Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond
Abstract
1. Introduction
1.1. Clinical Significance of Iron Homeostasis
1.2. Iron Transport and Cellular Uptake
2. Mitochondrial Iron Trafficking
2.1. Iron Delivery to Mitochondria
2.2. Iron Transport at the Outer Mitochondrial Membrane
2.3. Iron Transport at the Inner Mitochondrial Membrane
3. Sites of Mitochondrial Iron Utilization
3.1. Mitochondrial Iron Storage
3.2. Mitochondrial Iron–Sulfur Cluster Biogenesis and Transport
3.3. Heme Biosynthesis and Transport
4. Iron Export from Mitochondria
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2020, 513, 6–12. [Google Scholar] [CrossRef]
- Puig, S.; Askeland, E.; Thiele, D.J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 2005, 120, 99–110. [Google Scholar] [CrossRef]
- Siekevitz, P. Powerhouse of the cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Van der Bliek, A.; Sedensky, M.M.; Morgan, P.G. Cell biology of the mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ristow, M. Mitochondria and metabolic homeostasis. Antioxid. Redox Signal 2013, 19, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Swenson, S.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From synthesis to utilization: The ins and outs of mitochondrial heme. Cells 2020, 9, 579. [Google Scholar] [CrossRef]
- Braymer, J.J.; Lill, R. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Cloonan, S.M. Mitochondrial iron in human health and disease. Annu. Rev. Physiol. 2019, 81, 453–482. [Google Scholar] [CrossRef]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and iron: Current questions. Expert Rev. Hematol. 2016, 10, 65–79. [Google Scholar] [CrossRef]
- Walker, E.M.; Walker, S.M. Review: Effects of iron overload on the immune system. Ann. Clin. Labr. Sci. 2000, 30, 354–365. [Google Scholar]
- Rivella, S. Iron metabolism under conditions of ineffective erythropoiesis in beta-Thalassemia. Blood 2019, 133, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Gambale, A.; Iolascon, A.; Andolfo, I.; Russo, R. Diagnosis and management of congenital dyserythropoietic anemias. Expert Rev. Hematol. 2016, 9, 283–296. [Google Scholar] [CrossRef]
- Ducamp, S.; Fleming, M.D. The molecular genetics of sideroblastic anemia. Blood 2019, 133, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Vallumsetla, N.; Rachmilewitz, E.; Verma, A.; Ginzburg, Y. Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood 2014, 124, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.T.; Shang, M.; Wu, R.; Chang, H.; Khechaduri, A.; Sato, T.; Kamide, C.; Liu, T.; Prasad, S.V.N.; Ardehali, H. Increased heme levels in the heart lead to exacerbated ischemic injury. J. Am. Heart Assoc. 2015, 4, e002272. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Wu, R.; Shang, M.; Sato, T.; Chen, C.; Shapiro, J.S.; Liu, T.; Thakur, A.; Sawicki, K.T.; Prasad, S.V.N.; et al. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol. Med. 2016, 8, 247–267. [Google Scholar] [CrossRef]
- Tang, M.; Chen, Z.; Wu, D.; Chen, L. Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases. J. Cell. Physiol. 2018, 233, 9179–9190. [Google Scholar] [CrossRef]
- Horowitz, M.P.; Greenamyre, J.T. Mitochondrial iron metabolism and its role in neurodegeneration. J. Alzheimer’s Dis. 2010, 20, S551–S568. [Google Scholar] [CrossRef]
- Richardson, D.R.; Huang, M.L.; Whitnall, M.; Becker, E.M.; Ponka, P.; Rahmanto, Y.S. The ins and outs of mitochondrial iron-loading: The metabolic defect in Friedreich’s ataxia. J. Mol. Med. 2009, 88, 323–329. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. BioFactors 2019, 46, 5–20. [Google Scholar] [CrossRef]
- Anzovino, A.; Lane, D.J.R.; Huang, M.L.-H.; Richardson, D.R. Fixing frataxin: ‘Ironing out’ the metabolic defect in Friedreich’s ataxia. Br. J. Pharmac. 2014, 171, 2174–2190. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, Y.; Carrasco, C.M.; Campos, J.D.; Aguirre, P.; Núñez, M.T. Parkinson’s disease: The mitochondria-iron link. Parkinson’s Dis. 2016, 2016, 7049108. [Google Scholar] [CrossRef] [PubMed]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef]
- Simcox, J.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef]
- González-Domínguez, A.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron metabolism in obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, L.M.; Rivella, S. Cancer cells with irons in the fire. Free Radic. Biol. Med. 2014, 79, 337–342. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Favaro, E.; Ramachandran, A.; McCormick, R.; Gee, H.; Blancher, C.; Crosby, M.; Devlin, C.; Blick, C.; Buffa, F.; Li, J.-L.; et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS ONE 2010, 5, e10345. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-I.; Schwartz, J.M.; Maldonado, E.N.; Lemasters, J.J.; Nieminen, A.-L. Mitoferrin-2-dependent mitochondrial iron uptake sensitizes human head and neck squamous carcinoma cells to photodynamic therapy. J. Biol. Chem. 2013, 288, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hooda, J.; Cadinu, D.; Alam, M.; Shah, A.; Cao, T.M.; Sullivan, L.A.; Brekken, R.; Zhang, L. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS ONE 2013, 8, e63402. [Google Scholar] [CrossRef]
- Puy, H.; Gouya, L.; Deybach, J.-C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Rouault, T.A.; Tong, W.H. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008, 24, 398–407. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Ta, N.; Zhang, T.; Gao, W. The multifaceted regulation of mitochondria in ferroptosis. Life 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Chevion, M. A site-specific mechanism for free radical induced biological damage: The essential role of redox-active transition metals. Free Radic. Biol. Med. 1988, 5, 27–37. [Google Scholar] [CrossRef]
- Reddi, A.R.; Hamza, I. Heme mobilization in animals: A metallolipid’s journey. Acc. Chem. Res. 2016, 49, 1104–1110. [Google Scholar] [CrossRef]
- Duffy, S.P.; Shing, J.; Saraon, P.; Berger, L.C.; Eiden, M.V.; Wilde, A.; Tailor, C.S. The fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol. Cell. Biol. 2010, 30, 5318–5324. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.; Andrews, N. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Lawen, A.; Lane, D.J. Mammalian iron homeostasis in health and disease: Uptake, storage, transport, and molecular mechanisms of action. Antioxid. Redox Signal. 2013, 18, 2473–2507. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Ponka, P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1997, 1331, 1–40. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Kwok, E.Y.; Severance, S.; Kosman, D.J. Evidence for iron channeling in the Fet3p−Ftr1p high-affinity iron uptake complex in the yeast plasma membrane. Biochemistry 2006, 45, 6317–6327. [Google Scholar] [CrossRef]
- Singh, A.; Severance, S.; Kaur, N.; Wiltsie, W.; Kosman, D.J. Assembly, activation, and trafficking of the Fet3p-Ftr1p high affinity iron permease complex in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 13355–13364. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Peng, J.-B.; Cohen, A.; Nelson, H.; Nelson, N.; Hediger, M.A. Yeast SMF1 mediates H+-coupled iron uptake with concomitant uncoupled cation currents. J. Biol. Chem. 1999, 274, 35089–35094. [Google Scholar] [CrossRef]
- Dix, D.; Bridgham, J.; Broderius, M.; Wide, D. Characterization of the FET4 protein of yeast: Evidence for a direct role in the transport of iron. J. Biol. Chem. 1997, 272, 11770–11777. [Google Scholar] [CrossRef]
