Redox-Sensitive and Folate-Receptor-Mediated Targeting of Cervical Cancer Cells for Photodynamic Therapy Using Nanophotosensitizers Composed of Chlorin e6-Conjugated ?-Cyclodextrin via Diselenide Linkage
Abstract
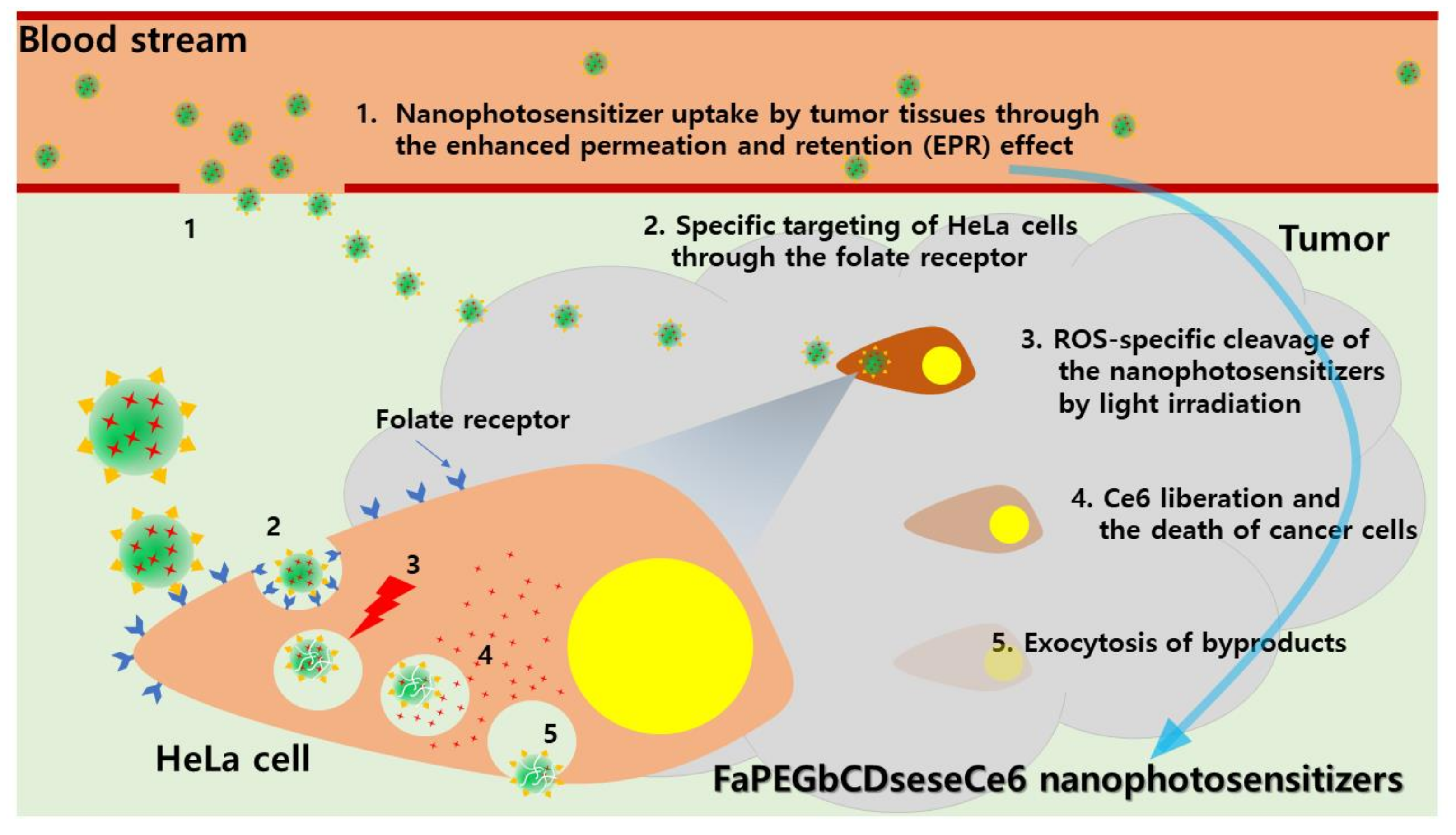
:1. Introduction
2. Materials and Methods
2.1. Chemicals
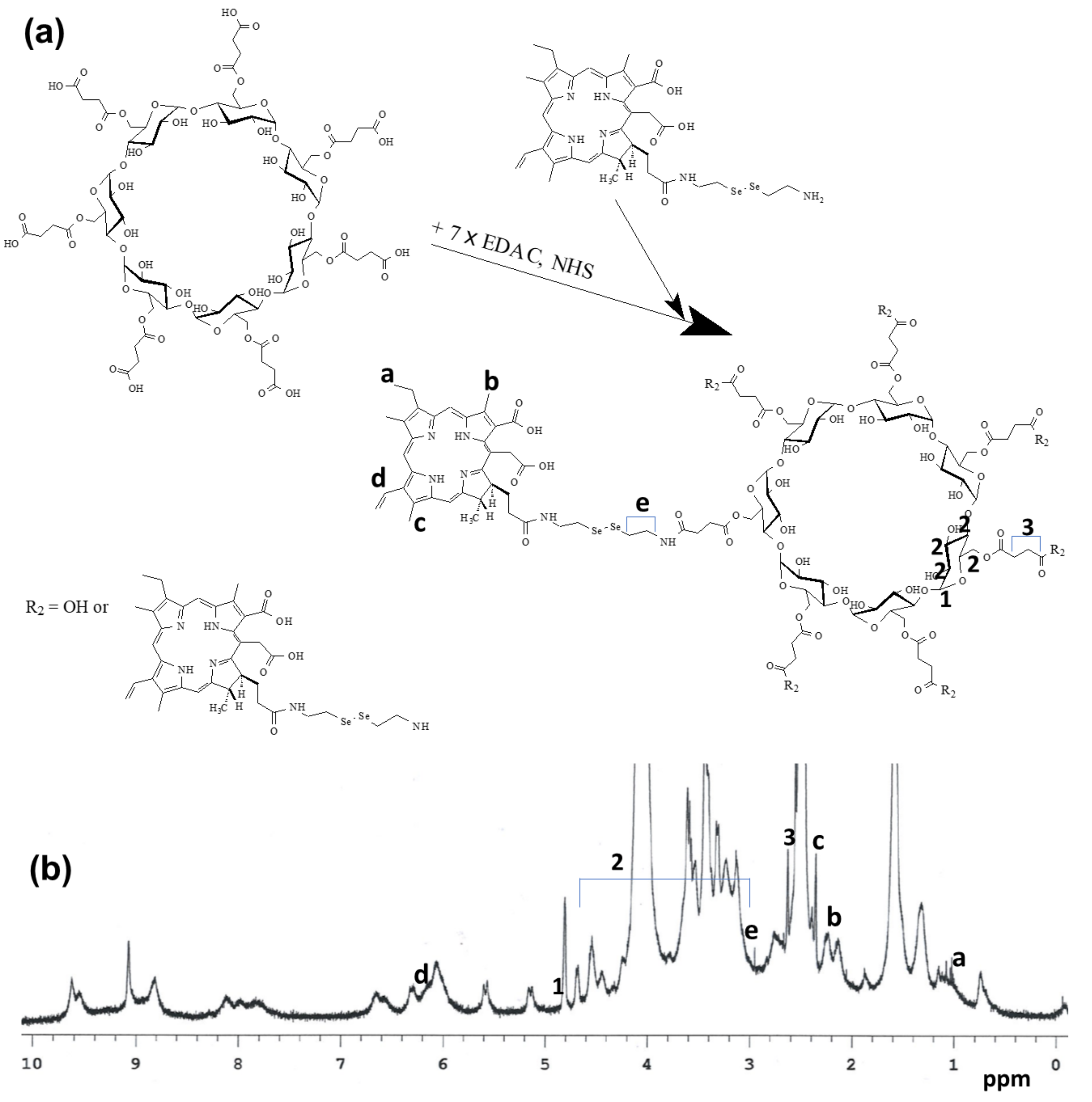
2.2. Synthesis of FaPEGbCDseseCe6 Conjugates
2.3. 1H Nuclear Magnetic Resonance (NMR) Spectra
2.4. Fabrication of FaPEGbCDseseCe6 Nanophotosensitizers
2.5. Transmission Electron Microscope
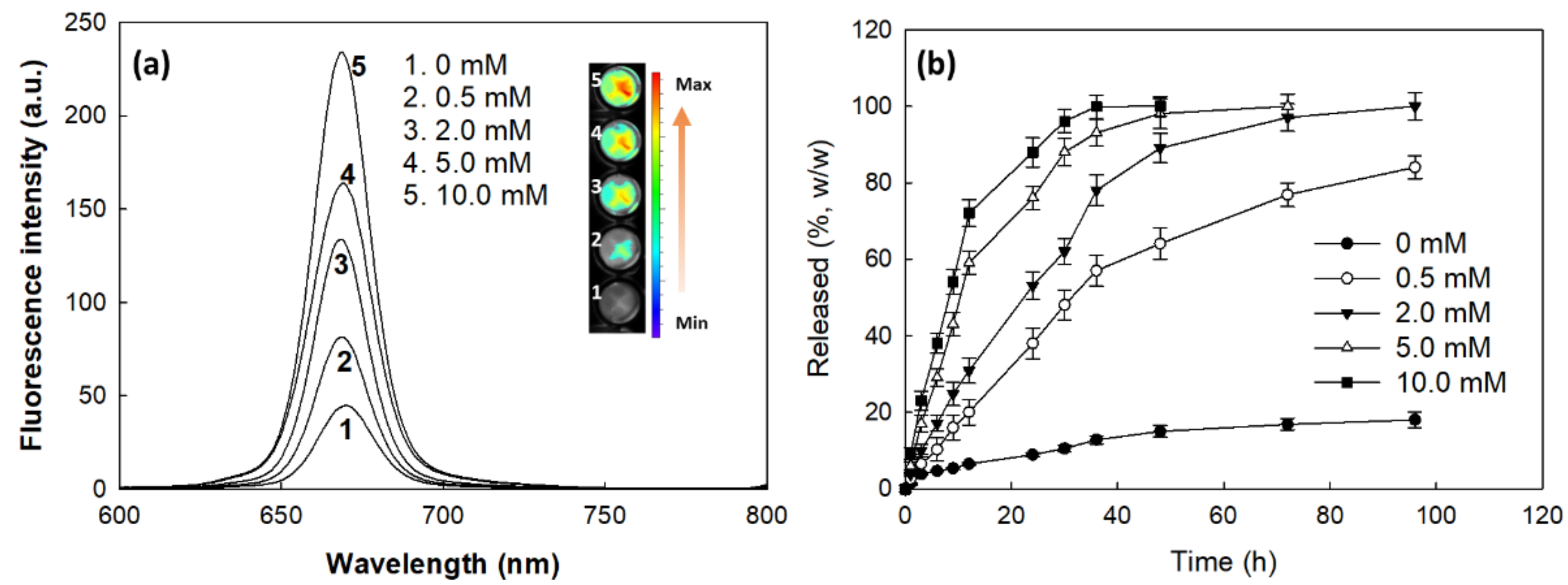
2.6. Fluorescence Spectrophotometer Measurement
2.7. Drug Release from Nanophotosensitizer
2.8. Cell Culture
2.9. Light Source for PDT Treatment
2.10. PDT Treatment of Cancer Cells
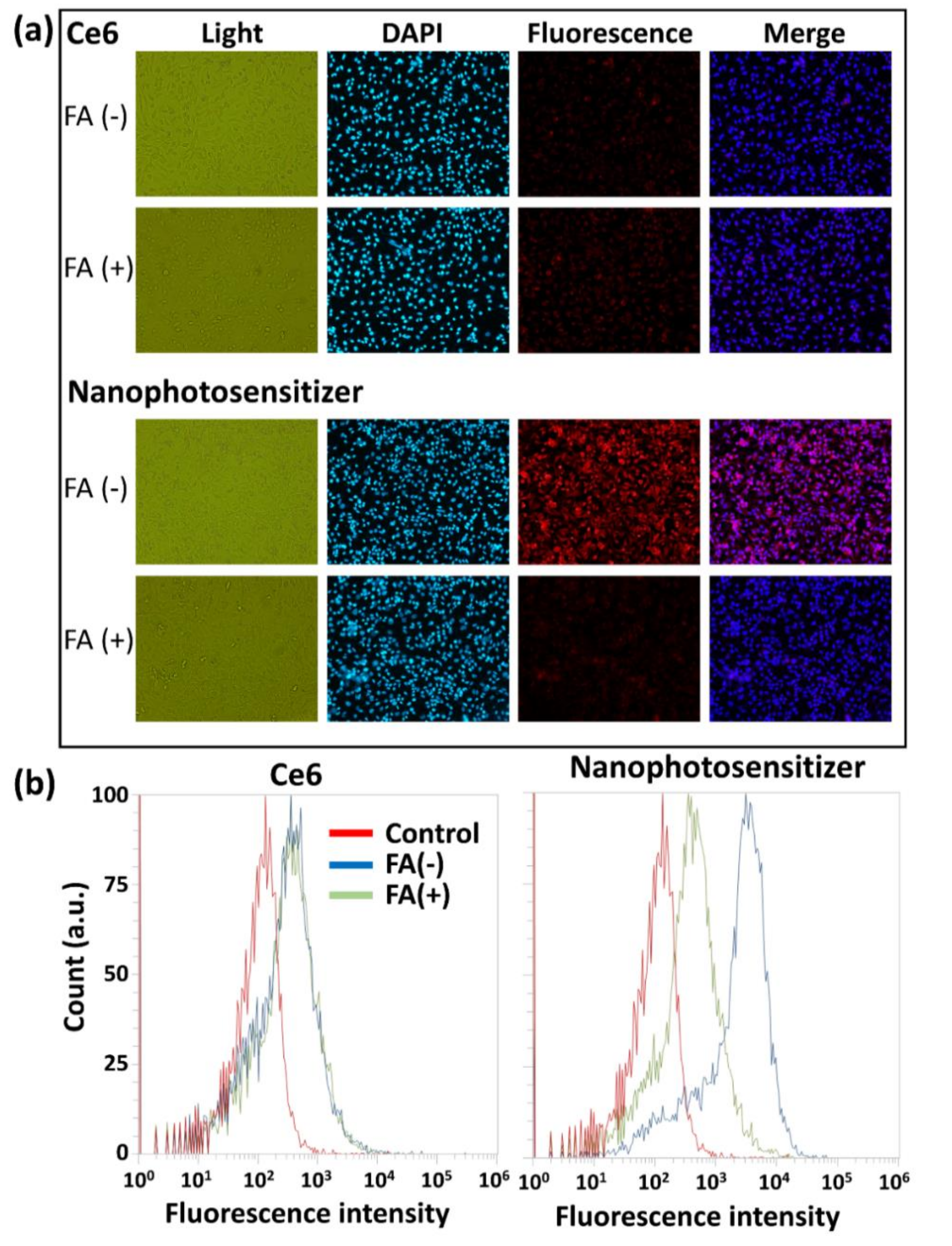
2.11. Intracellular Uptake of Nanophotosensitizers
2.12. Fluorescence Microscopy
2.13. Flow Cytometry
2.14. ROS Generation Assay
2.15. In Vivo Fluorescence Imaging and PDT Study
2.16. Statistical Analysis
3. Results
3.1. Synthesis of FaPEGbCDseseCe6 Conjugates
3.2. Fabrication and Characterization of Nanophotosensitizers of FaPEGbCDseseCe6 Conjugates
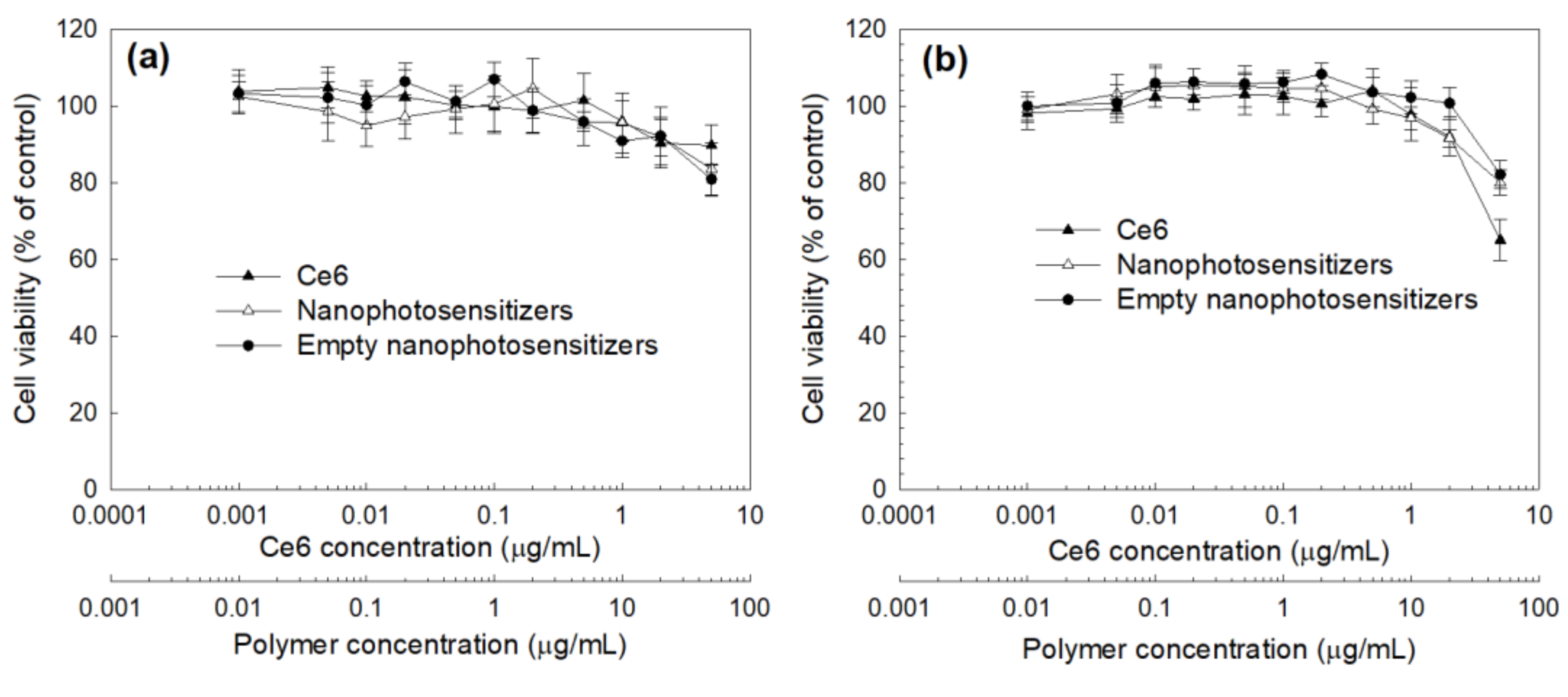
3.3. Cell Culture and PDT Study In Vitro
3.4. In Vivo Animal Tumoxenograft Study Using HeLa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Bosch, F.X.; de Sanjosé, S. The epidemiology of human papillomavirus infection and cervical cancer. Dis. Markers 2007, 23, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Naga Ch, P.; Gurram, L.; Chopra, S.; Mahantshetty, U. The management of locally advanced cervical cancer. Curr. Opin. Oncol. 2018, 30, 323–329. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mönig, S.; Chevallay, M.; Niclauss, N.; Zilli, T.; Fang, W.; Bansal, A.; Hoeppner, J. Early esophageal cancer: The significance of surgery, endoscopy, and chemoradiation. Ann. N. Y. Acad. Sci. 2018, 1434, 115–123. [Google Scholar] [CrossRef]
