Establishment of Pancreatobiliary Cancer Zebrafish Avatars for Chemotherapy Screening
Abstract
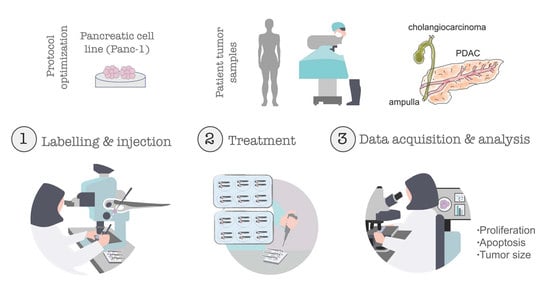
:1. Introduction
2. Materials and Methods
2.1. Animal Care and Handling
2.2. Zebrafish Lines
2.3. Patient Samples
2.4. Human Pancreatic Cancer Cell Line
2.5. Cell Culture
2.6. Cell Staining
2.7. Zebrafish Xenograft Microinjection
2.8. Xenografts Drug Administration
2.9. Xenograft Whole-Mount Immunofluorescence
2.10. Xenograft Imaging and Quantification
2.11. Histopathology
2.12. Statistical Analysis
3. Results
3.1. Characterization and Histomorphological Features of Panc-1 Zebrafish Xenografts
3.2. Pancreatic Cancer Zebrafish Xenografts Show Sensitivity to Standard Chemotherapy
3.3. Zebrafish Avatars Can Be Generated from Human Pancreatobiliary Tumors of Different Histotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer. 2002, 2, 897–909. [Google Scholar] [CrossRef]
- Oberstein, P.E.; Olive, K.P. Pancreatic cancer: Why is it so hard to treat? Adv. Gastroenterol. 2013, 6, 321–337. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.; Jacob, R.; Manne, U.; Paluri, R. Advances in pancreatic cancer biomarkers. Oncol. Rev. 2019, 13, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer. 2018, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crossFtalk in pancreatic cancer and tissue fibrosis. Mol. Cancer. 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Vinaixa, J.; Navarro, P. Immune evasion in pancreatic cancer: From mechanisms to therapy. Cancers 2018, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, V.; Sperduti, I.; Milella, M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 365, 768–769. [Google Scholar]
- Ahn, D.H.; Bekaii-Sabb, T. Ampullary Cancer: An Overview. Am. Soc. Clin. Oncol. Educ. B. 2014, 34, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different Clinicopathologic Findings in Two Histologic Types of Carcinoma of Papilla of Vater. Jpn. J. Cancer Res. 1994, 85, 161–166. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Estrada, M.F.; Mendes, R.V.; Fior, R. Zebrafish Avatars towards Personalized Medicine—A Comparative Review between.Avatar Models. Cells 2020, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Sun, L.; Liu, M.; Mao, Y. Patient-derived organoids: A promising model for personalized cancer treatment. Gastroenterol. Rep. 2018, 6, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Driehuis, E.; Hoeck, A.v.; Moore, K.; Kolders, S.; Francies, H.E.; Gulersonmez, M.C.; Stigter, E.C.A.; Burgering, B.; Geurts, V.; Gracanin, A.; et al. Pancreatic Cancer Organoids Recapitulate Disease and Allow Personalized Drug Screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590. [Google Scholar] [CrossRef]
- Fior, R.; Pόvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive pro filing of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Ferreira, S.; Póvoa, V.; Cardoso, M.J.; Vieira, S.; Stroom, J.; Fidalgo, P.; Rio-Tinto, R.; Parés, O.; Greco, C.; et al. Developments in zebrafish avatars as radiotherapy sensitivity reporters—Towards personalized medicine. EBioMedicine 2020, 51, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rebelo de Almeida, C.; Mendes, R.V.; Pezzarossa, A.; Gago, G.; Carvalho, C.; Alves, A.; Brito, M.J.; Cardoso, M.J.; Ribeiro, J.; Cardoso, F.; et al. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Commun. Biol. 2020, 3, 299. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, M.; Póvoa, V.; Fior, R. Generation of Zebrafish Larval Xenografts and Tumor Behavior Analysis. J. Vis. Exp. 2021, 172, e62373. [Google Scholar]