- Moore, R.E.; Kim, Y.; Philpott, C.C. The mechanism of ferrichrome transport through Arn1p and its metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2003, 100, 5664–5669. [Google Scholar] [CrossRef]
- Li, F.-Y.; Nikali, K.; Gregan, J.; Leibiger, I.; Leibiger, B.; Schweyen, R.; Larsson, C.; Suomalainen-Wartiovaara, A. Characterization of a novel human putative mitochondrial transporter homologous to the yeast mitochondrial RNA splicing proteins 3 and 4. FEBS Lett. 2001, 494, 79–84. [Google Scholar] [CrossRef]
- Philpott, C.C.; Ryu, M.-S. Special delivery: Distributing iron in the cytosol of mammalian cells. Front. Pharmacol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Arosio, P.; Levi, S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 783–792. [Google Scholar] [CrossRef]
- Ferreira, C.; Bucchini, D.; Martin, M.-E.; Levi, S.; Arosio, P.; Grandchamp, B.; Beaumont, C. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 2000, 275, 3021–3024. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Merlot, A.M.; Huang, M.H.-L.; Bae, D.-H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. Cellular iron uptake, trafficking, and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta 2015, 1853, 1130–1144. [Google Scholar] [CrossRef]
- Blaiseau, P.; Seguin, A.; Camadro, J.-M.; Lesuisse, E. Iron uptake in yeasts. In Iron Uptake and Homeostasis in Microorganisms; Caister Academic Press: Poole, UK, 2011. [Google Scholar]
- Anderson, G.J.; Vulpe, C.D. Mammalian iron transport. Cell. Mol. Life Sci. 2009, 66, 3241–3261. [Google Scholar] [CrossRef]
- Chen, C.; Paw, B.H. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Ryu, M.-S.; Frey, A.; Patel, S. Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 2017, 292, 12764–12771. [Google Scholar] [CrossRef]
- Vogt, A.-C.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.; Galy, B.; Hentze, M. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Rauen, U.; Springer, A.; Weisheit, D.; Petrat, F.; Korth, H.-G.; De Groot, H.; Sustmann, R. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. ChemBioChem 2007, 8, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Sheftel, A.D.; Kim, S.F.; Ponka, P.; Brown, P.; Levis, M.; Shurtleff, S.; Campana, D.; Downing, J.; Small, D. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood 2005, 105, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.; Sloan, K.E.; Ranjan, N.; Warda, A.S.; Kretschmer, J.; Blessing, C.; Hübner, B.; Seikowski, J.; Dennerlein, S.; Rehling, P.; et al. NSUN3 and ABH1 modify the wobble position of mt-tRNA Met to expand codon recognition in mitochondrial translation. EMBO J. 2016, 35, 2104–2119. [Google Scholar] [CrossRef]
- Fu, D.; Jordan, J.J.; Samson, L.D. Human ALKBH7 is required for alkylation and oxidation-induced programmed necrosis. Genes Dev. 2013, 27, 1089–1100. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 650–656. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends Biochem. Sci. 2015, 41, 274–286. [Google Scholar] [CrossRef]
- Muckenthaler, M.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Mims, M.P.; Guan, Y.; Pospisilova, D.; Horvathova, M.; Indrak, K.; Ponka, P.; Divoky, V.; Prchal, J.T. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 2005, 105, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; D’Apolito, M.; Servedio, V.; Cimmino, F.; Piga, A.; Camaschella, C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2). Blood 2006, 107, 349–354. [Google Scholar] [CrossRef]
- Beaumont, C.; Delaunay, J.; Hetet, G.; Grandchamp, B.; De Montalembert, M.; Tchernia, G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood 2006, 107, 4168–4170. [Google Scholar] [CrossRef]
- Hildick-Smith, G.J.; Cooney, J.D.; Garone, C.; Kremer, L.S.; Haack, T.B.; Thon, J.N.; Miyata, N.; Lieber, D.S.; Calvo, S.E.; Akman, H.O.; et al. Macrocytic anemia and mitochondriopathy resulting from a defect in sideroflexin 4. Am. J. Hum. Genet. 2013, 93, 906–914. [Google Scholar] [CrossRef]
- Farhan, S.M.K.; Wang, J.; Robinson, J.F.; Lahiry, P.; Siu, V.M.; Prasad, C.; Kronick, J.B.; Ramsay, D.A.; Rupar, C.A.; Hegele, R.A. Exome sequencing identifies NFS1 deficiency in a novel Fe-S cluster disease, infantile mitochondrial complex II/III deficiency. Mol. Genet. Genom. Med. 2014, 2, 73–80. [Google Scholar] [CrossRef]
- Hershkovitz, T.; Kurolap, A.; Tal, G.; Paperna, T.; Mory, A.; Staples, J.; Brigatti, K.W.; Gonzaga-Jauregui, C.; Dumin, E.; Saada, A.; et al. A recurring NFS1 pathogenic variant causes a mitochondrial disorder with variable intra-familial patient outcomes. Mol. Genet. Metab. Rep. 2020, 26, 100699. [Google Scholar] [CrossRef]
- Lim, S.C.; Friemel, M.; Marum, J.E.; Tucker, E.; Bruno, D.L.; Riley, L.G.; Christodoulou, J.; Kirk, E.; Boneh, A.; DeGennaro, C.M.; et al. Mutations in LYRM4, encoding iron–sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum. Mol. Genet. 2013, 22, 4460–4473. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Molto, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; De Michele, G.; Cavalcanti, F.; Painese, L.; Perretti, A.; Santoro, L.; Vita, G.; Toscano, A.; Amboni, M.; Grimaldi, G.; et al. Why do some Friedreich’s ataxia patients retain tendon reflexes? J. Neurol. 1999, 246, 353–357. [Google Scholar] [CrossRef]
- Bidichandani, I.S.; Ashizawa, T.; Patel, P. Atypical Friedreich ataxia caused by compound heterozygosity for a novel missense mutation and the GAA triplet-repeat expansion. Am. J. Hum. Genet. 1997, 60, 1251–1256. [Google Scholar]
- Zühlke, C.; Laccone, F.; Cossée, M.; Kohlschütter, A.; Koenig, M.; Schwinger, E. Mutation of the start codon in the FRDA1 gene: Linkage analysis of three pedigrees with the ATG to ATT transversion points to a unique common ancestor. Qual. Life Res. 1998, 103, 102–105. [Google Scholar] [CrossRef]
- Cossee, M.; Dürr, A.; Schmitt, M.; Dahl, N.; Trouillas, P.; Kostrzewa, M.; Nivelon-Chevallier, A.; Gustavson, K.H.; Kohlschütter, A.; Müller, U.; et al. Friedreich’s ataxia: Point mutations and clinical presentation of compound heterozygotes. Ann. Neurol. 1999, 45, 200–206. [Google Scholar] [CrossRef]
- Gellera, C.; Castellotti, B.; Mariotti, C.; Mineri, R.; Seveso, V.; DiDonato, S.; Taroni, F. Frataxin gene point mutations in Italian Friedreich ataxia patients. Neurogenetics 2007, 8, 289–299. [Google Scholar] [CrossRef]
- Evans-Galea, M.V.; Corben, L.A.; Hasell, J.; Galea, C.A.; Fahey, M.C.; Du Sart, D.; Delatycki, M.B. A novel deletion–insertion mutation identified in exon 3 of FXN in two siblings with a severe Friedreich ataxia phenotype. Neurogenetics 2011, 12, 307–313. [Google Scholar] [CrossRef]
- Mochel, F.; Knight, M.A.; Tong, W.-H.; Hernandez, D.; Ayyad, K.; Taivassalo, T.; Andersen, P.M.; Singleton, A.; Rouault, T.A.; Fischbeck, K.H.; et al. Splice Mutation in the iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am. J. Hum. Genet. 2008, 82, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Lind, L.; Thornell, L.-E.; Holmberg, M. Myopathy with lactic acidosis is linked to chromosome 12q23.3-24.11 and caused by an intron mutation in the ISCU gene resulting in a splicing defect. Hum. Mol. Genet. 2008, 17, 1666–1672. [Google Scholar] [CrossRef]