- Sun, L.; Sheng, X.; Jiang, J.; Li, X.; Liu, N.; Liu, Y.; Zhang, T.; Li, D.; Zhang, X.; Wei, P. Surgical morbidity and oncologic results after concurrent chemoradiation therapy for advanced cervical cancer. Int. J. Gynaecol. Obstet. 2014, 125, 111–115. [Google Scholar] [CrossRef]
- Favero, G.; Pierobon, J.; Genta, M.L.; Araújo, M.P.; Miglino, G.; Del Carmen Pilar Diz, M.; de Andrade Carvalho, H.; Fukushima, J.T.; Baracat, E.C.; Carvalho, J.P. Laparoscopic extrafascial hysterectomy (completion surgery) after primary chemoradiation in patients with locally advanced cervical cancer: Technical aspects and operative outcomes. Int. J. Gynecol. Cancer. 2014, 24, 608–614. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wu, W.; Li, H. Vaginal delivery of carboplatin-loaded thermosensitive hydrogel to prevent local cervical cancer recurrence in mice. Drug Deliv. 2016, 23, 3544–3551. [Google Scholar] [CrossRef]
- He, D.; Duan, C.; Chen, J.; Lai, L.; Chen, J.; Chen, D. The safety and efficacy of the preoperative neoadjuvant chemotherapy for patients with cervical cancer: A systematic review and meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 14693–14700. [Google Scholar]
- Wang, Y.; Wang, G.; Wei, L.H.; Huang, L.H.; Wang, J.L.; Wang, S.J.; Li, X.P.; Shen, D.H.; Bao, D.M.; Gao, J. Neoadjuvant chemotherapy for locally advanced cervical cancer reduces surgical risks and lymph-vascular space involvement. Chin. J. Cancer 2011, 30, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kang, S.B.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S. Comparison of chemoradiation with radiation as postoperative adjuvant therapy in cervical cancer patients with intermediate-risk factors. Eur. J. Surg. Oncol. 2009, 35, 192–196. [Google Scholar] [CrossRef]
- Rogers, L.; Siu, S.S.; Luesley, D.; Bryant, A.; Dickinson, H.O. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst. Rev. 2012, 5, CD007583. [Google Scholar] [CrossRef] [Green Version]
