Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury
Abstract
:1. Introduction
2. LVs in SCI Research and Therapies
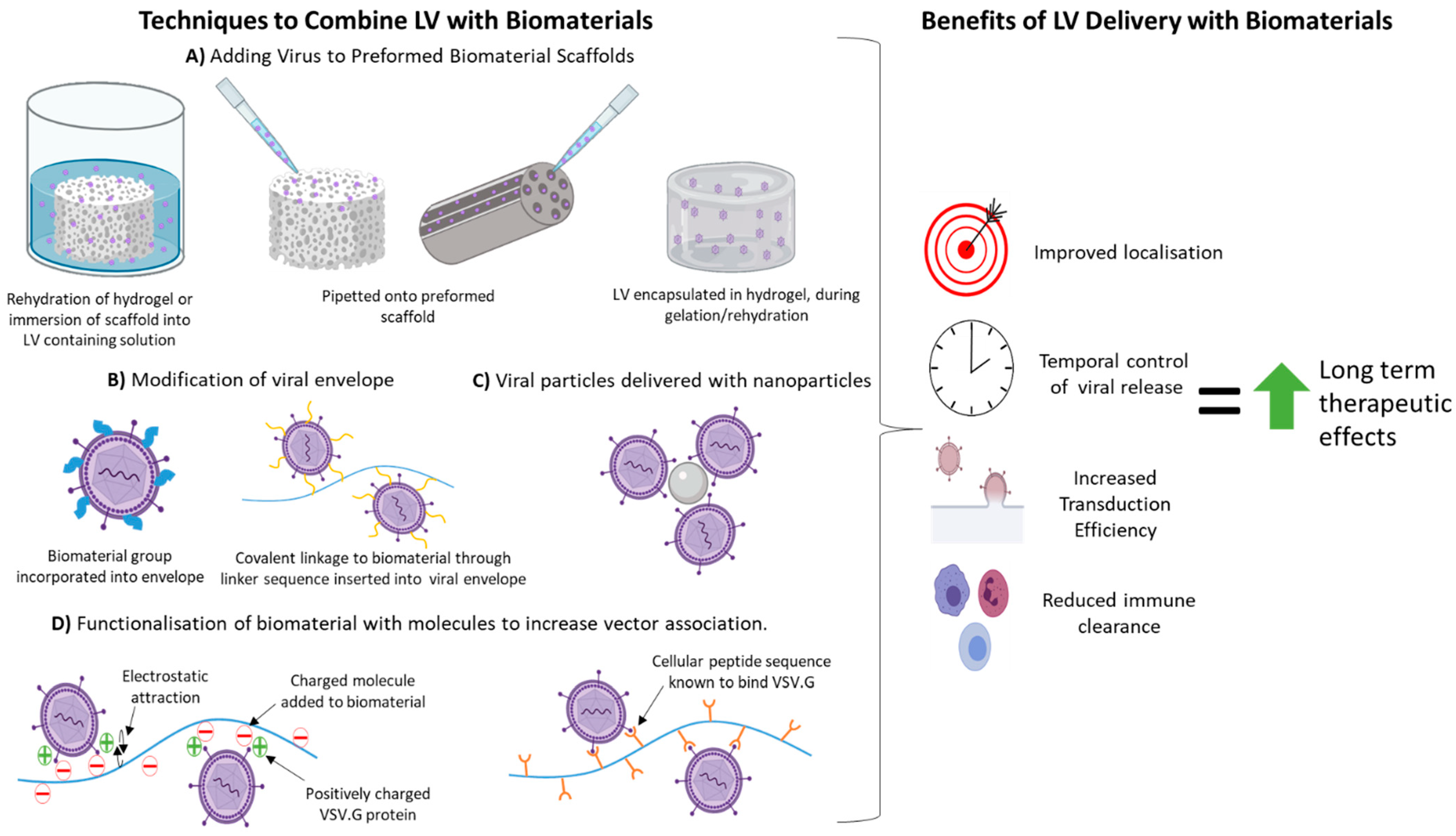
3. Benefits of LV Delivery with Biomaterials
4. Techniques Used to Combine LVs with Biomaterials Previously Used in SCI Regeneration Therapies
4.1. Non-Specific Binding/Assocaition with Biomaterial
| Non-Specific Binding/Association with Biomaterial | ||||
|---|---|---|---|---|
| Biomaterial | Attachment Details | Model | Vector Retention and Transduction Efficiency | Ref |
| PLG | Lyophilisation with sucrose. | HEK cells. Subcutaneous implantation in mice. |
| [63] |
| PEG or Gelatin | Pipetted into channels. | Intrathecal delivery to uninjured and thoracic spinal cord hemi-section injury in mice. |
| [17] |
| PCL | Pipetted onto scaffold and incubated 2 min before implantation. | Implantation into mouse periovarian fat pad. |
| [64] |
| PEG | PEG-mal hydrogel tubes with LV injected directly into tubes before implantation. | Mouse C5 1.15 mm lateral hemisection. |
| [65] |
4.2. Surface Modification/Functionaliseation of Biomaterial
| Surface Modification/Functionalisation of Biomaterials | ||||
|---|---|---|---|---|
| Biomaterial | Attachment Details | Model | Vector Retention and Transduction Efficiency | Ref |
| PLG | PS coated PLG microspheres formed into scaffold. LV pipetted onto scaffold before implantation. | Rat SC hemi-section model (spinal segment not given). |
| [48] |
| Chitosan or heparin immobilised onto PLG post fabrication using EDC/NHS chemistry. Virus pipetted onto modified/unmodified scaffold. | Implanted into mouse T9–T10 SC hemi-section lesion. |
| [46] | |
| Gelatin | Cysteine added as a cross linker to chitosan and heparin in EDC/NHS solution. For hydrogel incorporation, filtered solutions were flash frozen in nitrogen and lyophilised. Virus pipetted onto modified/unmodified hydrogel. | Implantation into mouse intrathecal space above thoracic spinal cord. |
| [17] |
| PEG | Low (1–10 kDa) and high (30–70 kDa) MW PLL were added to PEG acrylate hydrogels. Subsequently hydrogel incubated with virus solution. | HT1080 cells cultured on LV containing scaffolds functionalised with PLL of different MW. |
| [51] |
| Peptides sequences that bind VSV-G protein. Peptides incubated with virus first then this mixture attached to PEG hydrogel via acrylate—PEG—maleimide linker. | HT1080 cells added to LV containing peptide functionalised scaffolds. |
| [51] | |
| PEGDA | Non-covalent attachment of PLL to premade PEGDA cyrogel through emulsion in PLL solution. Covalent attachment of PLL to PEGDA through poly-acrylic linker and EDC-NHS chemistry. LV pipetted onto pre-made scaffolds. | Non-invasive NIHT3T3 cells seeded on scaffold and stained to test cell adhesion. Subcutaneous implantation of scaffolds in mice. |
| [47] |
4.3. Encapsulation of LVs within Hydrogels
| Encapsulation of LVs within Hydrogels | ||||
|---|---|---|---|---|
| Biomaterial | Attachment Details | Model | Vector Retention and Transduction Efficiency | Ref |
| PEG | Macroporous PEG hydrogel encapsulating gelatin microspheres. Microspheres were hydrated with LV containing solution before addition to PEG and gelation. | Subcutaneous implant in CD1 mice. |
| [71] |
| Collagen | LVs mixed with collagen during gelation. | C6 cells were seeded on gels containing LVs. |
| [44] |
| Collagen –vs- Chitosan/b-glycerol phosphate | LVs mixed with chitosan BGP or collagen before gelation. | LV elution measured following incubation of scaffolds in cell culture medium. |
| [49] |