- Sanaker, P.S.; Toompuu, M.; Hogan, V.E.; He, L.; Tzoulis, C.; Chrzanowska-Lightowlers, Z.M.; Taylor, R.W.; Bindoff, L.A. Differences in RNA processing underlie the tissue specific phenotype of ISCU myopathy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kollberg, G.; Tulinius, M.; Melberg, A.; Darin, N.; Andersen, O.; Holmgren, D.; Oldfors, A.; Holme, E. Clinical manifestation and a new ISCU mutation in iron–sulphur cluster deficiency myopathy. Brain 2009, 132, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Spiegal, R.; Saada, A.; Halvardson, J.; Soiferman, D.; Shaag, A.; Edvardson, S.; Horovitz, Y.; Khayat, M.; Shalev, S.A.; Feuk, L.; et al. Deleterious mutation in FDX1L gene is associated with a novel mitochondrial muscle myopathy. Eur. J. Hum. Genet. 2014, 22, 902–906. [Google Scholar] [CrossRef]
- Gurgel-Giannetti, J.; Lynch, D.S.; De Paiva, A.R.B.; Lucato, L.; Yamamoto, G.; Thomsen, C.; Basu, S.; Freua, F.; Giannetti, A.V.; Assis, B.D.R.D.; et al. A novel complex neurological phenotype due to a homozygous mutation in FDX2. Brain 2018, 141, 2289–2298. [Google Scholar] [CrossRef]
- Paul, A.; Drecourt, A.; Petit, F.; Deguine, D.D.; Vasnier, C.; Oufadem, M.; Masson, C.; Bonnet, C.; Masmoudi, S.; Mosnier, I.; et al. FDXR Mutations cause sensorial neuropathies and expand the spectrum of mitochondrial Fe-S-synthesis diseases. Am. J. Hum. Genet. 2017, 101, 630–637. [Google Scholar] [CrossRef]
- Van Waveren, H.G.D.; van Roermund, H.P.; Snijders, P.J. Hereditary sideroblastic anaemia and autosomal inheritance of erythrocyte dimorphism in a Dutch family. Eur. J. Haemat. 1987, 38, 405–409. [Google Scholar] [CrossRef]
- Schmitz-Abe, K.; Ciesielski, S.; Schmidt, P.J.; Campagna, D.R.; Rahimov, F.; Schilke, B.A.; Cuijpers, M.; Rieneck, K.; Lausen, B.; Linenberger, M.L.; et al. Congenital sideroblastic anemia due to mutations in the mitochondrial HSP70 homologue HSPA9. Blood 2015, 126, 2734–2738. [Google Scholar] [CrossRef]
- Camaschella, C.; Campanella, A.; De Falco, L.; Boschetto, L.; Merlini, R.; Silvestri, L.; Levi, S.; Iolascon, A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 2007, 110, 1353–1358. [Google Scholar] [CrossRef]
- Ye, H.; Jeong, S.Y.; Ghosh, M.C.; Kovtunovych, G.; Silvestri, L.; Ortillo, D.; Uchida, N.; Tisdale, J.; Camaschella, C.; Rouault, T.A. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J. Clin. Investig. 2010, 120, 1749–1761. [Google Scholar] [CrossRef]
- Liu, G.; Guo, S.; Anderson, G.J.; Camaschella, C.; Han, B.; Nie, G. Heterozygous missense mutations in the GLXR5 gene cause sideroblastic anemia in a Chinese patient. Blood 2014, 124, 2750–2751. [Google Scholar] [CrossRef]
- Shukla, A.; Hebbar, M.; Srivastava, A.; Kadavigere, R.; Upadhyai, P.; Kanthi, A.; Brandau, O.; Bielas, S.; Girisha, K.M. Homozygous p. (Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. J. Hum. Genet. 2017, 62, 723–727. [Google Scholar] [CrossRef]
- Torraco, A.; Stehling, O.; Stümpfig, C.; Rösser, R.; De Rasmo, D.; Fiermonte, G.; Verrigni, D.; Rizza, T.; Vozza, A.; Di Nottia, M.; et al. ISCA1 mutation in a patient with infantile-onset leukodystrophy causes defects in mitochondrial [4Fe-4S] proteins. Hum. Mol. Genet. 2018, 27, 2739–2754. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, Z.N.; Al-Dosary, M.; Alfadhel, M.; Faqeih, E.A.; Alsagob, M.; Kenana, R.; Almass, R.; Al-Harazi, O.S.; Al-Hindi, H.; Malibari, O.I.; et al. ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J. Med. Genet. 2015, 52, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, J.T.; Besse, A.; Alston, C.L.; Pang, K.; Appadurai, V.; Samanta, M.; Smpokou, P.; McFarland, R.; Taylor, R.W.; Bonnen, P.E. Loss-of-function mutations in ISCA2 disrupt 4Fe-4S cluster machinery and cause a fatal leukodystrophy with hyperglycinemia and mtDNA depletion. Hum. Mutat. 2018, 39, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Alfadhel, M.; Nashabat, M.; Alrifai, M.T.; Alshaalan, H.; Al Mutairi, F.; Al-Shahrani, S.A.; Plecko, B.; Almass, R.; Alsagob, M.; Almutairi, F.B.; et al. Further delineation of the phenotypic spectrum of ISCA2 defect: A report of ten new cases. Eur. J. Paediatr. Neurol. 2018, 22, 46–55. [Google Scholar] [CrossRef]
- Toldo, I.; Nosadini, M.; Boscardin, C.; Talenti, G.; Manara, R.; Lamantea, E.; Legati, A.; Ghezzi, D.; Perilongo, G.; Sartori, S. Neonatal mitochondrial leukoencephalopathy with brain and spinal involvement and high lactate: Expanding the phenotype of ISCA2 gene mutations. Metab. Brain Dis. 2018, 33, 805–812. [Google Scholar] [CrossRef]
- Bolar, N.A.; Vanlander, A.V.; Wilbrecht, C.; Van Der Aa, N.; Smet, J.; De Paepe, B.; Vandeweyer, G.; Kooy, F.; Eyskens, F.; De Latter, E.; et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum. Mol. Genet. 2013, 22, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Lossos, A.; Stümpfig, C.; Stevanin, G.; Gaussen, M.; Zimmerman, B.-E.; Mundwiller, E.; Asulin, M.; Chamma, L.; Sheffer, R.; Misk, A.; et al. Fe/S protein assembly gene IBA57 mutation causes hereditary spastic paraplegia. Neurology 2015, 84, 659–667. [Google Scholar] [CrossRef]
- Debray, F.-G.; Stümpfig, C.; Vanlander, A.V.; Dideberg, V.; Josse, C.; Caberg, J.-H.; Boemer, F.; Bours, V.; Stevens, R.; Seneca, S.; et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes fatal infantile leukodystrophy. J. Inherit. Metab. Dis. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Torraco, A.; Ardissone, A.; Invernizzi, F.; Rizza, T.; Fiermonte, G.; Niceta, M.; Zanetti, N.; Martinelli, D.; Vozza, A.; Verrigni, D.; et al. Novel mutations in IBA57 are associated with leukodystrophy and variable clinical phenotypes. J. Neurol. 2016, 264, 102–111. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Zhang, Z.; Zhou, L.; Jiang, Y.; Wang, J.; Xiao, J.; Wu, Y. Phenotypic spectrum of mutations in IBA57, a candidate gene for cavitating leukoencephalopathy. Clin. Genet. 2017, 93, 235–241. [Google Scholar] [CrossRef]
- Ishiyama, A.; Sakai, C.; Matsushima, Y.; Noguchi, S.; Mitsuhashi, S.; Endo, Y.; Hayashi, Y.K.; Saito, Y.; Nakagawa, E.; Komaki, H.; et al. IBA57 mutations abrogate iron-sulfur cluster assembly leading to cavitating leukoencephalopathy. Neurol. Genet. 2017, 3, e184. [Google Scholar] [CrossRef] [PubMed]
- Seyda, A.; Newbold, R.F.; Hudson, T.; Verner, A.; MacKay, N.; Winter, S.; Feigenbaum, A.; Malaney, S.; Gonzalez-Halphen, D.; Cuthbert, A.P.; et al. A novel syndrome affecting multiple mitochondrial functions, located by microcell-mediated transfer to chromosome 2p14-2p13. Am. J. Hum. Genet. 2001, 68, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sastre, A.; Tort, F.; Stehling, O.; Uzarska, M.A.; Arranz, J.A.; del Toro, M.; Labayru, M.T.; Landa, J.; Font, A.; Garcia-Villoria, J.; et al. A fatal mitochondrial disease is associated with defective NFU1 Function in the Maturation of a Subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 2011, 89, 656–667. [Google Scholar] [CrossRef]
- Calvo, S.E.; Tucker, E.; Compton, A.; Kirby, D.M.; Crawford, G.; Burtt, N.P.; Rivas, M.; Guiducci, C.; Bruno, D.L.; Goldberger, O.A.; et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 2010, 42, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Kevelam, S.H.; Rodenburg, R.J.; Wolf, N.I.; Ferreira, P.; Lunsing, R.J.; Nijtmans, L.G.; Mitchell, A.; Arroyo, H.A.; Rating, D.; Vanderver, A.; et al. NUBPL mutations in patients with complex I deficiency and a distinct MRI pattern. Neurology 2013, 80, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Kimonis, V.; al Dubaisi, R.; Maclean, A.; Hall, K.; Weiss, L.; Stover, A.E.; Schwartz, P.H.; Berg, B.; Cheng, C.; Parikh, S.; et al. NUBPL mitochondrial disease: New patients and review of the genetic and clinical spectrum. J. Med. Genet. 2020, 58, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Friederich, M.W.; Perez, F.A.; Knight, K.M.; Van Hove, R.A.; Yang, S.P.; Saneto, R.P.; Van Hove, J.L. Pathogenic variants in NUBPL result in failure to assemble the matrix arm of complex I and cause a complex leukoencephalopathy with thalamic involvement. Mol. Genet. Metab. 2019, 129, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R.; Friederich, M.W.; Swanson, M.A.; Shaikh, T.; Bhattacharya, K.; Scharer, G.H.; Aicher, J.; Creadon-Swindell, G.; Geiger, E.; MacLean, K.N.; et al. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain 2013, 137, 366–379. [Google Scholar] [CrossRef]