- Takekuma, M.; Kasamatsu, Y.; Kado, N.; Kuji, S.; Tanaka, A.; Takahashi, N.; Abe, M.; Hirashima, Y. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I-II cervical cancer: A review. J Obstet. Gynaecol. Res. 2017, 43, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetta, G.; Fei, F.; Mangioni, C. Chemotherapy with paclitaxel, ifosfamide, and cisplatin for the treatment of squamous cell cervical cancer: The experience of Monza. Semin. Oncol. 2000, 27, 23–27. [Google Scholar]
- Chung, C.W.; Kim, C.H.; Choi, K.H.; Yoo, J.J.; Kim, D.H.; Chung, K.D.; Jeong, Y.I.; Kang, D.H. Effect of surfactant on 5-aminolevulinic acid uptake and PpIX generation in human cholangiocarcinoma cell. Eur. J. Pharm. Biopharm. 2012, 80, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics. 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [Green Version]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light technology for efficient and effective photodynamic therapy: A critical review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Yoo, J.J.; Kim, C.; Chung, C.W.; Jeong, Y.I.; Kang, D.H. 5-aminolevulinic acid-incorporated poly(vinyl alcohol) nanofiber-coated metal stent for application in photodynamic therapy. Int. J. Nanomedicine 2012, 7, 1997–2005. [Google Scholar]
- Apalla, Z.; Sotiriou, E.; Chovarda, E.; Lefaki, I.; Devliotou-Panagiotidou, D.; Ioannides, D. Skin cancer: Preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br. J. Dermatol. 2010, 162, 171–175. [Google Scholar] [CrossRef]
- Ryu, J.H.; Jeong, Y.I.; Kim, H.Y.; Son, G.M.; Lee, H.L.; Chung, C.W.; Chu, C.W.; Kang, D.H. Enhanced photosensing and photodynamic treatment of colon cancer cells using methoxy poly(ethylene glycol)-conjugated chlorin e6. J. Nanosci. Nanotechnol. 2018, 18, 1131–1136. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Ali, M.Y.; Tariq, I.; Pinnapireddy, S.R.; Duse, L.; Goergen, N.; Wölk, C.; Hause, G.; Jedelská, J.; et al. Wavelength dependent photo-cytotoxicity to ovarian carcinoma cells using temoporfin loaded tetraether liposomes as efficient drug delivery system. Eur. J. Pharm. Biopharm. 2020, 150, 50–65. [Google Scholar] [CrossRef]
