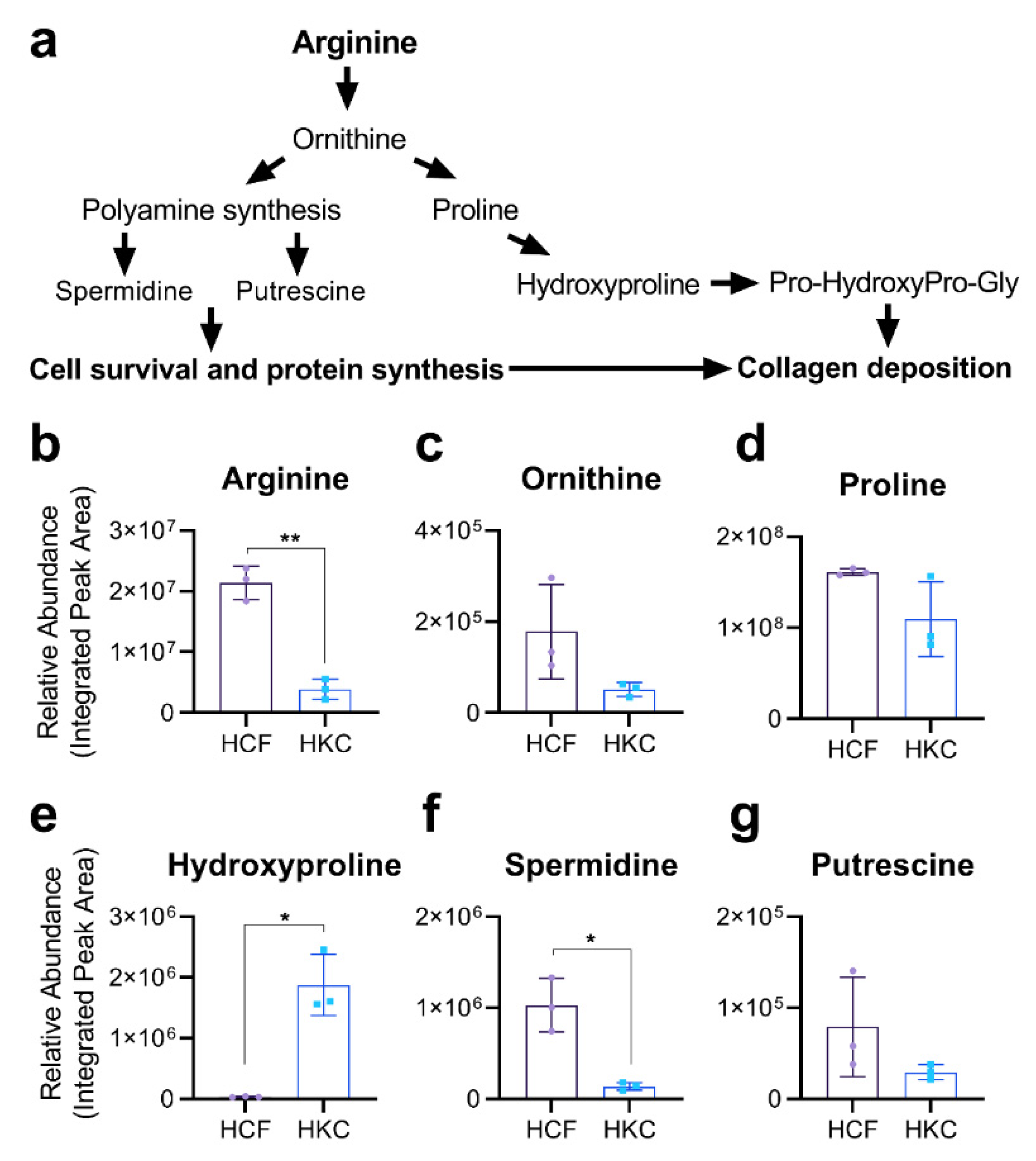
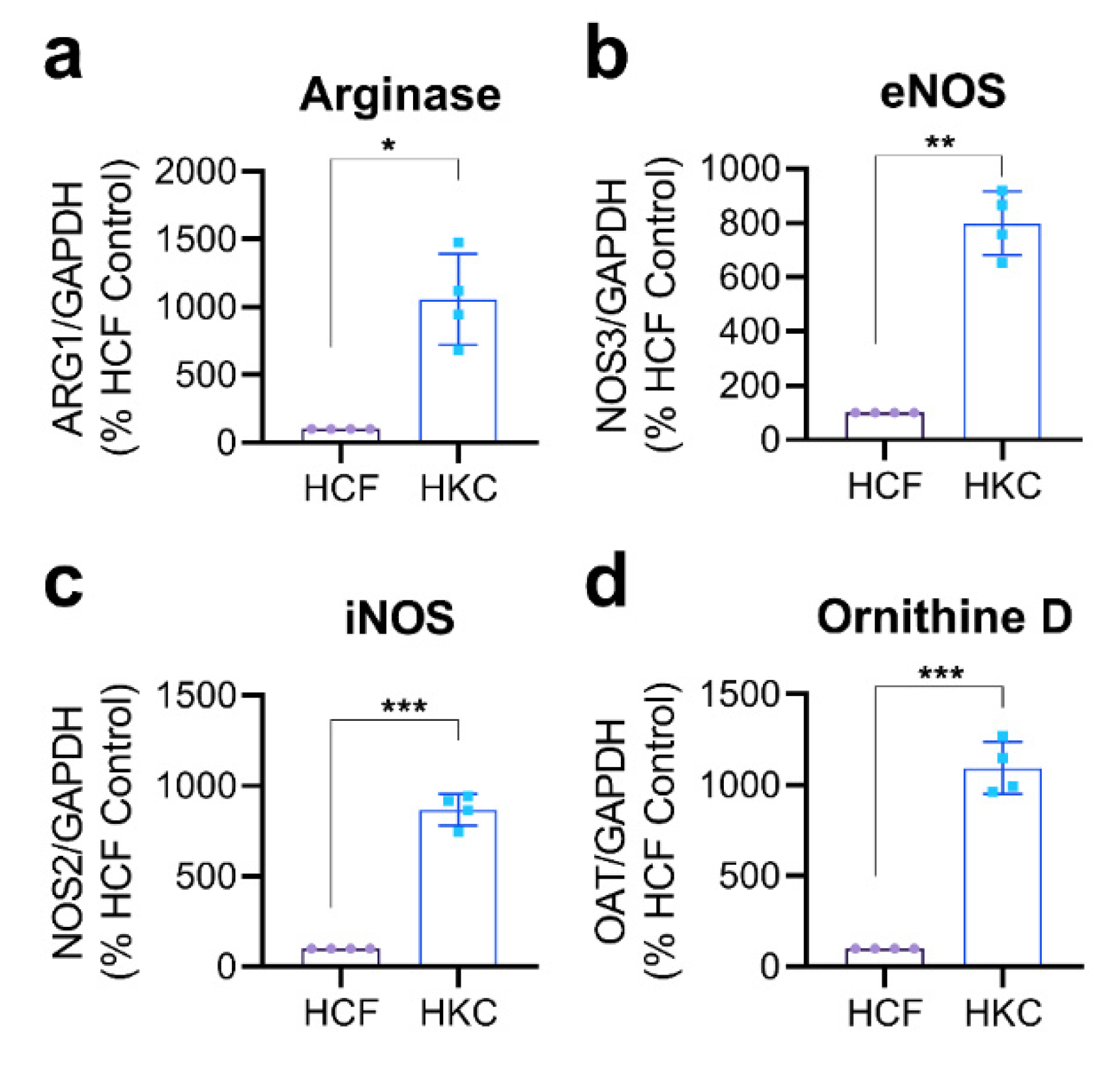
3.1. Arginine Metabolism in Corneal Fibroblasts
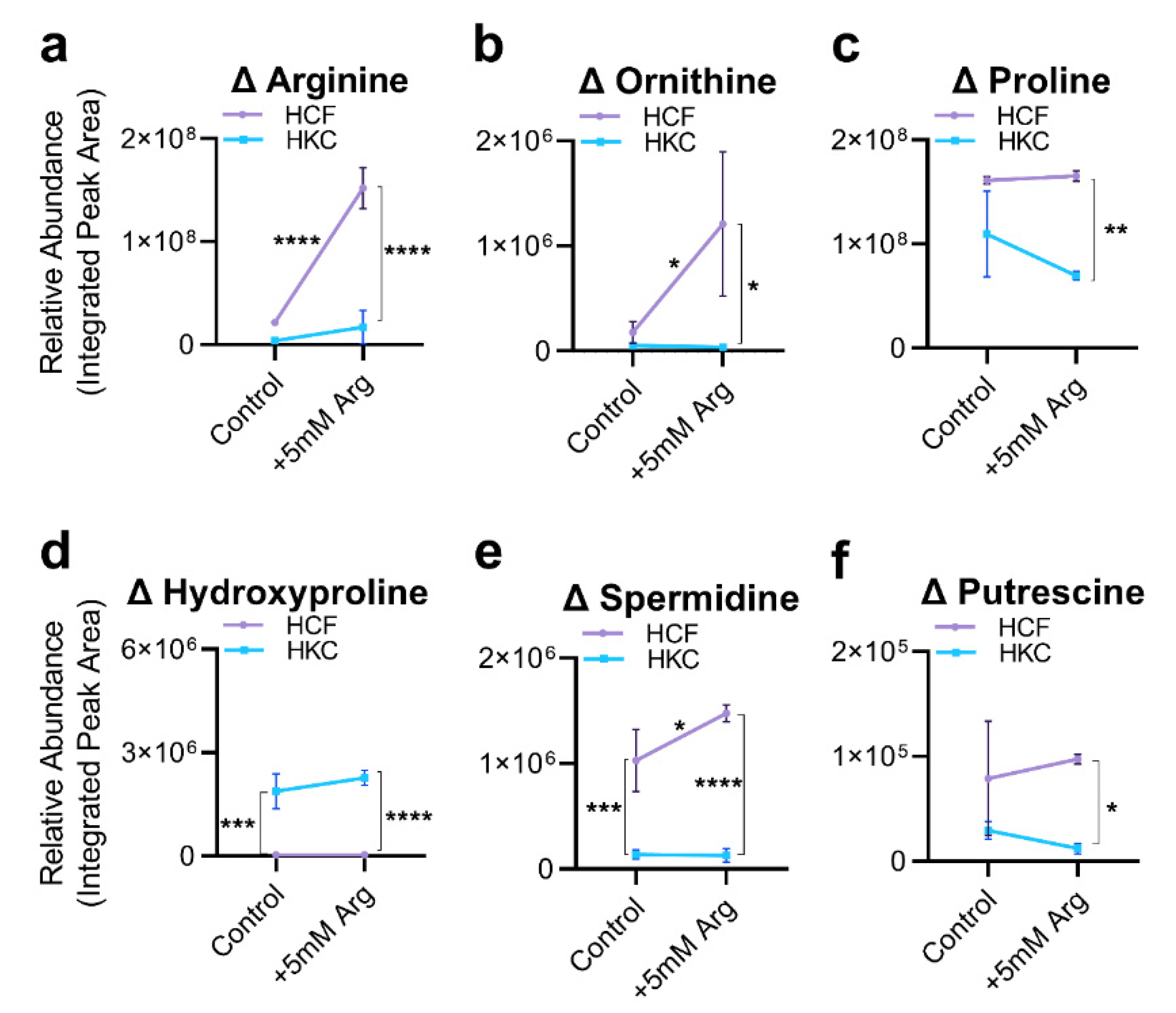
3.2. Arginine Uptake and Arginine-Related Metabolites
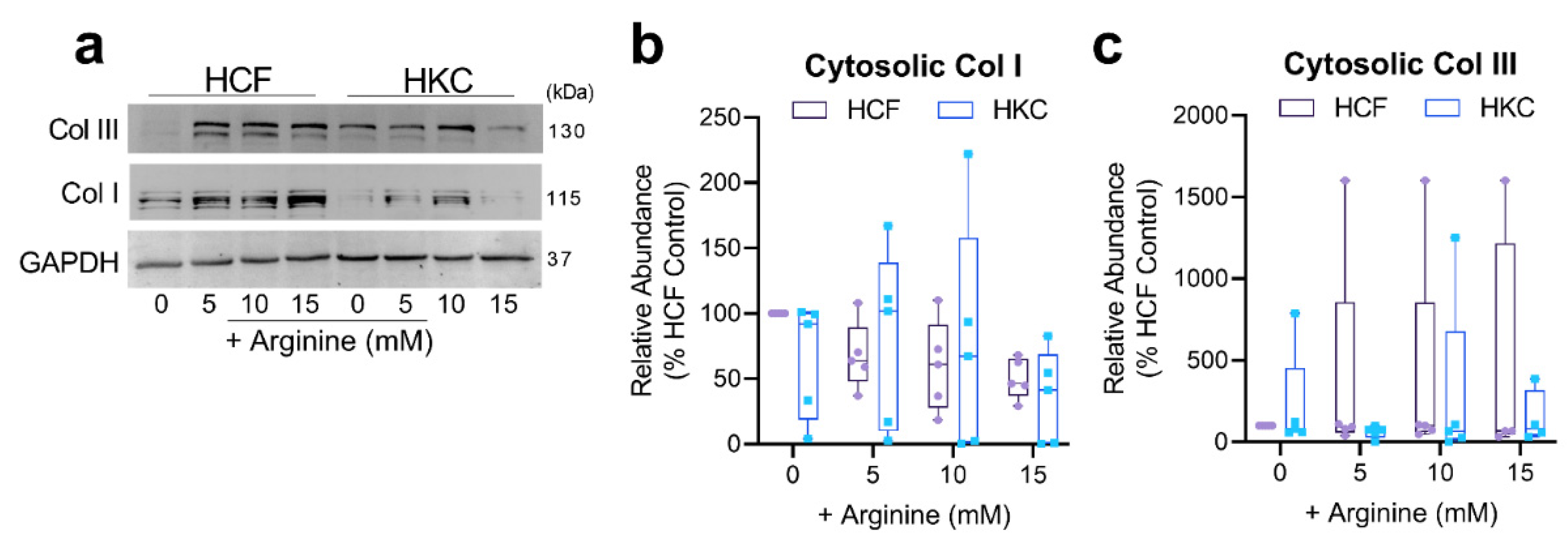
3.3. Arginine Supplementation and ECM Secretion and Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Yamada, E.; Hasegawa, T.; Yamada, R.-H. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate: I. Pyrroline-5-carboxylate synthase. Arch. Biochem. Biophys. 1991, 291, 1–8. [Google Scholar] [CrossRef]
- Flynn, N.; Meininger, C.; Haynes, T.; Wu, G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427–438. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929. [Google Scholar] [CrossRef] [PubMed]
- Bessman, S.P.; Carpenter, C.L. The creatine-creatine phosphate energy shuttle. Annu. Rev. Biochem. 1985, 54, 831–862. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. Enzym. Relat. Areas. Mol. Biol. 1979, 50, 177–242. [Google Scholar]
- Wang, S.; Tsun, Z.-Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Khan, A.; Coe, D.; Zaher, S.; Chai, J.-G.; Kropf, P.; Müller, I.; Larkin, D.F.P.; George, A.J.T. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur. J. Immunol. 2011, 41, 2997–3005. [Google Scholar] [CrossRef]