- Haack, T.B.; Rolinski, B.; Haberberger, B.; Zimmermann, F.; Schum, J.; Strecker, V.; Graf, E.; Athing, U.; Hoppen, T.; Wittig, I.; et al. Homozygous missense mutation in BOLA3 causes multiple mitochondrial dysfunctions syndrome in two siblings. J. Inherit. Metab. Dis. 2012, 36, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pagon, A.R.; Bird, T.D.; Detter, J.C.; Pierce, I. Hereditary sideroblastic anaemia and ataxia: An X linked recessive disorder. J. Med. Genet. 1985, 22, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Kispal, G.; Lange, H.; Fitzsimons, E.; Tolmie, J.; Lill, R.; Bishop, D.F. Human ABC7 transporter: Gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood 2000, 96, 3256–3264. [Google Scholar] [CrossRef]
- Maguire, A.; Hellier, K.; Hammans, S.; May, A. X-linked cerebellar ataxia and sideroblastic anaemia associated with a missense mutation in the ABC7 gene predicting V411L. Br. J. Haematol. 2001, 115, 910–917. [Google Scholar] [CrossRef]
- Lamoril, J.; Boulechfar, S.; de Verneuil, H.; Grandchamp, B.; Nordmann, Y.; Deybach, J.-C. Human Erythropoietic Protoporphyria: Two point mutations in the ferrochelatase gene. Biochem. Biophys. Res. Commun. 1991, 181, 594–599. [Google Scholar] [CrossRef]
- Nakahashi, Y.; Fujita, H.; Taketani, S.; Ishida, N.; Kappas, A.; Sassa, S. The molecular defect of ferrochelatase in a patient with erythropoietic protoporphyria. Proc. Natl. Acad. Sci. USA 1992, 89, 281–285. [Google Scholar] [CrossRef]
- Brenner, D.; Didier, J.M.; Frasier, F.; Christensen, S.R.; Evans, G.A.; Dailey, H.A. A molecular defect in human protoporphyria. Am. J. Hum. Genet. 1992, 50, 1203–1210. [Google Scholar]
- Sarkany, R.P.E.; Alexander, G.J.M.A.; Cox, T.M. Recessive inheritance of erythropoietic protoporphyria with liver failure. Lancet 1994, 343, 1394–1396. [Google Scholar] [CrossRef]
- Bloomer, J.; Bruzzone, C.; Zhu, L.; Scarlett, Y.; Magness, S.; Brenner, D. Molecular defects in ferrochelatase in patients with protoporphyria requiring liver transplantation. J. Clin. Investig. 1998, 102, 107–114. [Google Scholar] [CrossRef]
- Schneider-Yin, X.; Gouya, L.; Dorsey, M.; Rüfenacht, U.; Deybach, J.C.; Ferreira, G.C. Mutations in the iron-sulfur cluster ligands of the human ferrochelatase lead to erythropoietic protoporphyria. Blood 2000, 96, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Herrerro, C.; To-Figueras, J.; Badenas, C. Clinical, biochemical, and genetic study of 11 patients with erythropoietic protoporphyria including one with homozygous disease. Arch. Dermatol. 2007, 143, 1125–1129. [Google Scholar] [CrossRef]
- Valnot, I.; Von Kleist-Retzow, J.-C.; Barrientos, A.; Gorbatyuk, M.; Taanman, J.-W.; Mehaye, B.; Rustin, P.; Tzagoloff, A.; Munnich, A.; Rotig, A. A mutation in the human heme A: Farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Pankratz, N.; Nichols, W.C.; Uniacke, S.K.; Halter, C.; Murrell, J.; Rudolph, A.; Shults, C.W.; Conneally, P.M.; Foroud, T. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef]
- Coenen, M.J.H.; van den Heuvel, L.P.; Ugalde, C.; Brinke, M.T.; Nijtmans, L.G.J.; Trijbels, F.J.M.; Beblo, S.; Maier, E.M.; Muntau, A.C.; Smeitink, J.A.M. Cytochrome c oxidase biogenesis in a patient with a mutation in COX10 gene. Ann. Neurol. 2004, 56, 560–564. [Google Scholar] [CrossRef]
- Kennaway, N.G.; Carerro-Valenzuela, R.D.; Ewart, G.; Balan, V.K.; Lightowlers, R.; Zhang, Y.Z.; Powell, B.R.; Capaldi, R.A.; Buist, N.R. Isoforms of mammalian cytochrome c oxidase: Correlation with human cytochrome c oxidase deficiency. Pediat. Res. 1990, 28, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Mattman, A.; Carlson, C.G.; Glerum, D.M.; Hoffbuhr, K.C.; Leary, S.C.; Kennaway, N.G.; Shoubridge, E.A. Mutations in COX15 produce a defect in mitochondrial heme biosynthetic pathway, causing early onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003, 72, 101–114. [Google Scholar] [CrossRef]
- Alfadhel, M.; Lillquist, Y.P.; Waters, P.J.; Sinclair, G.; Struys, E.; McFadden, D.; Hendson, G.; Hyams, L.; Shoffner, J.; Vallance, H.D. Infantile cardioencephalopathy due to a COX15 gene defect: Report and review. Am. J. Med. Genet. Part A 2011, 155, 840–844. [Google Scholar] [CrossRef]
- Bugiani, M.; Tiranti, V.; Farina, L.; Uziel, G.; Zeviani, M. Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency. J. Med. Genet. 2005, 42, e28. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Zhang, A.-S.; Brown, C.; Shirihai, O.S.; Ponka, P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 2007, 110, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Roshan, T.M.; Kahawita, T.M.; Mason, A.B.; Sheftel, A.D.; Ponka, P. Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run” mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nag, S.; Mason, A.B.; Barroso, M.M. Endosome–mitochondria interactions are modulated by iron release from transferrin. J. Cell Biol. 2016, 214, 831–845. [Google Scholar] [CrossRef]
- Shvartsman, M.; Kikkeri, R.; Shanzer, A.; Cabantchik, Z.I. Non-transferrin-bound iron reaches mitochondria by a chelator-inaccessible mechanism: Biological and clinical implications. Am. J. Physiol. Cell Physiol. 2007, 293, C1383–C1394. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, M.; Fibach, E.; Cabantchik, Z.I. Transferrin-iron routing to the cytosol and mitochondria as studied by live and real-time fluorescence. Biochem. J. 2010, 429, 185–193. [Google Scholar] [CrossRef]
- Shvartsman, M.; Cabantchik, Z.I. Intracellular iron trafficking: Role of cytosolic ligands. BioMetals 2012, 25, 711–723. [Google Scholar] [CrossRef]
- Khalil, S.; Holy, M.; Grado, S.; Fleming, R.; Kurita, R.; Nakamura, Y.; Goldfarb, A. A specialized pathway for erythroid iron delivery through lysosomal trafficking of transferrin receptor 2. Blood Adv. 2017, 1, 1181–1194. [Google Scholar] [CrossRef]
- Hughes, C.E.; Coody, T.; Jeong, M.-Y.; Berg, J.; Winge, D.R.; Hughes, A.L. Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef]
- Weber, R.A.; Yen, F.; Nicholson, S.P.; Alwaseem, H.; Bayraktar, E.; Alam, M.; Timson, R.C.; La, K.; Abu-Remaileh, M.; Molina, H.; et al. maintaining iron homeostasis is the key role of lysosomal acidity for cell proliferation. Mol. Cell 2020, 77, 645–655.e7. [Google Scholar] [CrossRef]
- Kim, J.E.; Vali, S.W.; Nguyen, T.Q.; Dancis, A.; Lindahl, P.A. Mössbauer and LC-ICP-MS investigation of iron trafficking between vacuoles and mitochondria in vma2Δ Saccharomyces cerevisiae. J. Biol. Chem. 2021, 296, 100141. [Google Scholar] [CrossRef] [PubMed]