- Nath, S.; Saad, M.A.; Pigula, M.; Swain, J.W.R.; Hasan, T. Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease. Cancers 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, B.J.; Brewer, C.; Van Nostrand, K.; Berns, M.W.; McCullough, J.L.; Tadir, Y.; Manetta, A. Photodynamic therapy using topically applied dihematoporphyrin ether in the treatment of cervical intraepithelial neoplasia. Gynecol. Oncol. 1997, 64, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Wierrani, F.; Kubin, A.; Jindra, R.; Henry, M.; Gharehbaghi, K.; Grin, W.; Söltz-Szötz, J.; Alth, G.; Grünberger, W. 5-aminolevulinic acid-mediated photodynamic therapy of intraepithelial neoplasia and human papillomavirus of the uterine cervix--a new experimental approach. Cancer Detect. Prev. 1999, 23, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Trushina, O.I.; Novikova, E.G.; Sokolov, V.V.; Filonenko, E.V.; Chissov, V.I.; Vorozhtsov, G.N. Photodynamic therapy of virus-associated precancer and early stages cancer of cervix uteri. Photodiagnosis Photodyn. Ther. 2008, 5, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Rosin, F.C.P.; Teixeira, M.G.; Pelissari, C.; Corrêa, L. Resistance of oral cancer cells to 5-ALA-mediated photodynamic therapy. J. Cell Biochem. 2018, 119, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Muto, M.; Yoshimura, K.; Niimi, M.; Ezoe, Y.; Yoda, Y.; Yamamoto, Y.; Nishisaki, H.; Higashino, K.; Iishi, H. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat. Oncol. 