- Varanda, A.B.; Martins-Logrado, A.; Ferreira, M.G.; Fior, R. Zebrafish Xenografts Unveil Sensitivity to Olaparib beyond BRCA Status. Cancers 2020, 12, 1769. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell. 2008, 2, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Casale, F.; Canaparo, R.; Serpe, L.; Muntoni, E.; Pepa, C.D.; Costa, M.; Mairone, L.; Zara, G.P.; Fornari, G.; Eandi, M. Plasma concentrations of 5-fluorouracil and its metabolites in colon cancer patients. Pharmacol. Res. 2004, 50, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. 2000, 6, 1205–1218. [Google Scholar] [PubMed]
- Limacher, J.M.; Duclos, B.; Wihlm, J.; Leveque, D.; Dufour, P.; Eichler, F.; Keiling, R.; Bergerat, J.P. 945 Phase I study of high dose 5-fluorouracil and folinic acid in weekly continuous infusion. Euro. J. Cancer. 1995, 31, S197. [Google Scholar] [CrossRef]
- Chabot, G.G. Clinical pharmacokinetics of irinotecan. Clin. Pharmacokinet. 1997, 33, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.T.; Vermorken, J.B.; Rosing, H.; ten Bokkel Huinink, W.W.; Mandjes, I.; Pinedo, H.M.; Beijnen, J.H. Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: A European Cancer Centre (ECC) trial. Ann. Oncol. 1995, 6, 699–704. [Google Scholar]
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother Pharmacol. 2016, 78, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Möser, S.; Kalthoff, H.; Török, V.; Löhr, M.; Klöppel, G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: Towards the establishment of an in vitro research platform. Virchows Arch. 2003, 442, 444–452. [Google Scholar] [CrossRef]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and pancreatic cancer: Focus on metabolism, cytokines, and immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.E.; Humphreys, M.J.; Campbell, F.; Neoptolemos, J.P.; Boyd, M.T. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: Increased expression of matrix metalloproteinase-7 predicts poor survival. Clin. Cancer Res. 2004, 10, 2832–2845. [Google Scholar] [CrossRef] [Green Version]
- Bloomston, M.; Zervos, E.E. Rosemurgy, A.S. Matrix metalloproteinases and their role in pancreatic cancer: A review of preclinical studies and clinical trials. An. Surg. Oncol. 2002, 9, 668–674. [Google Scholar] [CrossRef]
- Awasthi, N.; Zhang, C.; Schwarz, A.M.; Hinz, S.; Wang, C.; Williams, N.S.; Schwarz, M.A.; Schwarz, R.E. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis 2013, 34, 2361–2369. [Google Scholar] [CrossRef] [Green Version]
- Khatri, I.; Bhasin, M.K. A Transcriptomics-Based Meta-Analysis Combined with Machine Learning Identifies a Secretory Biomarker Panel for Diagnosis of Pancreatic Adenocarcinoma. Front. Genet. 2020, 11, 572284. [Google Scholar] [CrossRef] [PubMed]
- Yong-Xian, G.; Xiao-Huan, L.; Fan, Z.; Guo-Fang, T. Gemcitabine inhibits proliferation and induces apoptosis in human pancreatic cancer PANC-1 cells. J. Can. Res. 2016, 12, 1–4. [Google Scholar]