- Yambire, K.F.; Rostosky, C.; Watanabe, T.; Pacheu-Grau, D.; Torres-Odio, S.; Sanchez-Guerrero, A.; Senderovich, O.; Meyron-Holtz, E.G.; Milosevic, I.; Frahm, J.; et al. Author response: Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. eLife 2019, 8, e51031. [Google Scholar] [CrossRef] [PubMed]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A. Low molecular weight intracellular iron transport compounds. Blood 1977, 50, 433–439. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Hart, D.O.; Goetz, D.H.; Green, M.R. A Mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010, 141, 1006–1017. [Google Scholar] [CrossRef]
- Lange, H.; Kispal, G.; Lill, R. Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. J. Biol. Chem. 1999, 274, 18989–18996. [Google Scholar] [CrossRef]
- Flatmark, T.; Romslo, I. Energy dependent accumulation of iron by isolated rat liver mitochondria. J. Biol. Chem. 1975, 250, 6433–6438. [Google Scholar] [CrossRef]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Frey, A.G.; Nandal, A.; Park, J.H.; Smith, P.M.; Yabe, T.; Ryu, M.-S.; Ghosh, M.C.; Lee, J.; Rouault, T.A.; Park, M.H.; et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl. Acad. Sci. USA 2014, 111, 8031–8036. [Google Scholar] [CrossRef] [PubMed]
- Yanatori, I.; Yasui, Y.; Tabuchi, M.; Kishi, F. Chaperone protein involved in transmembrane transport of iron. Biochem. J. 2014, 462, 25–37. [Google Scholar] [CrossRef]
- Li, H.; Mapolelo, D.T.; Dingra, N.N.; Naik, A.G.; Lees, N.S.; Hoffman, B.M.; Riggs-Gelasco, P.J.; Huynh, B.H.; Johnson, M.K.; Outten, C.E. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 2009, 48, 9569–9581. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Molik, S.; Godoy, J.R.; Uzarska, M.A.; Richter, N.; Seubert, A.; Zhang, Y.; Stubbe, J.; Pierrel, F.; Herrero, E.; et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010, 12, 373–385. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Mizrachi, D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012, 2, 164. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.A.; Ghio, A.J.; Garrick, L.M.; Garrick, M.; Zhao, L.; Fenton, R.; Thévenod, F. Evidence for mitochondrial localization of divalent metal transporter 1 (DMT1). FASEB J. 2014, 28, 2134–2145. [Google Scholar] [CrossRef]
- Wolff, N.A.; Garrick, M.; Zhao, L.; Garrick, L.M.; Ghio, A.J.; Thévenod, F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 2018, 8, 2134–2145. [Google Scholar] [CrossRef] [PubMed]
- Foury, F.; Roganti, T. Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J. Biol. Chem. 2002, 277, 24475–24483. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Stadler, J.A.; Richhardt, N.; Seubert, A.; Eickhorst, T.; Schweyen, R.J.; Lill, R.; Wiesenberger, G. A specific role of the yeast mitochondrial carriers Mrs3/4p in mitochondrial iron acquisition under iron-limiting conditions. J. Biol. Chem. 2003, 278, 40612–40620. [Google Scholar] [CrossRef] [PubMed]
- Nyhus, K.J.; Ozaki, L.S.; Jacobson, E.S. Role of mitochondrial carrier protein Mrs3/4 in iron acquisition and oxidative stress resistance of Cryptococcus neoformans. Med. Mycol. 2002, 40, 581–591. [Google Scholar] [CrossRef][Green Version]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Shimo, H.; Nagasaka, S.; Fujimoto, M.; Takanashi, H.; Tsutsumi, N.; An, G.; Nakanishi, H.; Nishizawa, N.K. The rice mitochondrial iron transporter is essential for plant growth. Nat. Commun. 2011, 2, 322. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, S.; Tan, G.; Ye, W.; Liu, D.; Qian, X.; Ding, Z.; Zhong, Y.; Zhang, J.; Jiang, D.; et al. Reduction of mitoferrin results in abnormal development and extended lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e29666. [Google Scholar] [CrossRef][Green Version]
- Metzendorf, C.; Wu, W.; Lind, M.I. Overexpression of Drosophila mitoferrin in l(2)mbn cells results in dysregulation of Fer1HCH expression. Biochem. J. 2009, 421, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, A.; Satrústegui, J. New mitochondrial carriers: An overview. Cell. Mol. Life Sci. 2005, 62, 2204–2227. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, M.; Baines, C.P. Physiological and pathological roles of mitochondrial SLC25 carriers. Biochem. J. 2013, 454, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.; Robinson, A.J. The conserved substrate binding site of mitochondrial carriers. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 1237–1248. [Google Scholar] [CrossRef]
- Palmieri, F. Diseases caused by defects of mitochondrial carriers: A review. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 564–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyver, E.R.; Knight, S.; Lesuisse, E.; Dancis, A. Frataxin and mitochondrial carrier proteins, Mrs3p and Mrs4p, cooperate in providing iron for heme synthesis. J. Biol. Chem. 2005, 280, 19794–19807. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyver, E.R.; Knight, S.; Pain, D.; Lesuisse, E.; Dancis, A. Mrs3p, Mrs4p, and frataxin provide iron for Fe-S cluster synthesis in mitochondria. J. Biol. Chem. 2006, 281, 22493–22502. [Google Scholar] [CrossRef]
- Courel, M.; Lallet, S.; Camadro, J.-M.; Blaiseau, P.-L. Direct activation of genes involved in intracellular iron use by the yeast iron-responsive transcription factor Aft2 without its paralog Aft1. Mol. Cell. Biol. 2005, 25, 6760–6771. [Google Scholar] [CrossRef]
- Rutherford, J.C.; Jaron, S.; Winge, D.R. Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J. Biol. Chem. 2003, 278, 27636–27643. [Google Scholar] [CrossRef]
- Froschauer, E.M.; Schweyen, R.J.; Wiesenberger, G. The yeast mitochondrial carrier proteins Mrs3p/Mrs4p mediate iron transport across the inner mitochondrial membrane. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Vest, K.E.; Wang, J.; Gammon, M.G.; Maynard, M.K.; White, O.L.; Cobine, J.A.; Mahone, W.K.; Cobine, P.A. Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae. Open Biol. 2016, 6, 150223. [Google Scholar] [CrossRef] [PubMed]
- Froschauer, E.M.; Rietzschel, N.; Hassler, M.R.; Binder, M.; Schweyen, R.J.; Lill, R.; Mühlenhoff, U.; Wiesenberger, G. The mitochondrial carrier Rim2 co-imports pyrimidine nucleotides and iron. Biochem. J. 2013, 455, 57–65. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Jia, X.; Ward, D.M.; Kaplan, J. Genetic and biochemical analysis of high iron toxicity in yeast. J. Biol. Chem. 2011, 286, 3851–3862. [Google Scholar] [CrossRef]
- Yoon, H.; Zhang, Y.; Pain, J.; Lyver, E.R.; Lesuisse, E.; Pain, D.; Dancis, A. Rim2, a pyrimidine nucleotide exchanger, is needed for iron utilization in mitochondria. Biochem. J. 2011, 440, 137–146. [Google Scholar] [CrossRef]
- Di Noia, M.A.; Todisco, S.; Cirigliano, A.; Rinaldi, T.; Agrimi, G.; Iacobazzi, V.; Palmieri, F. The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J. Biol. Chem. 2014, 289, 33137–33148. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; Blanco, E.; Palmieri, L.; Palmieri, F. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 20850–20857. [Google Scholar] [CrossRef]
- Van Dyck, E.; Jank, B.; Ragnini, A.; Schweyen, R.J.; Duyckaerts, C.; Sluse, F.; Foury, F. Overexpression of a novel member of the mitochondrial carrier family rescues defects in both DNA and RNA metabolism in yeast mitochondria. Mol. Genet. Genom. 1995, 246, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Traba, J.; Froschauer, E.M.; Wiesenberger, G.; Satrústegui, J.; Del Arco, A. Yeast mitochondria import ATP through the calcium-dependent ATP-Mg/Pi carrier Sal1p, and are ATP consumers during aerobic growth in glucose. Mol. Microbiol. 2008, 69, 570–585. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Hoffmann, B.; Richter, N.; Rietzschel, N.; Spantgar, F.; Stehling, O.; Uzarska, M.A.; Lill, R. Compartmentalization of iron between mitochondria and the cytosol and its regulation. Eur. J. Cell Biol. 2015, 94, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Anderson, S.A.; Gwynn, B.; Deck, K.M.; Chen, M.J.; Langer, N.B.; Shaw, G.C.; Huston, N.C.; Boyer, L.F.; Datta, S.; et al. Iron regulatory protein-1 protects against mitoferrin1-deficient porphyria. J. Biol. Chem. 2014, 289, 7835–7843. [Google Scholar] [CrossRef]