2012, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Liu, Q.; Liang, Z.; Wang, J.; Pang, M.; Huang, W.; Wu, W.; Hong, Z. Synthesis and biological evaluation of peptide-conjugated phthalocyanine photosensitizers with highly hydrophilic modifications. Org. Biomol. Chem. 2016, 14, 3409–3422. [Google Scholar] [CrossRef]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles loading porphyrin sensitizers in improvement of photodynamic therapy for ovarian cancer. Photodiagnosis Photodyn. Ther. 2020, 33, 102156. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Jeong, Y.I.; Kim, D.H.; Kwak, T.W.; Chung, C.W.; Kim, C.H.; Kang, D.H. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int. J. Pharm. 2013, 454, 74–81. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Cha, B.; Lee, H.L.; Song, Y.H.; Jung, Y.H.; Kwak, T.W.; Choi, C.; Jeong, G.W.; Nah, J.W.; Kang, D.H. Simple nanophotosensitizer fabrication using water-soluble chitosan for photodynamic therapy in gastrointestinal cancer cells. Int. J. Pharm. 2017, 532, 194–203. [Google Scholar] [CrossRef]
- Sugumaran, A.; Mathialagan, V. Colloidal nanocarriers as versatile targeted delivery systems for cervical cancer. Curr. Pharm. Des. 2020, 26, 5174–5187. [Google Scholar] [CrossRef]
- Ben Mihoub, A.; Larue, L.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of cyclodextrins in anticancer photodynamic therapy treatment. Molecules. 2018, 23, 1936. [Google Scholar] [CrossRef] [Green Version]
- Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Vanderesse, R.; Acherar, S. Inclusion complex vs. conjugation of hydrophobic photosensitizers with β-cyclodextrin: Improved disaggregation and photodynamic therapy efficacy against glioblastoma cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110604. [Google Scholar] [CrossRef]
- Loftsson, T. Drug permeation through biomembranes: Cyclodextrins and the unstirred water layer. Pharmazie 2012, 67, 363–370. [Google Scholar] [PubMed]