- Biswas, R.; Bugde, P.; He, J.; Merien, F.; Lu, J.; Liu, D.X.; Myint, K.; Liu, J.; McKeage, M.; Li, Y. Transport-Mediated Oxaliplatin Resistance Associated with Endogenous Overexpression of MRP2 in Caco-2 and PANC-1 Cells. Cancers (Basel). 2019, 11, 1330. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, C.; Buttenschoen, K.; Straeter, J.; Henne-Bruns, D.; Kornmann, M. Pre-clinical evaluation of the activity of irinotecan as a basis for regional chemotherapy. Anticancer Res. 2005, 25, 795–804. [Google Scholar]
- Morgan, M.A.; Meirovitz, A.; Davis, M.A.; Kollar, L.E.; Hassan, M.C.; Lawrence, T.S. Radiotherapy Combined with Gemcitabine and Oxaliplatin in Pancreatic Cancer Cells. Transl. Oncol. 2008, 1, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Halloran, C.M.; Ghaneh, P.; Shore, S.; Greenhalf, W.; Zumstein, L.; Wilson, D.; Neoptolemos, J.P.; Costello, E. 5-Fluorouracil or gemcitabine combined with adenoviral-mediated reintroduction of p16INK4A greatly enhanced cytotoxicity in Panc-1 pancreatic adenocarcinoma cells. J. Gene Med. 2004, 6, 514–525. [Google Scholar] [CrossRef]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.K.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2019, 128, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, F.U.; Marques, I.J.; Woltering, J.M.; Vlecken, D.H.; Aghdassi, A.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 2009, 137, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wei, H.; Hu, J.; Sun, S.; Long, J.; Wang, X. U0126 inhibits pancreatic cancer progression via the KRAS signaling pathway in a zebrafish xenotransplantation model. Oncol. Rep. 2015, 34, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Franco, G.; Usai, A.; Funel, N.; Palmeri, M.; Montesanti, I.E.R.; Bianchini, M.; Gianardi, D.; Furbetta, N.; Guadagni, S.; Vasile, E.; et al. Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine. World J. Gastroenterol. 2020, 26, 2792–2809. [Google Scholar] [CrossRef]




| Patient | Staging | Histologic Subtype | Tumor Size | R0 | Patient CT Regimen |
|---|---|---|---|---|---|
| #1 | T2N0 | pancreas ductal adenocarcinoma with areas of papillary cystic G2 | 38 mm | yes | 0 |
| #2 | T3N0 | pancreas ductal adenocarcinoma G2 | 56 mm | yes | FOLFIRINOX |
| #3 | T2N0 | pancreas ductal adenocarcinoma G1 (associated to IPMN) | 23 mm | no | FOLFIRINOX |
| #4 | T3N1 | ampulla adenocarcinoma, intestinal type (mucinous 40%) G2 | 45 mm | yes | FOLFOX |
| #5 | T3N0 | ampulla adenocarcinoma, biliopancreatic type G2 | 15 mm | yes | Gemcitabine |
| #6 | T3N1 | distal cholangiocarcinoma G2 | 17 mm | yes | 0 |
| #7 | T2N1 | pancreas ductal adenocarcinoma G2 | 34 mm | yes | 0 |
| #8 | T3N2M1 | pancreas ductal adenocarcinoma G3 | 48 mm | yes | FOLFIRINOX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares Barroso, M.; Costa, B.; Rebelo de Almeida, C.; Castillo Martin, M.; Couto, N.; Carvalho, T.; Fior, R. Establishment of Pancreatobiliary Cancer Zebrafish Avatars for Chemotherapy Screening. Cells 2021, 10, 2077. https://doi.org/10.3390/cells10082077
Tavares Barroso M, Costa B, Rebelo de Almeida C, Castillo Martin M, Couto N, Carvalho T, Fior R. Establishment of Pancreatobiliary Cancer Zebrafish Avatars for Chemotherapy Screening. Cells. 2021; 10(8):2077. https://doi.org/10.3390/cells10082077
Chicago/Turabian StyleTavares Barroso, Mariana, Bruna Costa, Cátia Rebelo de Almeida, Mireia Castillo Martin, Nuno Couto, Tânia Carvalho, and Rita Fior. 2021. "Establishment of Pancreatobiliary Cancer Zebrafish Avatars for Chemotherapy Screening" Cells 10, no. 8: 2077. https://doi.org/10.3390/cells10082077