- Barbul, A.; Lazarou, S.; Efron, D.; Wasserkrug, H.; Efron, G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 1990, 108, 331–336, discussion 336–337. [Google Scholar]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine supplementation and wound healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chinkes, D.L.; Wolfe, R.R. The anabolic effect of arginine on proteins in skin wound and muscle is independent of nitric oxide production. Clin. Nutr. 2008, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Trocki, O.; Wang, S.-l.; Gonce, S.J.; Joffe, S.N.; Alexander, J.W. Metabolic and immune effects of dietary arginine supplementation after burn. Arch. Surg. 1987, 122, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Gottschlich, M. Nutritional immunomodulation in burn patients. Crit. Care Med. 1990, 18, S149. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.J.; Zamora, S.A.; McMillan, D.D.; Fick, G.H.; Butzner, J.D.; Parsons, H.G.; Scott, R.B. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J. Pediatrics 2002, 140, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-M.; Sheridan, R.L.; Burke, J.F.; Chapman, T.E.; Tompkins, R.G.; Young, V.R. Kinetics of plasma arginine and leucine in pediatric burn patients. Am. J. Clin. Nutr. 1996, 64, 60–66. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.-M.; Ryan, C.M.; Castillo, L.; Lu, X.-M.; Beaumier, L.; Tompkins, R.G.; Young, V.R. Arginine and ornithine kinetics in severely burned patients: Increased rate of arginine disposal. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E509–E517. [Google Scholar] [CrossRef]
- Herndon, D.N.; Tompkins, R.G. Support of the metabolic response to burn injury. Lancet 2004, 363, 1895–1902. [Google Scholar] [CrossRef]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Linsenmayer, T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol. 1988, 106, 999–1008. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Kenney, M.; Nesburn, A.; Burgeson, R.; Butkowski, R.; Ljubimov, A. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea 1997, 16, 345–351. [Google Scholar] [CrossRef]
- Karamichos, D.; Zareian, R.; Guo, X.; Hutcheon, A.E.; Ruberti, J.W.; Zieske, J.D. Novel in vitro model for keratoconus disease. J. Funct. Biomater. 2012, 3, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Hutcheon, A.E.; Rich, C.B.; Trinkaus-Randall, V.; Asara, J.M.; Zieske, J.D. In vitro model suggests oxidative stress involved in keratoconus disease. Sci. Rep. 2014, 4, 4608. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Hutcheon, A.E.K.; Zieske, J.D. Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, e228–e238. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Guo, X.; Hutcheon, A.E.K.; Karamichos, D.; Ciolino, J.B. Methods for Investigating Corneal Cell Interactions and Extracellular Vesicles In Vitro. Curr. Protoc. Cell. Biol. 2020, 89, e114. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf, S.B.; Ricoult, S.J.H.; Yuan, M.; Xu, Y.; Peake, D.A.; Manning, B.D.; Asara, J.M. A relative quantitative positive/negative ion switching method for untargeted lipidomics via high resolution LC-MS/MS from any biological source. Metabolomics 2017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef]