- Lee, H.M.; Chung, C.W.; Kim, C.H.; Kim, D.H.; Kwak, T.W.; Jeong, Y.I.; Kang, D.H. Defensive mechanism in cholangiocarcinoma cells against oxidative stress induced by chlorin e6-based photodynamic therapy. Drug. Des. Devel. Ther. 2014, 8, 1451–1462. [Google Scholar] [PubMed] [Green Version]
- Hirsjärvi, S.; Passirani, C.; Benoit, J.P. Passive and active tumor targeting with nanocarriers. Curr. Drug Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumor microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- de Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in cancer Heterogeneity and Therapy. Oxid. Med. Cell Longev 2017, 2017, 2467940. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Glass, S.B.; Gonzalez-Fajardo, L.; Beringhs, A.O.; Lu, X. Redox potential and ROS-mediated nanomedicines for improving cancer therapy. Antioxid. Redox Signal. 2019, 30, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Ryu, H.; Kim, D.H.; Cheng, W.N.; Yoon, J.E.; Kang, S.; Han, S.G. Piperlongumine induces cell cycle arrest via reactive oxygen species accumulation and IKKβ suppression in human breast cancer cells. Antioxidants 2019, 8, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.L.; Hwang, S.C.; Nah, J.W.; Kim, J.; Cha, B.; Kang, D.H.; Jeong, Y.I. Redox- and pH-responsive nanoparticles release piperlongumine in a stimuli-sensitive manner to inhibit pulmonary metastasis of colorectal carcinoma cells. J. Pharm. Sci. 2018, 107, 2702–2712. [Google Scholar] [CrossRef]
- Wei, C.; Liang, B.; Li, Y.; Yan, B.; Zhou, Y.; Liu, Y.; Lang, M. A drug-free therapeutic system for cancer therapy by diselenide-based polymers themselves. Adv. Healthc. Mater. 2021, 10, e2001471. [Google Scholar] [CrossRef]
- Manda, G.; Isvoranu, G.; Comanescu, M.V.; Manea, A.; Debelec Butuner, B.; Korkmaz, K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015, 5, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Casas, A.; Di Venosa, G.; Hasan, T.; Batlle, A. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, Y.; Wu, J.; Duncanm, R.; Strohalm, J.; Ulbrich, K.; Akaike, T.; Maeda, H. Early phase tumor accumulation of macromolecules: A great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998, 89, 307–314. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Daruwalla, J.; Nikfarjam, M.; Greish, K.; Malcontenti-Wilson, C.; Muralidharan, V.; Christophi, C.; Maeda, H. In vitro and in vivo evaluation of tumor targeting styrene-maleic acid copolymer-pirarubicin micelles: Survival improvement and inhibition of liver metastases. Cancer Sci. 2010, 101, 1866–1874. [Google Scholar] [CrossRef]
- Youn, Y.S.; Bae, Y.H. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv, Rev. 2018, 130, 3–11. [Google Scholar] [CrossRef]
- Liu, C.; Ding, L.; Bai, L.; Chen, X.; Kang, H.; Hou, L.; Wang, J. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem. Biophys. Res. Commun. 2017, 491, 1083–1091. [Google Scholar] [CrossRef]
- Roy, A.G.; Robinson, J.M.; Sharma, P.; Rodriguez-Garcia, A.; Poussin, M.A.; Nickerson-Nutter, C.; Powell, D.J., Jr. Folate receptor beta as a direct and indirect target for antibody-based cancer immunotherapy. Int. J. Mol. Sci. 2021, 22, 5572. [Google Scholar] [CrossRef]
- Bai, L.X.; Ding, L.; Jiang, S.W.; Kang, H.J.; Gao, C.F.; Chen, C.; Zhou, Q.; Wang, J.T. Down-regulation of FRα inhibits proliferation and promotes apoptosis of cervical cancer cells in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 5667–5672. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Li, Y.; Lv, Q.; Chen, F.; Li, B.; Zhang, Z.; Guo, H.; Lu, D.; Wang, F.; et al. Evaluation of folate receptor-mediated tumor detection as a triage tool in cervical cancer screening. Int. J. Gynecol. Obstet. 2020, 150, 379–384. [Google Scholar] [CrossRef]