| Fibrin with/without Polybrene | LVs mixed with thrombin before mixing with fibrinogen (between 3.75–7.5 mg/mL). Polybrene added to some gels before gelation. | LV loaded fibrin gels spotted in a pattern and cells grown on top. Or NIH-3T3 cells or 293T cells seeded on top gels. |
| [41] |
| Alginate | Different ratios of low and high MW (LMW and HMW) alginate polymers (75/25 and 25/75 low/high MW), as well as high MW alginate alone were used to create gels. | LV loaded gels injected into left hind limb muscle of mice. |
| [50] |
| HyA | 3 scaffolds compared: (i) NP-HyA; HyA microspheres (with a thiol group) mixed with RGD-conjugated PEG to form nanoporous HyA hydrogel once in situ. (ii) Mac-HyA; NP-HyA hydrogels crosslinked around PEG microparticles that proteolytically degrade in situ to leave behind a macroporous architecture. (iii) HyA-MP; polydisperse HyA-PEG microparticles (from NP-HyA scaffold) assembled in situ. Precursor molecules were mixed with PLL and LVs before injection and gelation in vivo. | Injected into mouse left/right mammary fat pad with opposite pad acting as internal LV loaded NP-HyA control. |
| [73] |
| 3 scaffolds compared: (i) NP-HyA; HyA microspheres (with a thiol group) mixed with RGD-conjugated PEG to form nanoporous HyA hydrogels once in situ. (ii) Monodisperse HyA-PEG (mHyA-MP) or (iii) polydisperse (pHyA-MP) microparticles assembled in situ. Precursor molecules mixed with PLL and LVs before injection and gelation in vivo. | Injected after spinal cord T8-T10 clip compression injury in mice. |
| [72] | |
4.4. Modification of LV Envelope
| Modification of LV Envelope | ||||
|---|---|---|---|---|
| Strategy | Attachment Details | Model | Viral Transduction Efficacy and Retention | Ref |
| PEG conjugated to VSV.G envelope | LV was conjugated with SSPEG and CCPEG, respectively. 10 g of SSPEG or CCPEG polymer (activated by succinimidyl succinate) was added per µg of protein content in LV preparation. Conjugation reactions were performed at 25 °C with gentle stirring. Reactions were stopped by addition of 103 L-lysine. | PEGylated and unPEGylated LV were added to 293T cells in the presence of serum containing neutralising antibodies against unmodified LV-VSV.G and human serum with normal complement levels. Injection into tail vein of mice for bio-distribution. |
| [53,55] |
| Fibrinogen binding site inserted into VSV.G envelope | Introduction of FXIII recognition sequence and protease recognition sites into LV envelope protein sequence. This was achieved by inserting a 17 aa peptide sequence into pMD2.g plasmid (FXIII-LVs). Subsequent incubation of FXIII-LV with thrombin, Ca2+ and fibrinogen created a bridge between, FXIII and fibrin, covalently attaching LV to fibrin hydrogels before gelation. | Fibrin gel spots with wild type LVs or FXIII-LVs were printed onto tissue culture slides and a confluent layer of 293 T cells grown on top. |
| [43] |
4.5. Use of Nanoparticels in LV Delivery
| Use of Nanoparticles in LV Delivery | ||||
|---|---|---|---|---|
| Biomaterial | Attachment Method | Model | Vector Retention and Transduction Efficiency | Ref |
| HA NP + collagen hydrogel. | LVs mixed with HA NPs in PBS then added to collagen before gelation. | Subcutaneous implantation into mouse. |
| [44] |
| HA NP + PLG scaffolds | LVs mixed with HA NPs in PBS then loaded into channels of preformed PLG channel-bridge scaffold. | Implanted into Rat SC hemi-section T9-T10. |
| [18] |
| LVs pipetted on top of pre-made HA-NP/PLG scaffold compared to LVs mixed with HA NPs and LVs pipetted on top of PLG only scaffolds. | Implantation into mouse epididymal fat pad. |