- Wang, Y.; Langer, N.B.; Shaw, G.C.; Yang, G.; Li, L.; Kaplan, J.; Paw, B.H.; Bloomer, J.R. Abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. Exp. Hematol. 2011, 39, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Paradkar, P.N.; Li, L.; Pierce, E.L.; Langer, N.B.; Takahashi-Makise, N.; Hyde, B.B.; Shirihai, O.S.; Ward, D.M.; Kaplan, J.; et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16263–16268. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Shintre, C.A.; Althoff, T.; Gutierrez, V.; Segawa, M.; Saxberg, A.D.; Martinez, M.; Adamson, R.; Young, M.R.; Faust, B.; et al. ABCB10 exports mitochondrial biliverdin, driving metabolic maladaptation in obesity. Sci. Transl. Med. 2021, 13, eabd1869. [Google Scholar] [CrossRef]
- Huang, M.L.-H.; Becker, E.M.; Whitnall, M.; Rahmanto, Y.S.; Ponka, P.; Richardson, D.R. Elucidation of the mechanism of mitochondrial iron loading in Friedreich’s ataxia by analysis of a mouse mutant. Proc. Natl. Acad. Sci. USA 2009, 106, 16381–16386. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Puccio, H. Dysregulation of cellular iron metabolism in Friedreich ataxia: From primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front. Pharmacol. 2014, 5, 130. [Google Scholar] [CrossRef]
- Martelli, A.; Schmucker, S.; Reutenauer, L.; Mathieu, J.R.; Peyssonnaux, C.; Karim, Z.; Puy, H.; Galy, B.; Hentze, M.; Puccio, H. Iron regulatory protein 1 sustains mitochondrial iron loading and function in frataxin deficiency. Cell Metab. 2015, 21, 311–323. [Google Scholar] [CrossRef]
- Crooks, D.R.; Natarajan, T.G.; Jeong, S.Y.; Chen, C.; Park, S.Y.; Huang, H.; Ghosh, M.C.; Tong, W.-H.; Haller, R.G.; Wu, C.; et al. Elevated FGF21 secretion, PGC-1α and ketogenic enzyme expression are hallmarks of iron-sulfur cluster depletion in human skeletal muscle. Hum. Mol. Gen. 2014, 23, 24–39. [Google Scholar] [CrossRef]
- Delatycki, M.B.; Camakaris, J.; Brooks, H.; Evans-Whipp, T.; Thorburn, D.R.; Williamson, R.; Forrest, S.M. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann. Neurol. 1999, 45, 673–675. [Google Scholar] [CrossRef]
- Puccio, H.; Simon, D.; Cossée, M.; Criqui-Filipe, P.; Tiziano, F.D.; Melki, J.; Hindelang, C.; Matyas, R.; Rustin, P.; Koenig, M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001, 27, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Kim, J.-S.; Kon, K.; Jaeschke, H.; Ikejima, K.; Watanabe, S.; Lemasters, J.J. Translocatoin of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 2008, 48, 1644–1654. [Google Scholar] [CrossRef]
- Lockhart, P.; Holtom, B.; Lincoln, S.; Hussey, J.; Zimprich, A.; Gasser, T.; Wszolek, Z.K.; Hardy, J.; Farrer, M. The human sideroflexin 5 (SFXN5) gene: Sequence, expression analysis and exclusion as a candidate for PARK3. Gene 2002, 285, 229–237. [Google Scholar] [CrossRef]
- Fleming, M.D.; Campagna, D.R.; Haslett, J.N.; Trenor III, C.C.; Andrews, N.C. A mutation in a mitochondrial transmembrane protein is responsibe for the pleiotropic hematological and skeletal phenotype of flexed-tail (f/f) mice. Genes Dev. 2001, 15, 652–657. [Google Scholar] [CrossRef]
- Kory, N.; Wyant, G.A.; Prakash, G.; de Bos, J.U.; Bottanelli, F.; Pacold, M.E.; Chan, S.H.; Lewis, C.A.; Wang, T.; Keys, H.R.; et al. SFXN1 is a mitochondrial serine transporter required for one-carbon metabolism. Science 2018, 362, eaat9528. [Google Scholar] [CrossRef]
- Acoba, M.G.; Alpergin, E.S.S.; Renuse, S.; Fernández-Del-Río, L.; Lu, Y.-W.; Khalimonchuk, O.; Clarke, C.F.; Pandey, A.; Wolfgang, M.J.; Claypool, S.M. The mitochondrial carrier SFXN1 is critical for complex III integrity and cellular metabolism. Cell Rep. 2021, 34, 108869. [Google Scholar] [CrossRef]
- Paul, B.T.; Tesfay, L.; Winkler, C.R.; Torti, F.M.; Torti, S.V. Sideroflexin 4 affects Fe-S cluster biogenesis, iron metabolism, mitochondrial respiration and heme biosynthetic enzymes. Sci. Rep. 2019, 9, 19634. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Corsi, B.; Bosisio, M.; Invernizzi, R.; Volz, A.; Sanford, D.; Arosio, P.; Drysdale, J. A Human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 2001, 276, 24437–24440. [Google Scholar] [CrossRef]
- Levi, S.; Arosio, P. Mitochondrial ferritin. Int. J. Biochem. Cell Biol. 2004, 36, 1887–1889. [Google Scholar] [CrossRef]
- Cazzola, M.; Invernizzi, R.; Bergamaschi, G.; Levi, S.; Corsi, B.; Travaglino, E.; Rolandi, V.; Biasiotto, G.; Drysdale, J.; Arosio, P. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood 2003, 101, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, P.; Biasiotto, G.; Sanvito, F.; Olivieri, S.; Arosio, P.; Levi, S. Mitochondrial ferritin expression in adult mouse tissues. J. Histochem. Cytochem. 2007, 55, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Santambrogio, P.; Levi, S.; Arosio, P.; Chasteen, N.D. Unique iron binding and oxidation properties of human mitochondrial ferritin: A comparative analysis with human H-chain ferritin. J. Mol. Biol. 2005, 347, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Corsi, B.; Cozzi, A.; Arosio, P.; Drysdale, J.; Santambrogio, P.; Campanella, A.; Biasiotto, G.; Albertini, A.; Levi, S. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J. Biol. Chem. 2002, 277, 22430–22437. [Google Scholar] [CrossRef]
- Campanella, A.; Rovelli, E.; Santambrogio, P.; Cozzi, A.; Taroni, F.; Levi, S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: Hypothesis for a protective role in Friedreich ataxia. Hum. Mol. Genet. 2008, 18, 1–11. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. ARS 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Lindahl, P.A.; Moore, M.J. Labile low-molecular-mass metal complexes in mitochondria: Trials and tribulations of a burgeoning field. Biochemistry 2016, 55, 4140–4153. [Google Scholar] [CrossRef]
- Qi, W.; Cowan, J. Mechanism of glutaredoxin—ISU [2Fe-2S] cluster exchange. Chem. Commun. 2011, 47, 4989–4991. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Iron speciation in the cytosol: An overview. Dalton Trans. 2013, 42, 3220–3229. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pierik, A.J.; Lill, R. Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1. Science 2014, 343, 1137–1140. [Google Scholar] [CrossRef]
- Schaedler, T.; Thornton, J.D.; Kruse, I.; Schwarzländer, M.; Meyer, A.J.; van Veen, H.W.; Balk, J. A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J. Biol. Chem. 2014, 289, 23264–23274. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(iii) citrate speciation in aqueous solution. Dalton Trans. 2009, 8616–8625. [Google Scholar] [CrossRef]
- Maio, N.; Rouault, T.A. Iron–sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1853, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, P.; Sazanov, L. Organization of iron-sulfur clusters in respiratory complex I. Science 2005, 309, 771–774. [Google Scholar] [CrossRef]
- Sazanov, L.; Hinchliffe, P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 2006, 311, 1430–1436. [Google Scholar] [CrossRef]