- Locasale, J.W.; Melman, T.; Song, S.; Yang, X.; Swanson, K.D.; Cantley, L.C.; Wong, E.T.; Asara, J.M. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteom. MCP 2012, 11, M111.014688. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Connon, C.J. Approaches to corneal tissue engineering: Top-down or bottom-up? Procedia Eng. 2015, 110, 15–20. [Google Scholar] [CrossRef]
- McKay, T.B.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Karamichos, D. Modeling the cornea in 3-dimensions: Current and future perspectives. Exp. Eye. Res. 2020, 197, 108127. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Arginase inhibitors: A rational approach over one century. Med. Res. Rev. 2017, 37, 475–513. [Google Scholar] [CrossRef]
- Barbul, A. Proline precursors to sustain mammalian collagen synthesis. J. Nutr. 2008, 138, 2021S–2024S. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino. Acids. 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino. Acids. 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. 1, 4-Diaminobutane (putrescine), spermidine, and spermine. Annu. Rev. Biochem. 1976, 45, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, P.S.; Rao, P.N.; Nishioka, K. Putrescine biosynthesis in mammalial cells: Essential for DNA synthesis but not for mitosis. Biochem. Biophys. Res. Commun. 1977, 74, 1125–1133. [Google Scholar] [CrossRef]
- Russell, D.; Snyder, S.H. Amine synthesis in rapidly growing tissues: Ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. USA 1968, 60, 1420–1427. [Google Scholar] [CrossRef]