| [52] | |
| Fibrin alone Or fibrin + HA-NP | LVs mixed HA NP. LV-NP complexes mixed with fibrinogen and thrombin solution prior to gelation. | LV loaded gels implanted subcutaneously into mice. |
| [74] |
| PEG hydrogel functionalised with HCNPs | PEG gels with HCNPs (3:1 heparin: chitosan) incorporated into PEG gels during formation. LVs pipetted on top premade hydrogels. | Subcutaneous implantation in mice. |
| [45] |
5. Studies Combining LVs with Biomaterials as Regenerative Therapies for SCI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NSCISC. National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance; University of Alabama at Birmingham: Birmingham, AL, USA, 2021. [Google Scholar]
- Finnerup, N.B. Neuropathic pain and spasticity: Intricate consequences of spinal cord injury. Spinal Cord 2017, 55, 1046–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, H. Chapter 35—Traumatic spinal cord injury. In Handbook of Clinical Neurology; Barnes, M.P., Good, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 110, pp. 411–426. [Google Scholar]
- Krause, J.S.; Dismuke-Greer, C.E.; Reed, K.S.; Li, C. Employment status, hours working, and gainful earnings after spinal cord injury: Relationship with pain, prescription medications for pain, and nonprescription opioid use. Spinal Cord 2020, 58, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Kim, H.J.; Ehsanipour, A.; Bierman, R.D.; Kaarela, O.; Xue, C.; Khademhosseini, A.; Seidlits, S.K. Regenerative Therapies for Spinal Cord Injury. Tissue Eng. Part B Rev. 2019, 25, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Walthers, C.M.; Seidlits, S.K. Gene delivery strategies to promote spinal cord repair. Biomark. Insights 2015, 10, 11–29. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Guerrero, A.R.; Johnson, W.E.B.; Masri, W.E.; Baba, H. Gene therapy strategies for the treatment of spinal cord injury. Ther. Deliv. 2014, 5, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Gower, R.M.; Shepard, J.A.; Shea, L.D. Hydrogels for lentiviral gene delivery. Expert Opin. Drug Deliv. 2013, 10, 499–509. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Hum. Gene Ther. 2017, 28, 99–111. [Google Scholar] [CrossRef]
- Thomas, A.M.; Palma, J.L.; Shea, L.D. Sponge-mediated lentivirus delivery to acute and chronic spinal cord injuries. J. Control. Release 2015, 204, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, H.M.; Aviles, M.O.; Shin, S.; Holland, S.J.; Zelivyanskaya, M.L.; Fast, A.G.; Ko, S.Y.; Margul, D.J.; Bartels, A.K.; Boehler, R.M.; et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 2012, 33, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Meunier, A.; Pohl, M. Lentiviral vectors for gene transfer into the spinal cord glial cells. Gene Ther. 2009, 16, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Merten, O.-W.; Hebben, M.; Bovolenta, C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016, 3, 16017. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Khazaei, M.; Ahuja, C.S.; Yokota, K.; Ahlfors, J.-E.; Liu, Y.; Wang, J.; Shibata, S.; Chio, J.; Hettiaratchi, M.H.; et al. Human Oligodendrogenic Neural Progenitor Cells Delivered with Chondroitinase ABC Facilitate Functional Repair of Chronic Spinal Cord Injury. Stem Cell Rep. 2018, 11, 1433–1448. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, J.; Tran, K.; Burt, D.R.; Zhong, L.; Gao, G. Slow Infusion of Recombinant Adeno-Associated Viruses into the Mouse Cerebrospinal Fluid Space. Hum. Gene Ther. Methods 2018, 29, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Li, S.; Gregorevic, P.; Fall, B.M.; Chamberlain, J.S. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol. Ther. 2010, 18, 206–213. [Google Scholar] [CrossRef]