- Rudolf, J.; Makrantoni, V.; Ingledew, W.J.; Stark, M.; White, M.F. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol. Cell 2006, 23, 801–808. [Google Scholar] [CrossRef]
- Netz, D.; Stith, C.M.; Stümpfig, M.; Köpf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.J.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2012, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yarunin, A.; Panse, V.G.; Petfalski, E.; Dez, C.; Tollervey, D.; Hurt, E.C. Functional link between ribosome formation and biogenesis of iron–sulfur proteins. EMBO J. 2005, 24, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Biogenesis of iron-sulfur clusters in mammalian cells: New insights and relevance to human disease. Dis. Model. Mech. 2012, 5, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1868, 118863. [Google Scholar] [CrossRef]
- Paul, V.D.; Lill, R. Biogenesis of cytosolic and nuclear iron–sulfur proteins and their role in genome stability. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 1528–1539. [Google Scholar] [CrossRef]
- Lill, R. From the discovery to molecular understanding of cellular iron-sulfur protein biogenesis. Biol. Chem. 2020, 401, 855–876. [Google Scholar] [CrossRef]
- Rawat, S.; Stemmler, T.L. Key Players and Their Role During Mitochondrial Iron-Sulfur Cluster Biosynthesis. Chemistry 2011, 17, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Rouault, T. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.C.; Bornhövd, C.; Prokisch, H.; Neupert, W.; Hell, K. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 2005, 25, 174–183. [Google Scholar] [CrossRef]
- Pandey, A.; Golla, R.; Yoon, H.; Dancis, A.; Pain, D. Persulfide formation on mitochondrial cysteine desulfurase: Enzyme activation by a eukaryote-specific interacting protein and Fe–S cluster synthesis. Biochem. J. 2012, 448, 171–187. [Google Scholar] [CrossRef]
- Van Vranken, J.G.; Jeong, M.-Y.; Wei, P.; Chen, Y.-C.; Gygi, S.P.; Winge, D.R.; Rutter, J. The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. eLife 2016, 5, e17828. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The bioplex network: A systemic exploration of the human interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Stehling, O.; Pierik, A.J.; Elsasser, H.-P.; Muhlenhoff, U.; Webert, H.; Hobler, A.; Hannemann, F.; Bernhardt, R.; Lill, R. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 11775–11780. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ghosh, M.; Kovtunovych, G.; Crooks, D.R.; Rouault, T.A. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 484–492. [Google Scholar] [CrossRef]
- Webert, H.; Freibert, S.-A.; Gallo, A.; Heidenreich, T.; Linne, U.; Amlacher, S.; Hurt, E.; Mühlenhoff, U.; Banci, L.; Lill, R. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 2014, 5, 5013. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Proteau, A.; Villarroya, M.M.; Moukadiri, I.; Zhang, L.; Trempe, J.-F.; Matte, A.; Armengod, M.E.; Cygler, M. Structural basis for Fe–S cluster assembly and tRNA thiolation mediated by IscS protein–protein interactions. PLoS Biol. 2010, 8, e1000354. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; McCabe, J.W.; Russell, D.H.; Barondeau, D.P. Molecular mechanism of ISC iron–sulfur cluster biogenesis revealed by high-resolution native mass spectrometry. J. Am. Chem. Soc. 2020, 142, 6018–6029. [Google Scholar] [CrossRef]
- Gervason, S.; Larkem, D.; Ben Mansour, A.; Botzanowski, T.; Müller, C.S.; Pecqueur, L.; Le Pavec, G.; Delaunay-Moisan, A.; Brun, O.; Agramunt, J.; et al. Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin. Nat. Commun. 2019, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.; Mühlenhoff, U.; Lill, R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003, 4, 906–911. [Google Scholar] [CrossRef]
- Colin, F.; Martelli, A.; Clémancey, M.; Latour, J.-M.; Gambarelli, S.; Zeppieri, L.; Birck, C.; Page, A.; Puccio, H.; de Choudens, S.O. Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J. Am. Chem. Soc. 2013, 135, 733–740. [Google Scholar] [CrossRef]
- Parent, A.; Elduque, X.; Cornu, D.; Belot, L.; Le Caer, J.-P.; Grandas, A.; Toledano, M.B.; D’Autréaux, B. Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat. Commun. 2015, 6, 5686. [Google Scholar] [CrossRef]
- Fox, N.G.; Das, D.; Chakrabarti, M.; Lindahl, P.A.; Barondeau, D.P. Frataxin accelerates [2Fe-2S] cluster formation on the human Fe–S assembly complex. Biochemistry 2015, 54, 3880–3889. [Google Scholar] [CrossRef]
- Fox, N.G.; Chakrabarti, M.; McCormick, S.P.; Lindahl, P.A.; Barondeau, D.P. The human iron–sulfur assembly complex catalyzes the synthesis of [2Fe-2S] clusters on ISCU2 that can be transferred to acceptor molecules. Biochemistry 2015, 54, 3871–3879. [Google Scholar] [CrossRef]
- Patra, S.; Barondeau, D.P. Mechanism of activation of the human cysteine desulfurase complex by frataxin. Proc. Natl. Acad. Sci. USA 2019, 116, 19421–19430. [Google Scholar] [CrossRef]
- Das, D.; Patra, S.; Bridwell-Rabb, J.; Barondeau, D.P. Mechanism of frataxin “bypass” in human iron-sulfur cluster biosynthesis with implications for Friedreich’s ataxia. J. Biol. Chem. 2019, 294, 9276–9284. [Google Scholar] [CrossRef]
- Schmucker, S.; Martelli, A.; Colin, F.; Page, A.; Wattenhofer-Donzé, M.; Reutenauer, L.; Puccio, H. Mammalian frataxin: An essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS ONE 2011, 6, e16199. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, S.; Puccio, H. Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Hum. Mol. Genet. 2010, 19, R103–R110. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Puccio, H. Frataxin: A protein in search for a function. J. Neurochem. 2013, 126, 43–52. [Google Scholar] [CrossRef]
- Adinolfi, S.; Iannuzzi, C.; Prischi, F.; Pastore, C.; Iametti, S.; Martin, S.R.; Bonomi, F.; Pastore, A. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol. 2009, 16, 390–396. [Google Scholar] [CrossRef]
- Prischi, F.; Konarev, P.; Iannuzzi, C.; Pastore, C.; Adinolfi, S.; Martin, S.R.; Svergun, D.I.; Pastore, A. Structural bases for the interaction of frataxin with the central components of iron–sulphur cluster assembly. Nat. Commun. 2010, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Gakh, O.; Bedekovics, T.; Duncan, S.F.; Smith IV, D.Y.; Berkholz, D.S.; Isaya, G. Normal and Friedreich ataxia cells express different isoforms of frataxin with complementary roles in iron-sulfur cluster assembly. J. Biol. Chem. 2010, 285, 38486–38501. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Barondeau, D. Human frataxin is an allosteric switch that activates the Fe−S cluster biosynthetic complex. Biochemistry 2010, 49, 9132–9139. [Google Scholar] [CrossRef]
- Bridwell-Rabb, J.; Fox, N.G.; Tsai, C.-L.; Winn, A.M.; Barondeau, D.P. Human frataxin activates Fe–S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 2014, 53, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Cowan, J.A. Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J. Biol. Chem. 2004, 279, 25943–25946. [Google Scholar] [CrossRef]
- Bencze, K.Z.; Yoon, T.; Millan-Pacheco, C.; Bradley, P.B.; Pastor, N.; Cowan, J.A.; Stemmler, T. Human frataxin: Iron and ferrochelatase binding surface. Chem. Commun. 2007, 1798–1800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, K.; Frederick, R.O.; Kim, J.H.; Reinen, N.M.; Tonelli, M.; Markley, J.L. Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU). J. Biol. Chem. 2013, 288, 28755–28770. [Google Scholar] [CrossRef] [PubMed]