- Sastre, M.; Regunathan, S.; Galea, E.; Reis, D.J. Agmatinase activity in rat brain: A metabolic pathway for the degradation of agmatine. J. Neurochem. 1996, 67, 1761–1765. [Google Scholar] [CrossRef]
- Pegg, A.E.; Williams-Ashman, H. Biosynthesis of putrescine in the prostate gland of the rat. Biochem. J. 1968, 108, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.J.; Talbot, J.; Ward, S. pH-sensitive control of arginase by Mn (II) ions at submicromolar concentrations. Arch. Biochem. Biophys. 1991, 286, 217–221. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Campo, M.L.; Soler, G. Physico-chemical properties of hepatocyte plasma-membrane-bound arginase. Arch. Int. Physiol. Biochim. Biophys. 1991, 99, 413–417. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, T.B.; Priyadarsini, S.; Rowsey, T.; Karamichos, D. Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells 2021, 10, 2076. https://doi.org/10.3390/cells10082076
McKay TB, Priyadarsini S, Rowsey T, Karamichos D. Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells. 2021; 10(8):2076. https://doi.org/10.3390/cells10082076
Chicago/Turabian StyleMcKay, Tina B., Shrestha Priyadarsini, Tyler Rowsey, and Dimitrios Karamichos. 2021. "Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus" Cells 10, no. 8: 2076. https://doi.org/10.3390/cells10082076
APA StyleMcKay, T. B., Priyadarsini, S., Rowsey, T., & Karamichos, D. (2021). Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells, 10(8), 2076. https://doi.org/10.3390/cells10082076