- Lee, S.J.; Shim, Y.H.; Oh, J.S.; Jeong, Y.I.; Park, I.K.; Lee, H.C. Folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide) graft copolymer nanoparticles for folate-receptor-mediated drug delivery. Nanoscale Res. Lett. 2015, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Tsubone, T.M.; Zhang, Z.; Goyal, R.; Santacruz, C.; Martins, W.K.; Kohnm, J.; Baptista, M.S. Porphyrin-loaded TyroSpheres for the intracellular delivery of drugs and photoinduced oxidant species. Mol. Pharm. 2020, 17, 2911–2924. [Google Scholar] [CrossRef]
- Martins, W.K.; Santos, N.F.; Rocha, C.D.S.; Bacellar, I.O.L.; Tsubone, T.M.; Viotto, A.C.; Matsukuma, A.Y.; Abrantes, A.B.D.P.; Siani, P.; Dias, L.; et al. Parallel damage in mitochondria and lysosomes is an efficient way to photoinduce cell death. Autophagy 2019, 15, 259–279. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, M.W.; Jeong, Y.-I.; Yang, H.S. Redox-Sensitive and Folate-Receptor-Mediated Targeting of Cervical Cancer Cells for Photodynamic Therapy Using Nanophotosensitizers Composed of Chlorin e6-Conjugated ?-Cyclodextrin via Diselenide Linkage. Cells 2021, 10, 2190. https://doi.org/10.3390/cells10092190
Kim H, Kim MW, Jeong Y-I, Yang HS. Redox-Sensitive and Folate-Receptor-Mediated Targeting of Cervical Cancer Cells for Photodynamic Therapy Using Nanophotosensitizers Composed of Chlorin e6-Conjugated ?-Cyclodextrin via Diselenide Linkage. Cells. 2021; 10(9):2190. https://doi.org/10.3390/cells10092190
Chicago/Turabian StyleKim, Howard, Mi Woon Kim, Young-IL Jeong, and Hoe Saeng Yang. 2021. "Redox-Sensitive and Folate-Receptor-Mediated Targeting of Cervical Cancer Cells for Photodynamic Therapy Using Nanophotosensitizers Composed of Chlorin e6-Conjugated ?-Cyclodextrin via Diselenide Linkage" Cells 10, no. 9: 2190. https://doi.org/10.3390/cells10092190
APA StyleKim, H., Kim, M. W., Jeong, Y.-I., & Yang, H. S. (2021). Redox-Sensitive and Folate-Receptor-Mediated Targeting of Cervical Cancer Cells for Photodynamic Therapy Using Nanophotosensitizers Composed of Chlorin e6-Conjugated ?-Cyclodextrin via Diselenide Linkage. Cells, 10(9), 2190. https://doi.org/10.3390/cells10092190