- Uhrigshardt, H.; Singh, A.; Kovtunovych, G.; Ghosh, M.; Rouault, T.A. Characterization of the human HSC20, an unusual DnaJ type III protein, involved in iron-sulfur cluster biogenesis. Hum. Mol. Gen. 2010, 19, 3816–3834. [Google Scholar] [CrossRef] [PubMed]
- Angerer, H.; Radermacher, M.; Mańkowska, M.; Steger, M.; Zwicker, K.; Heide, H.; Wittig, I.; Brandt, U.; Zickermann, V. The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. Proc. Natl. Acad. Sci. USA 2014, 111, 5207–5212. [Google Scholar] [CrossRef]
- Uzarska, M.A.; Dutkiewicz, R.; Freibert, S.-A.; Lill, R.; Mühlenhoff, U. The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol. Biol. Cell 2013, 24, 1830–1841. [Google Scholar] [CrossRef]
- Maio, N.; Singh, A.; Uhrigshardt, H.; Saxena, N.; Tong, W.-H.; Rouault, T.A. Cochaperone binding to LYR motifs confers specificity of iron sulfur cluster delivery. Cell Metab. 2014, 19, 445–457. [Google Scholar] [CrossRef]
- Sánchez, E.; Lobo, T.; Fox, J.L.; Zeviani, M.; Winge, D.R.; Fernández-Vizarra, E. LYRM7/MZM1L is a UQCRFS1 chaperone involved in the last steps of mitochondrial Complex III assembly in human cells. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Cortopassi, G. HSC20 interacts with frataxin and is involved in iron–sulfur cluster biogenesis and iron homeostasis. Hum. Mol. Genet. 2011, 21, 1457–1469. [Google Scholar] [CrossRef]
- Weiler, B.D.; Brück, M.-C.; Kothe, I.; Bill, E.; Lill, R.; Mühlenhoff, U. Mitochondrial [4Fe-4S] protein assembly involves reductive [2Fe-2S] cluster fusion on ISCA1–ISCA2 by electron flow from ferredoxin FDX2. Proc. Natl. Acad. Sci. USA 2020, 117, 20555–20565. [Google Scholar] [CrossRef]
- Gelling, C.; Dawes, I.W.; Richhardt, N.; Lill, R.; Mühlenhoff, U. Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes. Mol. Cell. Biol. 2008, 28, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U.; Richter, N.; Pines, O.; Pierik, A.J.; Lill, R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe-4S] proteins. J. Biol. Chem. 2017, 292, 17979. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Wilbrecht, C.; Stehling, O.; Niggemeyer, B.; Elsässer, H.-P.; Mühlenhoff, U.; Lill, R. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol. Biol. Cell 2012, 23, 1157–1166. [Google Scholar] [CrossRef]
- Kim, K.-D.; Chung, W.-H.; Kim, H.-J.; Lee, K.-C.; Roe, J.-H. Monothiol glutaredoxin Grx5 interacts with Fe–S scaffold proteins Isa1 and Isa2 and supports Fe–S assembly and DNA integrity in mitochondria of fission yeast. Biochem. Biophys. Res. Commun. 2010, 392, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Stehling, O.; Pierik, A.J.; Netz, D.J.A.; Kerscher, S.; Elsässer, H.-P.; Wittig, I.; Balk, J.; Brandt, U.; Lill, R. Human Ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol. Cell. Biol. 2009, 29, 6059–6073. [Google Scholar] [CrossRef] [PubMed]
- Beilschmidt, L.K.; Puccio, H.M. Mammalian Fe–S cluster biogenesis and its implication in disease. Biochimie 2014, 100, 48–60. [Google Scholar] [CrossRef]
- Cameron, J.; Janer, A.; Levandovskiy, V.; Mackay, N.; Rouault, T.A.; Tong, W.-H.; Ogilvie, I.; Shoubridge, E.A.; Robinson, B.H. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of mutliple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am. J. Hum. Gen. 2011, 89, 486–495. [Google Scholar] [CrossRef]
- Uzarska, M.A.; Nasta, V.; Weiler, B.D.; Spantgar, F.; Ciofi-Baffoni, S.; Saviello, M.R.; Gonnelli, L.; Mühlenhoff, U.; Banci, L.; Lill, R. Author response: Mitochondrial Bol1 and Bol3 function as assembly factors for specific iron-sulfur proteins. eLife 2016, 5, e16673. [Google Scholar] [CrossRef] [PubMed]
- Melber, A.; Na, U.; Vashisht, A.; Weiler, B.D.; Lill, R.; Wohlschlegel, J.A.; Winge, D.R. Author response: Role of Nfu1 and Bol3 in iron-sulfur cluster transfer to mitochondrial clients. eLife 2016, 5, e15991. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pain, J.; Dancis, A.; Pain, D. Mitochondria export iron–sulfur and sulfur intermediates to the cytoplasm for iron–sulfur cluster assembly and tRNA thiolation in yeast. J. Biol. Chem. 2019, 294, 9489–9502. [Google Scholar] [CrossRef] [PubMed]
- Mesecke, N.; Terziyska, N.; Kozany, C.; Baumann, F.; Neupert, W.; Hell, K.; Herrmann, J.M. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 2005, 121, 1059–1069. [Google Scholar] [CrossRef]
- Qi, W.; Li, J.; Cowan, J.A. A structural model for glutathione-complexed iron–sulfur cluster as a substrate for ABCB7-type transporters. Chem. Commun. 2014, 50, 3795–3798. [Google Scholar] [CrossRef] [PubMed]
- Kispal, G.; Csere, P.; Guiard, B.; Lill, R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett. 1997, 418, 346–350. [Google Scholar] [CrossRef]
- Pondarre, C.; Campagna, D.R.; Antiochos, B.; Sikorski, L.; Mulhern, H.; Fleming, M. Abcb7, the gene responsible for X-linked sideroblastic anemia with ataxia, is essential for hematopoiesis. Blood 2006, 109, 3567–3569. [Google Scholar] [CrossRef]
- Boultwood, J.; Pellagatti, A.; Nikpour, M.; Pushkaran, B.; Fidler, C.; Cattan, H.; Littlewood, T.J.; Malcovati, L.; Della Porta, M.G.; Jädersten, M.; et al. The role of the iron transporter ABCB7 in refractory anemia with ring sideroblasts. PLoS ONE 2008, 3, e1970. [Google Scholar] [CrossRef] [PubMed]
- Cavadini, P.; Biasiotto, G.; Poli, M.; Levi, S.; Verardi, R.; Zanella, I.; Derosas, M.; Ingrassia, R.; Corrado, M.; Arosio, P. RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood 2006, 109, 3552–3559. [Google Scholar] [CrossRef]
- Pondarré, C.; Antiochos, B.B.; Campagna, D.R.; Clarke, S.L.; Greer, E.; Deck, K.M.; McDonald, A.; Han, A.-P.; Medlock, A.; Kutok, J.L.; et al. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron–sulfur cluster biogenesis. Hum. Mol. Genet. 2006, 15, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Galluhn, D.; Langer, T. Reversible assembly of the ATP-binding cassette transporter Mdl1 with the F1F0-ATP synthase in mitochondria. J. Biol. Chem. 2004, 279, 38338–38345. [Google Scholar] [CrossRef]
- Young, L.; Leonhard, K.; Tatsuta, T.; Trowsdale, J.; Langer, T. Role of the ABC Transporter Mdl1 in peptide export from mitochondria. Science 2001, 291, 2135–2138. [Google Scholar] [CrossRef]
- Chloupková, M.; LeBard, L.S.; Koeller, D.M. MDL1 is a high copy suppressor of ATM1: Evidence for a role in resistance to oxidative stress. J. Mol. Biol. 2003, 331, 155–165. [Google Scholar] [CrossRef]
- Poulos, T. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed]
- Mosure, S.A.; Strutzenberg, T.S.; Shang, J.; Munoz-Tello, P.; Solt, L.A.; Griffin, P.R.; Kojetin, D.J. Structural basis for heme-dependent NCoR binding to the transcriptional repressor REV-ERBβ. Sci. Adv. 2021, 7, eabc6479. [Google Scholar] [CrossRef] [PubMed]
- Piel, R., III; Dailey, H.A., Jr.; Medlock, A.E. The mitochondrial heme metabolon: Insights into the complex(ity) of heme synthesis and distribution. Mol. Gen. Metab. 2019, 128, 198–203. [Google Scholar] [CrossRef]
- Celis, A.; DuBois, J.L. Making and breaking heme. Curr. Opin. Struct. Biol. 2019, 59, 19–28. [Google Scholar] [CrossRef]
- Hamza, I.; Dailey, H.A. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 1617–1632. [Google Scholar] [CrossRef]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the mitochondrial heme metabolism complex. PLoS ONE 2015, 10, e0135896. [Google Scholar] [CrossRef]
- Martinez-Guzman, O.; Willoughby, M.M.; Saini, A.; Dietz, J.V.; Bohovych, I.; Medlock, A.E.; