Hidradenitis Suppurativa: Where We Are and Where We Are Going
Abstract
:1. Introduction
2. Materials and Methods
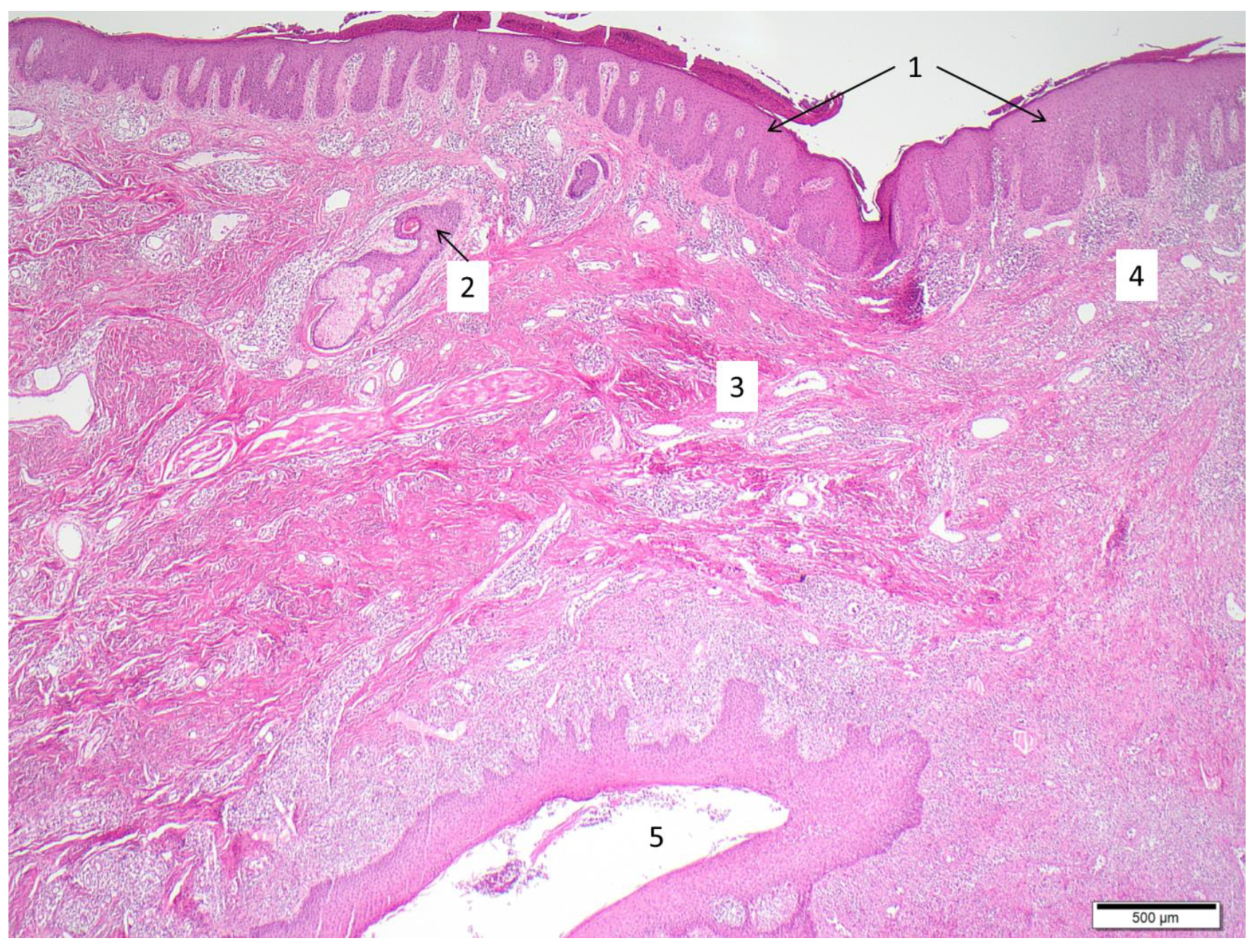
3. Diagnosis
3.1. Clinical Aspects
3.2. Severity Assessment
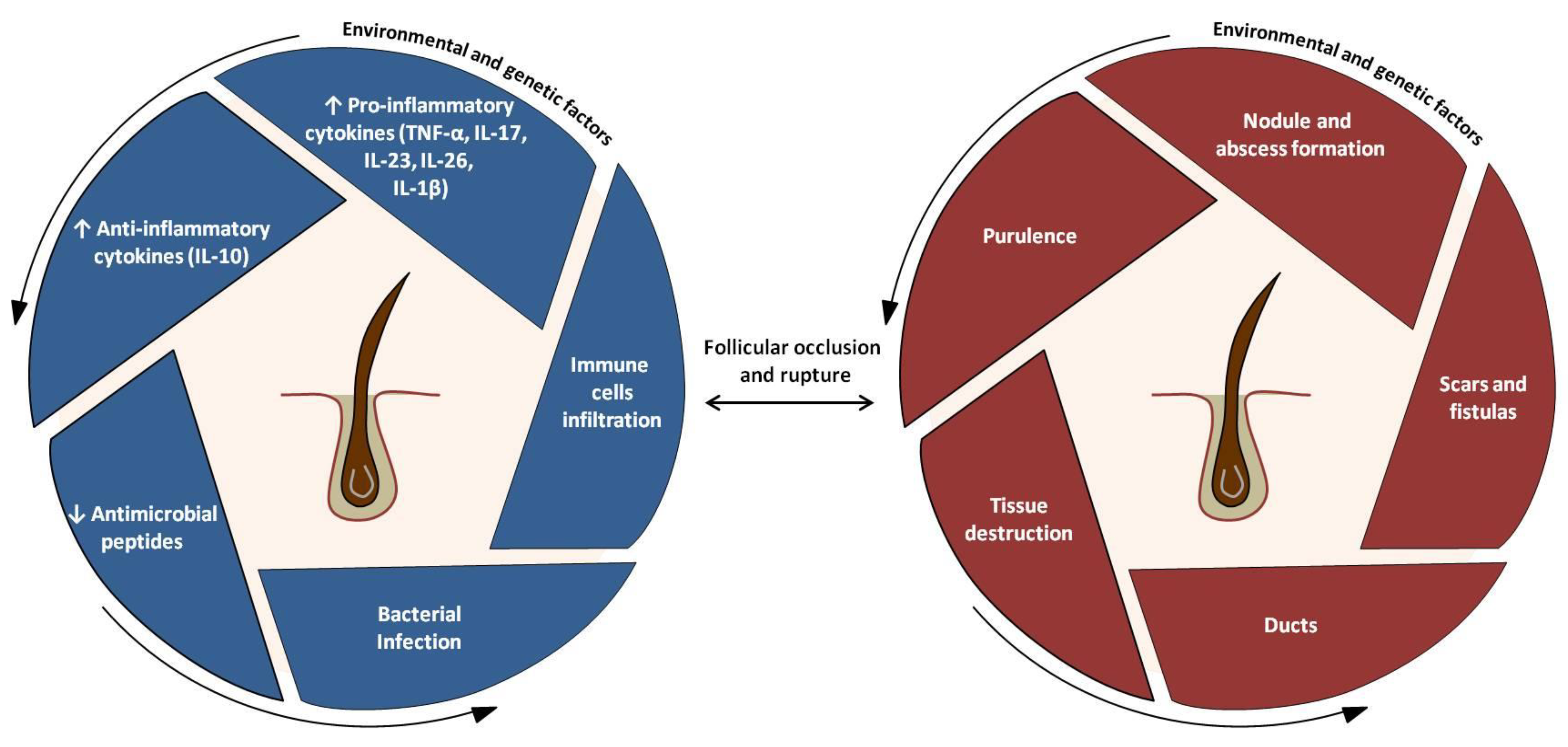
4. Etiology and Pathogenesis
4.1. Genetics
4.2. Lifestyle Factors
4.3. Pathogenic Events
4.4. Main Cytokine Networks in HS
5. Treatments
5.1. From Conventional Therapies to Molecular Treatments
| Hurley Staging (HS Severity) | Clinical Features | Recommended Treatments | Surgery |
|---|---|---|---|
| Stage I (mild) | Single or multiple abscesses without sinus tracts and scarring | Patient education: maintaining hygiene standards, cessation of smoking, losing weight * Topical clindamycin, antisepticsIntralesional corticosteroids | Incision and drainage Deroofing Laser |
| Stage II (moderate) | Recurrent abscesses with sinus tracts and scarring | Systemic antibiotics: tetracycline, clindamycin + rifampicin, rifampicin + moxifloxacin + metronidazole, ertapenem Immune modulators: prednisolone, retinoids, dapsone, cyclosporine | Local intervention Deroofing Laser |
| Stage III (severe) | Diffuse or multiple interconnected sinus tracts and abscesses across the entire area | Biologics: adalimumab, bimekizumab (phase III)/secukinumab (phase III), others (Table 2) | Extensive radical surgical excision |
5.2. Predictive Markers of Therapeutic Response
5.3. Future Treatments and Personalized Therapies
| Intervention/Compound | Target | Type | Phase | Number of Studies | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Avacopan | C5a receptor | systemic drug | 2 | 1 | NCT03852472 |
| Bimekizumab | IL-17A, IL-17F | systemic drug | 3 | 2 | NCT04242446, NCT04242498 |
| Iscalimab (CFZ533), LYS006 | CD40, LTA4H (respectively) | systemic drug | 2 | 1 | NCT03827798 |
| CSL324 | G-CSF receptor | systemic drug | 1 | 1 | NCT03972280 |
| Imsidolimab | IL-36 receptor | systemic drug | 2 | 1 | NCT04856930 |
| INCB05470 | JAK-1 | systemic drug | 2 | 1 | NCT04476043 |
| KT-474 | IRAK4 | systemic drug | 1 | 1 | NCT04772885 |
| LY3041658 | ELR + CXC chemokines | systemic drug | 2 | 1 | NCT04493502 |
| Metformin | unknown (anti-inflammatory) | systemic drug | 3 | 1 | NCT04649502 |
| PF-06650833, PF-06700841, PF-06826647 | IRAK4, TYK2+JAK1, TYK2 (respectively) | systemic drug | 2 | 1 | NCT04092452 |
| Risankizumab | IL-23p19 | systemic drug | 2 | 1 | NCT03926169 |
| Secukinumab | IL-17A | systemic drug | 3 | 3 | NCT04179175, NCT03713619, NCT03713632 |
| Spesolimab | IL-36 receptor | systemic drug | 2 | 2 | NCT04876391, NCT04762277 |
| Tofacitinib | pan-JAK | systemic drug | 2 | 1 | NCT04246372 |
| Upadacitinib | JAK-1 | systemic drug | 2 | 1 | NCT04430855 |
| Local therapy (wound dressings, creams, gels, sclerotherapy, AMP, triamcinolone) | local therapy | all | 9 | NCT04648631, NCT04194541, NCT04388163, NCT04354012, NCT04541550, NCT02805595, NCT04582669, NCT04756336, NCT04414514 | |
| Laser treatment | Laser | n.a. | 2 | NCT04508374, NCT03054155 | |
| Surgery (different procedures +/−ADA or NPWT+/−i) | Surgery | n.a. | 4 | NCT04526561, NCT04325607, NCT03784313, NCT03221621 | |
| Other (acupuncture, HS management, electronic reporting) | Other | n.a./4 | 3 | NCT04218422, NCT04200690, NCT04132388 |
6. Perspectives of Translational Studies in HS
6.1. In Vivo Animal Models
6.2. Ex Vivo Human Models
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Megna, M.; Timoshchuk, E.A.; Patruno, C.; Balato, N.; Fabbrocini, G.; Monfrecola, G. Hidradenitis suppurativa: From pathogenesis to diagnosis and treatment. Clin. Cosmet. Investig. Dermatol. 2017, 10, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinkel, C.; Thomsen, S.F. Hidradenitis Suppurativa: Causes, Features, and Current Treatments. J. Clin. Aesthet. Dermatol. 2018, 11, 17–23. [Google Scholar]
- Pink, A.; Anzengruber, F.; Navarini, A.A. Acne and hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 619–631. [Google Scholar] [CrossRef]
- Constantinou, C.A.; Fragoulis, G.E.; Nikiphorou, E. Hidradenitis suppurativa: Infection, autoimmunity, or both? Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19895488. [Google Scholar] [CrossRef] [Green Version]