- Vranckx, L.S.; Demeulemeester, J.; Debyser, Z.; Gijsbers, R. Towards a Safer, More Randomized Lentiviral Vector Integration Profile Exploring Artificial LEDGF Chimeras. PLoS ONE 2016, 11, e0164167. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.R.W.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 Integration in the Human Genome Favors Active Genes and Local Hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef] [Green Version]
- McCarty, D.M.; Young, S.M., Jr.; Samulski, R.J. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 2004, 38, 819–845. [Google Scholar] [CrossRef]
- Deyle, D.R.; Russell, D.W. Adeno-associated virus vector integration. Curr. Opin. Mol. Ther. 2009, 11, 442–447. [Google Scholar] [PubMed]
- Burnside, E.R.; De Winter, F.; Didangelos, A.; James, N.D.; Andreica, E.-C.; Layard-Horsfall, H.; Muir, E.M.; Verhaagen, J.; Bradbury, E.J. Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain 2018, 141, 2362–2381. [Google Scholar] [CrossRef]
- Das, A.T.; Tenenbaum, L.; Berkhout, B. Tet-On Systems For Doxycycline-inducible Gene Expression. Curr. Gene Ther. 2016, 16, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blits, B.; Bunge, M.B. Direct Gene Therapy for Repair of the Spinal Cord. J. Neurotrauma 2006, 23, 508–520. [Google Scholar] [CrossRef]
- Kruzik, A.; Fetahagic, D.; Hartlieb, B.; Dorn, S.; Koppensteiner, H.; Horling, F.M.; Scheiflinger, F.; Reipert, B.M.; de la Rosa, M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol. Ther. Methods Clin. Dev. 2019, 14, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Annoni, A.; Gregori, S.; Naldini, L.; Cantore, A. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell. Immunol. 2019, 342, 103802. [Google Scholar] [CrossRef] [PubMed]
- Trono, D. Lentiviral vectors: Turning a deadly foe into a therapeutic agent. Gene Ther 2000, 7, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Tolmachov, O.; Tolmachova, T.; Al-Allaf, F. Designing Lentiviral Gene Vectors; IntechOpen: London, UK, 2011. [Google Scholar]
- Loy, D.N.; Crawford, C.H.; Darnall, J.B.; Burke, D.A.; Onifer, S.M.; Whittemore, S.R. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J. Comp. Neurol. 2002, 445, 308–324. [Google Scholar] [CrossRef]
- Haggerty, A.E.; Maldonado-Lasunción, I.; Oudega, M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed. Mater. 2018, 13, 044105. [Google Scholar] [CrossRef] [Green Version]
- Koichi, M.; Noriko, M.; Takashi, S. Gene Delivery into the Central Nervous System (CNS) Using AAV Vectors; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Leibinger, M.; Zeitler, C.; Gobrecht, P.; Andreadaki, A.; Gisselmann, G.; Fischer, D. Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice. Nat. Commun. 2021, 12, 391. [Google Scholar] [CrossRef]
- Stepankova, K.; Jendelova, P.; Machova Urdzikova, L. Planet of the AAVs: The Spinal Cord Injury Episode. Biomedicines 2021, 9, 613. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.-Y.; Wang, B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.D.; Lei, P.; Padmashali, R.M.; Andreadis, S.T. Fibrin-mediated lentivirus gene transfer: Implications for lentivirus microarrays. J. Control. Release 2010, 144, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.F.; Cen, J.S.; Zhong, Q.; Chen, L.; Wang, J.; Deng, D.Y.; Wan, Y. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials 2013, 34, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Padmashali, R.M.; Andreadis, S.T. Engineering fibrinogen-binding VSV-G envelope for spatially- and cell-controlled lentivirus delivery through fibrin hydrogels. Biomaterials 2011, 32, 3330–3339. [Google Scholar] [CrossRef]
- Shin, S.; Shea, L.D. Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol. Ther. 2010, 18, 700–706. [Google Scholar] [CrossRef]
- Thomas, A.M.; Gomez, A.J.; Palma, J.L.; Yap, W.T.; Shea, L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials 2014, 35, 8687–8693. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.M.; Shea, L.D. Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. J. Control. Release Off. J. Control. Release Soc. 2013, 170, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrimali, P.; Peter, M.; Singh, A.; Dalal, N.; Dakave, S.; Chiplunkar, S.V.; Tayalia, P. Efficient in situ gene delivery via PEG diacrylate matrices. Biomater. Sci. 2018, 6, 3241–3250. [Google Scholar] [CrossRef]
- Shin, S.; Tuinstra, H.M.; Salvay, D.M.; Shea, L.D. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials 2010, 31, 4353–4359. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.S.; Nikolskaya, N.; Choileáin, S.N.; Hennessy, N.; O’Brien, T.; Strappe, P.M.; Gorelov, A.; Rochev, Y. Thermosensitive hydrogel for prolonged delivery of lentiviral vector expressing neurotrophin-3 in vitro. J. Gene Med. 2011, 13, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Stilhano, R.S.; Madrigal, J.L.; Wong, K.; Williams, P.A.; Martin, P.K.M.; Yamaguchi, F.S.M.; Samoto, V.Y.; Han, S.W.; Silva, E.A. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. J. Control. Release 2016, 237, 42–49. [Google Scholar] [CrossRef]
- Skoumal, M.; Seidlits, S.; Shin, S.; Shea, L. Localized lentivirus delivery via peptide interactions. Biotechnol. Bioeng. 2016, 113, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R.M.; Shin, S.; Fast, A.G.; Gower, R.M.; Shea, L.D. A PLG/HAp composite scaffold for lentivirus delivery. Biomaterials 2013, 34, 5431–5438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croyle, M.A.; Callahan, S.M.; Auricchio, A.; Schumer, G.; Linse, K.D.; Wilson, J.M.; Brunner, L.J.; Kobinger, G.P. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004, 78, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munis, A.M.; Mattiuzzo, G.; Bentley, E.M.; Collins, M.K.; Eyles, J.E.; Takeuchi, Y. Use of Heterologous Vesiculovirus G Proteins Circumvents the Humoral Anti-envelope Immunity in Lentivector-Based In Vivo Gene Delivery. Mol. Ther. Nucleic Acids 2019, 17, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Croyle, M.A.; Yu, Q.-C.; Wilson, J.M. Development of a Rapid Method for the PEGylation of Adenoviruses with Enhanced Transduction and Improved Stability under Harsh Storage Conditions. Hum. Gene Ther. 2000, 11, 1713–1722. [Google Scholar] [CrossRef]
- Jang, J.-H.; Schaffer, D.V.; Shea, L.D. Engineering biomaterial systems to enhance viral vector gene delivery. Mol. Ther. 2011, 19, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A.; Seifalian, A.M. Translational Regenerative Therapies for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1776. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Huang, C.; Zhao, Z.; Wang, A.; Li, P.; Fan, Y.; Zhou, G. Nano-hydroxyapatite(n-HA) involved in the regeneration of rat nerve injury triggered by overloading stretch. Med. Nov. Technol. Devices 2019, 4, 100022. [Google Scholar] [CrossRef]
- Taraballi, F.; Sushnitha, M.; Tsao, C.; Bauza, G.; Liverani, C.; Shi, A.; Tasciotti, E. Biomimetic Tissue Engineering: Tuning the Immune and Inflammatory Response to Implantable Biomaterials. Adv. Healthc. Mater. 2018, 7, 1800490. [Google Scholar] [CrossRef]
- Ribeiro-Samy, S.; Silva, N.A.; Correlo, V.M.; Fraga, J.S.; Pinto, L.; Teixeira-Castro, A.; Leite-Almeida, H.; Almeida, A.; Gimble, J.M.; Sousa, N. Development and characterization of a PHB-HV-based 3 D scaffold for a tissue engineering and cell-therapy combinatorial approach for spinal cord injury regeneration. Macromol. Biosci. 2013, 13, 1576–1592. [Google Scholar] [CrossRef] [Green Version]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal Delivery of Polyethylene Glycol-coated Gold Nanoparticles Promotes Functional Recovery After Spinal Cord Injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010, 213, 1513. [Google Scholar] [CrossRef]
- Shin, S.; Salvay, D.M.; Shea, L.D. Lentivirus delivery by adsorption to tissue engineering scaffolds. J. Biomed. Mater. Res. A 2010, 93, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushnell, G.G.; Rao, S.S.; Hartfield, R.M.; Zhang, Y.; Oakes, R.S.; Jeruss, J.S.; Shea, L.D. Microporous scaffolds loaded with immunomodulatory lentivirus to study the contribution of immune cell populations to tumor cell recruitment in vivo. Biotechnol. Bioeng. 2020, 117, 210–222. [Google Scholar] [CrossRef]
- Ciciriello, A.J.; Smith, D.R.; Munsell, M.K.; Boyd, S.J.; Shea, L.D.; Dumont, C.M. IL-10 lentivirus-laden hydrogel tubes increase spinal progenitor survival and neuronal differentiation after spinal cord injury. Biotechnol. Bioeng. 2021, 118, 2609–2625. [Google Scholar] [CrossRef]
- Brunger, J.M.; Huynh, N.P.T.; Guenther, C.M.; Perez-Pinera, P.; Moutos, F.T.; Sanchez-Adams, J.; Gersbach, C.A.; Guilak, F. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E798–E806. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, R.; Tralka, T.S.; Willingham, M.C.; Pastan, I. Inhibition of VSV binding and infectivity by phosphatidylserine: Is phosphatidylserine a VSV-binding site? Cell 1983, 32, 639–646. [Google Scholar] [CrossRef]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 2009, 10, 1402–1409. [Google Scholar] [CrossRef]
- Bart, J.; Tiggelaar, R.; Yang, M.; Schlautmann, S.; Zuilhof, H.; Gardeniers, H. Room-temperature intermediate layer bonding for microfluidic devices. Lab Chip 2009, 9, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Chang, L.-J. Kinetic Analyses of Stability of Simple and Complex Retroviral Vectors. Virology 2001, 280, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Shepard, J.A.; Virani, F.R.; Goodman, A.G.; Gossett, T.D.; Shin, S.; Shea, L.D. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials 2012, 33, 7412–7421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsanipour, A.; Sathialingam, M.; Rad, L.M.; de Rutte, J.; Bierman, R.D.; Liang, J.; Xiao, W.; Di Carlo, D.; Seidlits, S.K. Injectable, macroporous scaffolds for delivery of therapeutic genes to the injured spinal cord. APL Bioeng. 2021, 5, 016104. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, Hyaluronic Acid-Based Scaffolds with Macroporous Architecture for Gene Delivery. Cell. Mol. Bioeng. 2019, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.E.; Shin, S.; Shea, L.D. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. J. Control. Release 2012, 157, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gros, T.; Sakamoto, J.S.; Blesch, A.; Havton, L.A.; Tuszynski, M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 2010, 31, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Dumont, C.M.; Park, J.; Ciciriello, A.J.; Guo, A.; Tatineni, R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Polycistronic Delivery of IL-10 and NT-3 Promotes Oligodendrocyte Myelination and Functional Recovery in a Mouse Spinal Cord Injury Model. Tissue Eng. Part A 2020, 26, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Margul, D.J.; Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Johnson, M.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Combinatorial lentiviral gene delivery of pro-oligodendrogenic factors for improving myelination of regenerating axons after spinal cord injury. Biotechnol. Bioeng. 2019, 116, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuinstra, H.M.; Margul, D.J.; Goodman, A.G.; Boehler, R.M.; Holland, S.J.; Zelivyanskaya, M.L.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Long-Term Characterization of Axon Regeneration and Matrix Changes Using Multiple Channel Bridges for Spinal Cord Regeneration. Tissue Eng. Part A 2013, 20, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Natural Biomaterials and LV Therapy for SCI | ||||
|---|---|---|---|---|
| Biomaterial | Therapeutic Strategy | Injury Model | Results | Ref |
| Agarose | Agarose channel scaffold with LV-NT3 injected rostral to implantation site. | Implanted immediately after C4–5 2 mm long section removal in rat. |
| [75] |
| HyA | HyA-PEG microspheres mixed with PLL and LV-BDNF or LV-NT3 before injection. | Injected immediately after spinal cord T8-T10 clip compression injury in mice. |
| [72] |
| Synthetic Biomaterials and LV Therapy for SCI | ||||
|---|---|---|---|---|
| Biomaterial | Therapeutic Strategy | Injury Model | Results | Ref |
| PF-127 | LV-Lingo1-shRNA mixed with liquid PF127. LV-Lingo-shRNA bolus treatment (with 25% more LV than scaffold +LV). | Implanted immediately after T10 2 mm transection injury in rats. |
| [42] |
| PEG | LV-Shh loaded PEG sponges pipetted into macropores. | Intrathecal delivery above T9-10 2.25 mm hemi-section immediately or 4 weeks after injury in mice. |
| [17] |
| PEG hydrogel tubes with LV-IL10 injected directly into tubes before implantation. | Mouse C5 1.15-mm lateral hemisection. |
| [65] | |
| PLG | Multi-channel PLG bridge with LV-NT3 or LV-BDNF pipetted into channels. | Implanted immediately after T9-10 4 mm hemi-section in rat. |
| [18,78] |
| Multi-channel PLG bridge with heparin coating loaded with LV-Shh. | Implanted immediately after T9-10 2.25 mm hemi-section in rats. |
| [46] | |
| Multi-channel PLG bridge loaded with LV-Noggin and/or LV-PDGF. | Implanted immediately after C5 1.5 mm hemi-section in rats. |
| [77] | |
| Multi-channel PLG bridge loaded with LV-IL10, LV-NT3 or LVs with a polycistronic mRNA encoding both IL-10 and NT-3 (LV-IL10-NT3). | Implanted immediately after T9-10 2.5 mm hemisection in rats. |
| [76] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shortiss, C.; Howard, L.; McMahon, S.S. Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury. Cells 2021, 10, 2102. https://doi.org/10.3390/cells10082102
Shortiss C, Howard L, McMahon SS. Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury. Cells. 2021; 10(8):2102. https://doi.org/10.3390/cells10082102
Chicago/Turabian StyleShortiss, Ciara, Linda Howard, and Siobhan S. McMahon. 2021. "Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury" Cells 10, no. 8: 2102. https://doi.org/10.3390/cells10082102
APA StyleShortiss, C., Howard, L., & McMahon, S. S. (2021). Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury. Cells, 10(8), 2102. https://doi.org/10.3390/cells10082102