- Schneider-Burrus, S.; Jost, A.; Peters, E.M.J.; Witte-Haendel, E.; Sterry, W.; Sabat, R. Association of Hidradenitis Suppurativa with Body Image. JAMA Dermatol. 2018, 154, 447–451. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Finlay, A.Y.; Tomas-Aragones, L.; Poot, F.; Sampogna, F.; Marron, S.E.; Zemskov, S.V.; Abeni, D.; Tzellos, T.; Szepietowski, J.C.; et al. Quality of Life in Hidradenitis Suppurativa: An Update. Int. J. Environ. Res. Public Health 2021, 18, 6131. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, M.A.; Milrad, S.F.; Kirby, J.R.S.; Lev-Tov, H. Psychosocial impact of hidradenitis suppurativa: A practical guide for clinicians. J. Dermatol. Treat. 2021, 14, 1–23. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Diaz-Calvillo, P.; Rodriguez-Pozo, J.A.; Cuenca-Barrales, C.; Martinez-Lopez, A.; Arias-Santiago, S.; Molina-Leyva, A. The Burden of Hidradenitis Suppurativa Signs and Symptoms in Quality of Life: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6709. [Google Scholar] [CrossRef] [PubMed]
- Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 985–998. [Google Scholar] [CrossRef]
- Lipsker, D.; Severac, F.; Freysz, M.; Sauleau, E.; Boer, J.; Emtestam, L.; Matusiak, Ł.; Prens, E.; Velter, C.; Lenormand, C.; et al. The ABC of Hidradenitis Suppurativa: A Validated Glossary on how to Name Lesions. Dermatology 2016, 232, 137–142. [Google Scholar] [CrossRef]
- Marasca, C.; Tranchini, P.; Marino, V.; Annunziata, M.C.; Napolitano, M.; Fattore, D.; Fabbrocini, G. The pharmacology of antibiotic therapy in hidradenitis suppurativa. Expert Rev. Clin. Pharmacol. 2020, 13, 521–530. [Google Scholar] [CrossRef]
- Revuz, J.E.; Jemec, G.B.E. Diagnosing Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Agut-Busquet, E.; Romaní, J.; Ribera, M.; Luelmo, J. Hidradenitis suppurativa of the nape: Description of an atypical phenotype related to severe early-onset disease in men. J. Dermatol. 2019, 46, 149–153. [Google Scholar] [CrossRef]
- Esmann, S.; Dufour, D.N.; Jemec, G.B. Questionnaire-based diagnosis of hidradenitis suppurativa: Specificity, sensitivity and positive predictive value of specific diagnostic questions. Br. J. Dermatol. 2010, 163, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Pasquinucci, S.; Caracciolo, S.; Piccolo, D.; Cazzaniga, S.; Fantini, F.; Binello, L.; Pintori, G.; Naldi, L. The Hidradenitis suppurativa patient journey in Italy: Current status, unmet needs and opportunities. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Manfredini, M.; Massoli, L.; Carillo, C.; Barozzi, A.; Amendolagine, G.; Ruina, G.; Musmeci, D.; Libanore, M.; Curtolo, A.; et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 930–936. [Google Scholar] [CrossRef]
- Canoui-Poitrine, F.; Le Thuaut, A.; Revuz, J.E.; Viallette, C.; Gabison, G.; Poli, F.; Pouget, F.; Wolkenstein, P.; Bastuji-Garin, S. Identification of three hidradenitis suppurativa phenotypes: Latent class analysis of a cross-sectional study. J. Investig. Dermatol. 2013, 133, 1506–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zee, H.H.; Jemec, G.B. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J. Am. Acad. Dermatol. 2015, 73, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; Jfri, A.; Koster, S.B.L.; Gomez-Palencia, P.; Solera, M.; Alfaro-Rubio, A.; Hueso, L.; Sanz-Motilva, V. Defining hidradenitis suppurativa phenotypes based on the elementary lesion pattern: Results of a prospective study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1309–1318. [Google Scholar] [CrossRef]
- Thorlacius, L. Severity staging of hidradenitis suppurativa: Is Hurley classification the answer? Br. J. Dermatol. 2019, 181, 243–244. [Google Scholar] [CrossRef]
- Sartorius, K.; Emtestam, L.; Jemec, G.B.E.; Lapins, J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br. J. Dermatol. 2009, 161, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, K.; Killasli, H.; Heilborn, J.; Jemec, G.B.E.; Lapins, J.; Emtestam, L. Interobserver variability of clinical scores in hidradenitis suppurativa is low: Hidradenitis suppurativa scoring variability. Br. J. Dermatol. 2010, 162, 1261–1268. [Google Scholar] [CrossRef]
- Kimball, A.B.; Sobell, J.M.; Zouboulis, C.C.; Gu, Y.; Williams, D.A.; Sundaram, M.; Teixeira, H.D.; Jemec, G.B. HiSCR (Hidradenitis Suppurativa Clinical Response): A novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Faleri, S.; Franceschini, C.; Caro, R.D.; Chimenti, S.; Bianchi, L. AISI: A New Disease Severity Assessment Tool for Hidradenitis Suppurativa. Wounds 2015, 27, 258–264. [Google Scholar] [PubMed]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Hessam, S.; Scholl, L.; Sand, M.; Schmitz, L.; Reitenbach, S.; Bechara, F.G. A Novel Severity Assessment Scoring System for Hidradenitis Suppurativa. JAMA Dermatol. 2018, 154, 330–335. [Google Scholar] [CrossRef]
- Wortsman, X.; Moreno, C.; Soto, R.; Arellano, J.; Pezo, C.; Wortsman, J. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol. Surg. 2013, 39, 1835–1842. [Google Scholar] [CrossRef]
- Marasca, C.; Marasca, D.; Megna, M.; Annunziata, M.C.; Fabbrocini, G. Ultrasound: An indispensable tool to evaluate the outcome of surgical approaches in patients affected by hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e413–e414. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Thorlacius, L.; Villumsen, B.; Ingram, J.; Garg, A.; Christensen, K.; Butt, M.; Esmann, S.; Tan, J.; Jemec, G. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: Development and validation of a measure for clinical trials. Br. J. Dermatol. 2019, 183, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Marrón, S.; Gómez-Barrera, M.; Aragonés, L.T.; Díaz, R.D.; Rull, E.V.; Álvarez, M.M.; Puig, L. Development and Preliminary Validation of the HSQoL-24 Tool to Assess Quality of Life in Patients with Hidradenitis Suppurativa. Actas Dermo-Sifiliogr. 2019, 110, 554–560. [Google Scholar] [CrossRef]
- Kimball, A.B.; Sundaram, M.; Banderas, B.; Foley, C.; Shields, A.L. Development and initial psychometric evaluation of patient-reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J. Dermatol. Treat. 2018, 29, 152–164. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Bettoli, V.; De Pità, O.; Dini, V.; Fabbrocini, G.; Monfrecola, G.; Musumeci, M.; Parodi, A.; Sampogna, F.; Pennella, A.; et al. HIDRAdisk: An innovative visual tool to assess the burden of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2018, 33, e24–e26. [Google Scholar] [CrossRef] [Green Version]
- Fabbrocini, G.; Marasca, C.; Megna, M.; Peris, K.; HS Quality of Life Study Group. Age and gender influence on HIDRAdisk outcomes in adalimumab-treated hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 25–27. [Google Scholar] [CrossRef]
- Ingram, J.R.; Abbott, R.; Ghazavi, M.; Alexandroff, A.B.; McPhee, M.; Burton, T.; Clarke, T. The Hidradenitis Suppurativa Priority Setting Partnership. Br. J. Dermatol. 2014, 171, 1422–1427. [Google Scholar] [CrossRef]
- Knaysi, G.A., Jr.; Cosman, B.; Crikelair, G.F. Hidradenitis suppurativa. JAMA 1968, 203, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, J.S.; Guilbert, P.R.; Fitzsimmons, E.M. Evidence of genetic factors in hidradenitis suppurativa. Br. J. Dermatol. 1985, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, J.S.; Fitzsimmons, E.M.; Gilbert, G. Familial hidradenitis suppurativa: Evidence in favour of single gene transmission. J. Med. Genet. 1984, 21, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Der Werth, J.M.; Williams, H.C.; Raeburn, J.A. The clinical genetics of hidradenitis suppurativa revisited. Br. J. Dermatol. 2000, 142, 947–953. [Google Scholar] [CrossRef]
- Gao, M.; Wang, P.G.; Cui, Y.; Yang, S.; Zhang, Y.H.; Lin, D.; Zhang, K.Y.; Liang, Y.H.; Sun, L.D.; Yan, K.L.; et al. Inversa acne (hidradenitis suppurativa): A case report and identification of the locus at chromosome 1p21.1–1q25.3. J. Investig. Dermatol. 2006, 126, 1302–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pink, A.E.; Simpson, M.A.; Desai, N.; Dafou, D.; Hills, A.; Mortimer, P.; Smith, C.H.; Trembath, R.C.; Barker, J.N.W. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J. Investig. Dermatol. 2012, 132, 2459–2461. [Google Scholar] [CrossRef] [Green Version]
- Ingram, J.R.; Wood, M.; John, B.; Butler, R.; Anstey, A.V. Absence of pathogenic γ-secretase mutations in a South Wales cohort of familial and sporadic hidradenitis suppurativa (acne inversa). Br. J. Dermatol. 2013, 168, 874–876. [Google Scholar] [CrossRef]
- Nomura, Y.; Nomura, T.; Suzuki, S.; Takeda, M.; Mizuno, O.; Ohguchi, Y.; Abe, R.; Murata, Y.; Shimizu, H. A novel NCSTN mutation alone may be insufficient for the development of familial hidradenitis suppurativa. J. Dermatol. Sci. 2014, 74, 180–182. [Google Scholar] [CrossRef] [PubMed]
- van Straalen, K.R.; Prens, E.P.; Willemsen, G.; Boomsma, D.I.; van der Zee, H.H. Contribution of Genetics to the Susceptibility to Hidradenitis Suppurativa in a Large, Cross-sectional Dutch Twin Cohort. JAMA Dermatol. 2020, 156, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Freud, T.; Harman-Boehm, I.; Polishchuk, I.; Cohen, A.D. Hidradenitis suppurativa and metabolic syndrome: A comparative cross-sectional study of 3207 patients. Br. J. Dermatol. 2015, 173, 464–470. [Google Scholar] [CrossRef]
- Bettoli, V.; Naldi, L.; Cazzaniga, S.; Zauli, S.; Atzori, L.; Borghi, A.; Capezzera, R.; Caproni, M.; Cardinali, C.; De Vita, V.; et al. Overweight, diabetes and disease duration influence clinical severity in hidradenitis suppurativa-acne inversa: Evidence from the national Italian registry. Br. J. Dermatol. 2016, 174, 195–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malara, A.; Hughes, R.; Jennings, L.; Sweeney, C.M.; Lynch, M.; Awdeh, F.; Timoney, I.; Tobin, A.M.; Lynam-Loane, K.; Tobin, L.; et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.J.; Mekkes, J.R.; Zouboulis, C.C.; Scheinfeld, N.; Kimball, A.; Sundaram, M.; Gu, Y.; Okun, M.M.; Kerdel, F. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br. J. Dermatol. 2014, 171, 1561–1565. [Google Scholar] [CrossRef]
- Garg, A.; Birabaharan, M.; Strunk, A. Prevalence of type 2 diabetes mellitus among patients with hidradenitis suppurativa in the United States. J. Am. Acad. Dermatol. 2018, 79, 71–76. [Google Scholar] [CrossRef]
- Phan, K.; Charlton, O.; Smith, S.D. Hidradenitis suppurativa and diabetes mellitus: Updated systematic review and adjusted meta-analysis. Clin. Exp. Dermatol. 2019, 44, e126–e132. [Google Scholar] [CrossRef]
- Bui, T.L.; Silva-Hirschberg, C.; Torres, J.; Armstrong, A.W. Hidradenitis suppurativa and diabetes mellitus: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 395–402. [Google Scholar] [CrossRef]
- Sivanand, A.; Gulliver, W.P.; Josan, C.K.; Alhusayen, R.; Fleming, P.J. Weight Loss and Dietary Interventions for Hidradenitis Suppurativa: A Systematic Review. J. Cutan. Med. Surg. 2020, 24, 64–72. [Google Scholar] [CrossRef]
- Monfrecola, G.; Balato, A.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Donnarumma, M.; Lembo, S.; Fabbrocini, G. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: A possible metabolic loop. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1631–1633. [Google Scholar] [CrossRef] [Green Version]
- Marasca, C.; Balato, A.; Annunziata, M.C.; Cacciapuoti, S.; Fabbrocini, G. Insulin resistance, mTOR and hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e106–e107. [Google Scholar] [CrossRef]
- Horváth, B.; Janse, I.C.; Sibbald, G.R. Pain management in patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2015, 73, S47–S51. [Google Scholar] [CrossRef]
- Browne, B.A.; Holder, E.P.; Rupnick, L. Nonsteroidal anti-inflammatory drugs and necrotizing fasciitis. Am. J. Health Syst. Pharm. 1996, 53, 265–269. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bayer, C.R.; Aldape, M.J.; Stevens, D.L. The roles of injury and nonsteroidal anti-inflammatory drugs in the development and outcomes of severe group A streptococcal soft tissue infections. Curr. Opin. Infect. Dis. 2015, 28, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, P.; Mathur, M. Hidradenitis suppurativa and smoking: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 1006–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, G.; Prens, E.P. Inflammatory Mechanisms in Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Deilhes, F.; Rouquet, R.M.; Gall, Y.; Aquilina, C.; Paul, C.; Konstantinou, M.P. Profile of smoking dependency in hidradenitis suppurativa patients and smoking cessation outcomes. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e790–e791. [Google Scholar] [CrossRef] [PubMed]
- Bukvić Mokos, Z.; Miše, J.; Balić, A.; Marinović, B. Understanding the Relationship between Smoking and Hidradenitis Suppurativa. Acta Dermatovenerol. Croat. 2020, 28, 9–13. [Google Scholar] [PubMed]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- von Laffert, M.; Helmbold, P.; Wohlrab, J.; Fiedler, E.; Stadie, V.; Marsch, W.C. Hidradenitis suppurativa (acne inversa): Early inflammatory events at terminal follicles and at interfollicular epidermis. Exp. Dermatol. 2010, 19, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Plewig, G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: An approach to pathogenesis by evidence from translational biology. Exp. Dermatol. 2013, 22, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Guet-Revillet, H.; Coignard-Biehler, H.; Jais, J.P.; Quesne, G.; Frapy, E.; Poirée, S.; Le Guern, A.S.; Le Flèche-Matéos, A.; Hovnanian, A.; Consigny, P.H.; et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg. Infect. Dis. 2014, 20, 1990–1998. [Google Scholar] [CrossRef]
- Guet-Revillet, H.; Jais, J.P.; Ungeheuer, M.N.; Coignard-Biehler, H.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Lortholary, O.; Nassif, X.; et al. The Microbiological Landscape of Anaerobic Infections in Hidradenitis Suppurativa: A Prospective Metagenomic Study. Clin. Infect. Dis. 2017, 65, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.M.; Cook, L.C.; Zhan, X.; Banerjee, K.; Cong, Z.; Imamura-Kawasawa, Y.; Gettle, S.L.; Longenecker, A.L.; Kirby, J.S.; Nelson, A.M. Loss of Skin Microbial Diversity and Alteration of Bacterial Metabolic Function in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 716–720. [Google Scholar] [CrossRef]
- Naik, H.B.; Jo, J.H.; Paul, M.; Kong, H.H. Skin Microbiota Perturbations Are Distinct and Disease Severity-Dependent in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 922–925.e3. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef]
- Guenin-Macé, L.; Morel, J.D.; Doisne, J.M.; Schiavo, A.; Boulet, L.; Mayau, V.; Goncalves, P.; Duchatelet, S.; Hovnanian, A.; Bondet, V.; et al. Dysregulation of tryptophan catabolism at the host-skin microbiota interface in hidradenitis suppurativa. JCI Insight 2020, 5, e140598. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nisticò, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, C.; Hänni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, H.H.; de Ruiter, L.; van den Broecke, D.G.; Dik, W.A.; Laman, J.D.; Prens, E.P. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-α and IL-1β. Br. J. Dermatol. 2011, 164, 1292–1298. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Laman, J.D.; de Ruiter, L.; Dik, W.A.; Prens, E.P. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: An in situ and ex vivo study. Br. J. Dermatol. 2012, 166, 298–305. [Google Scholar] [CrossRef]
- Kelly, G.; Hughes, R.; McGarry, T.; van den Born, M.; Adamzik, K.; Fitzgerald, R.; Lawlor, C.; Tobin, A.M.; Sweeney, C.M.; Kirby, B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 2015, 173, 1431–1439. [Google Scholar] [CrossRef]
- Witte-Händel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mößner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef]
- Shah, A.; Alhusayen, R.; Amini-Nik, S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm. Res. 2017, 66, 931–945. [Google Scholar] [CrossRef]
- Choi, F.; Lehmer, L.; Ekelem, C.; Mesinkovska, N.A. Dietary and metabolic factors in the pathogenesis of hidradenitis suppurativa: A systematic review. Int. J. Dermatol. 2020, 59, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Caiazzo, G.; Annunziata, M.C.; Marasca, C.; Scala, E.; Cacciapuoti, S.; Fabbrocini, G. Anti-TNF-α therapy modulates mTORC1 signalling in hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e43–e45. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, H.; Ghosh, P.; Derin, R.; Buchholz, M.; Sasaki, C.; Madara, K.; Longo, D.L. Interleukin-12-induced interferon-gamma production by human peripheral blood T cells is regulated by mammalian target of rapamycin (mTOR). J. Biol. Chem. 2005, 280, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Yin, J.; Duan, J.; Liu, G.; Tan, B.; Yang, G.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. mTORC1 signaling and IL-17 expression: Defining pathways and possible therapeutic targets. Eur. J. Immunol. 2016, 46, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Dmitriev, A.; König, A.; Lang, V.; Diehl, S.; Kaufmann, R.; Pinter, A.; Buerger, C. mTORC1—A potential player in the pathogenesis of hidradenitis suppurativa? J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, E.; Di Caprio, R.; Cacciapuoti, S.; Caiazzo, G.; Fusco, A.; Tortorella, E.; Fabbrocini, G.; Balato, A. A new T helper 17 cytokine in hidradenitis suppurativa: Antimicrobial and proinflammatory role of interleukin-26. Br. J. Dermatol. 2019, 181, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Vigne, S.; Palmer, G.; Martin, P.; Lamacchia, C.; Strebel, D.; Rodriguez, E.; Olleros, M.L.; Vesin, D.; Garcia, I.; Ronchi, F.; et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood 2012, 120, 3478–3487. [Google Scholar] [CrossRef] [Green Version]
- Di Caprio, R.; Balato, A.; Caiazzo, G.; Lembo, S.; Raimondo, A.; Fabbrocini, G.; Monfrecola, G. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch. Dermatol. Res. 2017, 309, 673–678. [Google Scholar] [CrossRef]
- Larochette, V.; Miot, C.; Poli, C.; Beaumont, E.; Roingeard, P.; Fickenscher, H.; Jeannin, P.; Delneste, Y. IL-26, a Cytokine With Roles in Extracellular DNA-Induced Inflammation and Microbial Defense. Front. Immunol. 2019, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Boniotto, M.; Guguin, A.; Surenaud, M.; Jean-Louis, F.; Tisserand, P.; Ortonne, N.; Hersant, B.; Bosc, R.; Poli, F.; et al. Intrinsic Defect in Keratinocyte Function Leads to Inflammation in Hidradenitis Suppurativa. J. Investig. Dermatol. 2016, 136, 1768–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.; Banerjee, A.; Berger, P.Z.; Gross, A.; McNish, S.; Amdur, R.; Shanmugam, V.K. Inherent differences in keratinocyte function in hidradenitis suppurativa: Evidence for the role of IL-22 in disease pathogenesis. Immunol. Investig. 2018, 47, 57–70. [Google Scholar] [CrossRef]
- Scala, E.; Balato, A.; Marasca, C.; Di Caprio, R.; Raimondo, A.; Cacciapuoti, S.; Fabbrocini, G. New insights into mechanism of Notch signaling in hidradenitis suppurativa. G. Ital. Dermatol. Venereol. 2020, 155, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Bechara, F.G.; Dickinson-Blok, J.L.; Gulliver, W.; Horvath, B.; Hughes, R.; Kimball, A.B.; Kirby, B.; Martorell, A.; Podda, M.; et al. Hidradenitis suppurativa/acne inversa: A practical framework for treatment optimization-systematic review and recommendations from the HS ALLIANCE working group. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032. [Google Scholar] [CrossRef]
- Clemmensen, O.J. Topical treatment of hidradenitis suppurativa with clindamycin. Int. J. Dermatol. 1983, 22, 325–328. [Google Scholar] [CrossRef]
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J. Am. Acad. Dermatol. 2019, 81, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Gener, G.; Canoui-Poitrine, F.; Revuz, J.E.; Faye, O.; Poli, F.; Gabison, G.; Pouget, F.; Viallette, C.; Wolkenstein, P.; Bastuji-Garin, S. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: A series of 116 consecutive patients. Dermatology 2009, 219, 148–154. [Google Scholar] [CrossRef]
- Join-Lambert, O.; Coignard-Biehler, H.; Jais, J.P.; Delage, M.; Guet-Revillet, H.; Poirée, S.; Duchatelet, S.; Jullien, V.; Hovnanian, A.; Lortholary, O.; et al. Efficacy of ertapenem in severe hidradenitis suppurativa: A pilot study in a cohort of 30 consecutive patients. J. Antimicrob. Chemother. 2016, 71, 513–520. [Google Scholar] [CrossRef]
- Mendes-Bastos, P.; Martorell, A.; Magina, S. Ertapenem for the treatment of Hidradenitis suppurativa: How much do we need it? Actas Dermosifiliogr. 2018, 109, 582–583. [Google Scholar] [CrossRef]
- Braunberger, T.L.; Nartker, N.T.; Nicholson, C.L.; Nahhas, A.F.; Parks-Miller, A.; Hanna, Z.; Jayaprakash, R.; Ramesh, M.S.; Rambhatla, P.V.; Hamzavi, I.H. Ertapenem—A potent treatment for clinical and quality of life improvement in patients with hidradenitis suppurativa. Int. J. Dermatol. 2018, 57, 1088–1093. [Google Scholar] [CrossRef]
- Fearfield, L.A.; Staughton, R.C. Severe vulval apocrine acne successfully treated with prednisolone and isotretinoin. Clin. Exp. Dermatol. 1999, 24, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Yazdanyar, S.; Boer, J.; Ingvarsson, G.; Szepietowski, J.C.; Jemec, G.B. Dapsone therapy for hidradenitis suppurativa: A series of 24 patients. Dermatology 2011, 222, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.J.; Light, M.J. Successful treatment of hidradenitis suppurativa with acitretin. J. Am. Acad. Dermatol. 1988, 19, 355–356. [Google Scholar] [CrossRef]
- Rose, R.F.; Goodfield, M.J.; Clark, S.M. Treatment of recalcitrant hidradenitis suppurativa with oral ciclosporin. Clin. Exp. Dermatol. 2006, 31, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.B.; Shabeeb, N.; Nicholson, C.L.; Braunberger, T.L.; Peacock, A.; Hamzavi, I.H. Emerging medical treatments for hidradenitis suppurativa. J. Am. Acad. Dermatol. 2020, 83, 554–562. [Google Scholar] [CrossRef]
- Posch, C.; Monshi, B.; Quint, T.; Vujic, I.; Lilgenau, N.; Rappersberger, K. The role of wide local excision for the treatment of severe hidradenitis suppurativa (Hurley grade III): Retrospective analysis of 74 patients. J. Am. Acad. Dermatol. 2017, 77, 123–129.e5. [Google Scholar] [CrossRef]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J. Am. Acad Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, E.; Clements, S.; Driscoll, M. A concise clinician’s guide to therapy for hidradenitis suppurativa. Int. J. Womens Dermatol. 2019, 6, 80–84. [Google Scholar] [CrossRef]
- Lee, E.Y.; Alhusayen, R.; Lansang, P.; Shear, N.; Yeung, J. What is hidradenitis suppurativa? Can. Fam. Physician 2017, 63, 114–120. [Google Scholar] [PubMed]
- van der Zee, H.H.; Prens, E.P.; Boer, J. Deroofing: A tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J. Am. Acad. Dermatol. 2010, 63, 475–480. [Google Scholar] [CrossRef]
- Hazen, P.G.; Hazen, B.P. Hidradenitis suppurativa: Successful treatment using carbon dioxide laser excision and marsupialization. Dermatol. Surg. 2010, 36, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G.B.; Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 1998, 39, 971–974. [Google Scholar] [CrossRef]
- Join-Lambert, O.; Coignard, H.; Jais, J.P.; Guet-Revillet, H.; Poirée, S.; Fraitag, S.; Jullien, V.; Ribadeau-Dumas, F.; Thèze, J.; Le Guern, A.S.; et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology 2011, 222, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Frew, J.W.; Giamarellos-Bourboulis, E.J.; Jemec, G.B.E.; Del Marmol, V.; Marzano, A.V.; Nikolakis, G.; Sayed, C.J.; Tzellos, T.; Wolk, K.; et al. Target molecules for future hidradenitis suppurativa treatment. Exp. Dermatol. 2021, 30, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.; Svensson, H. Surgical treatment of hidradenitis suppurativa. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 305–309. [Google Scholar] [CrossRef]
- Ingram, J.R.; Woo, P.N.; Chua, S.L.; Ormerod, A.D.; Desai, N.; Kai, A.C.; Hood, K.; Burton, T.; Kerdel, F.; Garner, S.E.; et al. Interventions for hidradenitis suppurativa: A Cochrane systematic review incorporating GRADE assessment of evidence quality. Br. J. Dermatol. 2016, 174, 970–978. [Google Scholar] [CrossRef]
- Montaudié, H.; Seitz-Polski, B.; Cornille, A.; Benzaken, S.; Lacour, J.P.; Passeron, T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J. Am. Acad. Dermatol. 2017, 76, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Hong, F.; Conlon, D.M.; Sidur, L.; Smith, K.M.; Fang, Y.; Cuff, C.A.; Kaymakcalan, Z.; Ruzek, M.C. Potential predictive biomarkers of adalimumab response in patients with hidradenitis suppurativa. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G.B.E.; Okun, M.M.; Forman, S.B.; Gulliver, W.P.F.; Prens, E.P.; Mrowietz, U.; Armstrong, A.W.; Geng, Z.; Gu, Y.; Williams, D.A.; et al. Adalimumab medium-term dosing strategy in moderate-to-severe hidradenitis suppurativa: Integrated results from the phase III randomized placebo-controlled PIONEER trials. Br. J. Dermatol. 2019, 181, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Blok, J.L.; Li, K.; Brodmerkel, C.; Horvátovich, P.; Jonkman, M.F.; Horváth, B. Ustekinumab in hidradenitis suppurativa: Clinical results and a search for potential biomarkers in serum. Br. J. Dermatol. 2016, 174, 839–846. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Expression of miRNA-155, miRNA-223, miRNA-31, miRNA-21, miRNA-125b, and miRNA-146a in the Inflammatory Pathway of Hidradenitis Suppurativa. Inflammation 2017, 40, 464–472. [Google Scholar] [CrossRef]
- Derruau, S.; Gobinet, C.; Untereiner, V.; Sockalingum, G.D.; Nassif, A.; Viguier, M.; Piot, O.; Lorimier, S. New insights into hidradenitis suppurativa diagnosis via salivary infrared biosignatures: A pilot study. J. Biophotonics 2021, 14, e202000327. [Google Scholar] [CrossRef]
- van Straalen, K.R.; Schneider-Burrus, S.; Prens, E.P. Current and future treatment of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, e178–e187. [Google Scholar] [CrossRef]
- Marasca, C.; Megna, M.; Balato, A.; Balato, N.; Napolitano, M.; Fabbrocini, G. Secukinumab and hidradenitis suppurativa: Friends or foes? JAAD Case Rep. 2019, 5, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Ribero, S.; Ramondetta, A.; Fabbrocini, G.; Bettoli, V.; Potenza, C.; Chiricozzi, A.; Licciardello, M.; Marzano, A.V.; Bianchi, L.; Rozzo, G.; et al. Effectiveness of Secukinumab in the treatment of moderate-severe hidradenitis suppurativa: Results from an Italian multicentric retrospective study in a real-life setting. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e441–e442. [Google Scholar] [CrossRef]
- Kerdel, F.R.; Azevedo, F.A.; Kerdel Don, C.; Don, F.A.; Fabbrocini, G.; Kerdel, F.A. Apremilast for the Treatment of Mild-to-Moderate Hidradenitis Suppurativa in a Prospective, Open-Label, Phase 2 Study. J. Drugs Dermatol. 2019, 18, 170–176. [Google Scholar] [PubMed]
- Folkes, A.S.; Hawatmeh, F.Z.; Wong, A.; Kerdel, F.A. Emerging drugs for the treatment of hidradenitis suppurativa. Expert Opin. Emerg. Drugs 2020, 25, 201–211. [Google Scholar] [CrossRef]
- Holm, J.G.; Jørgensen, A.R.; Yao, Y.; Thomsen, S.F. Certolizumab pegol for hidradenitis suppurativa: Case report and literature review. Dermatol. Ther. 2020, 2, e14494. [Google Scholar] [CrossRef]
- Wohlmuth-Wieser, I.; Alhusayen, R. Treatment of hidradenitis suppurativa with certolizumab pegol during pregnancy. Int. J. Dermatol. 2020, 60, e140–e141. [Google Scholar] [CrossRef]
- Esme, P.; Akoglu, G.; Caliskan, E. Rapid Response to Certolizumab Pegol in Hidradenitis Suppurativa: A Case Report. Skin Appendage Disord. 2021, 7, 58–61. [Google Scholar] [CrossRef]
- Kok, Y.; Nicolopoulos, J.; Dolianitis, C. Tildrakizumab as a potential long-term therapeutic agent for severe Hidradenitis Suppurativa: A 15 months experience of an Australian institution. Australas. J. Dermatol. 2021, 62, e313–e316. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Ruggiero, A.; Di Guida, A.; Patrì, A.; Fabbrocini, G.; Marasca, C. Ixekizumab: An efficacious treatment for both psoriasis and hidradenitis suppurativa. Dermatol. Ther. 2020, 33, e13756. [Google Scholar] [CrossRef]
- Yoshida, Y.; Oyama, N.; Iino, S.; Shimizu, C.; Hasegawa, M. Long-standing refractory hidradenitis suppurativa responded to a brodalumab monotherapy in a patient with psoriasis: A possible involvement of Th17 across the spectrum of both diseases. J. Dermatol. 2021, 48, 916–920. [Google Scholar] [CrossRef]
- Tricarico, P.M.; Boniotto, M.; Genovese, G.; Zouboulis, C.C.; Marzano, A.V.; Crovella, S. An Integrated Approach to Unravel Hidradenitis Suppurativa Etiopathogenesis. Front. Immunol. 2019, 10, 892. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; Wen, W.; Sun, J.; Su, B.; Liu, B.; Ma, D.; Lv, D.; Wen, Y.; Qu, T.; et al. Gamma-secretase gene mutations in familial acne inversa. Science 2010, 330, 1065. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Laman, J.D.; Prens, E.P. Can animal skin diseases or current transgenic mice serve as a model for hidradenitis suppurativa? Dermatology 2012, 225, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, L.; Huang, Y.; Liu, K.; Lu, C.; Si, N.; Wang, R.; Liu, Y.; Zhang, X. Keratin 5-Cre-driven deletion of Ncstn in an acne inversa-like mouse model leads to a markedly increased IL-36a and Sprr2 expression. Front. Med. 2020, 14, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Adalsteinsson, J.A.; Gudjonsson, J.E.; Ward, N.L. Research Techniques Made Simple: Murine Models of Human Psoriasis. J. Investig. Dermatol. 2018, 138, e1–e8. [Google Scholar] [CrossRef]
- Sundberg, J.P.; McElwee, K.; Brehm, M.A.; Su, L.; King, L.E., Jr. Animal Models for Alopecia Areata: What and Where? J. Investig. Dermatol. Symp. Proc. 2015, 17, 23–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartey, Q.Q.; Miller, R.J.; Pinsker, B.L.; Okoh, U.J.; Shipman, W.D.; George, B.A.; Nwizu, C.C.; Barnes, L.A.; Kerns, M.L.; Caffrey, J.A.; et al. Lessons learned from the development of a hidradenitis suppurativa xenograft mouse model. Clin. Exp. Dermatol. 2020, 45, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Ex vivo human models of hidradenitis suppurativa/acne inversa for laboratory research and drug screening. Br. J. Dermatol. 2019, 181, 244–246. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; Ardon, C.B.; van der Zee, H.H.; Lubberts, E.; Prens, E.P. The anti-inflammatory potency of biologics targeting tumour necrosis factor-α, interleukin (IL)-17A, IL-12/23 and CD20 in hidradenitis suppurativa: An ex vivo study. Br. J. Dermatol. 2019, 181, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Vossen, A.R.J.V.; van Straalen, K.R.; Florencia, E.F.; Prens, E.P. Lesional Inflammatory Profile in Hidradenitis Suppurativa Is Not Solely Driven by IL-1. J. Investig. Dermatol. 2020, 140, 1463–1466.e2. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Yoshida, G.J.; Wu, Y.; Xia, L.; Schneider, M.R. Sebaceous gland: Milestones of 30-year modelling research dedicated to the “brain of the skin”. Exp. Dermatol. 2020, 29, 1069–1079. [Google Scholar] [CrossRef]
- Kodzius, R.; Schulze, F.; Gao, X.; Schneider, M.R. Organ-on-Chip Technology: Current State and Future Developments. Genes 2017, 8, 266. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. https://doi.org/10.3390/cells10082094
Scala E, Cacciapuoti S, Garzorz-Stark N, Megna M, Marasca C, Seiringer P, Volz T, Eyerich K, Fabbrocini G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells. 2021; 10(8):2094. https://doi.org/10.3390/cells10082094
Chicago/Turabian StyleScala, Emanuele, Sara Cacciapuoti, Natalie Garzorz-Stark, Matteo Megna, Claudio Marasca, Peter Seiringer, Thomas Volz, Kilian Eyerich, and Gabriella Fabbrocini. 2021. "Hidradenitis Suppurativa: Where We Are and Where We Are Going" Cells 10, no. 8: 2094. https://doi.org/10.3390/cells10082094
APA StyleScala, E., Cacciapuoti, S., Garzorz-Stark, N., Megna, M., Marasca, C., Seiringer, P., Volz, T., Eyerich, K., & Fabbrocini, G. (2021). Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells, 10(8), 2094. https://doi.org/10.3390/cells10082094










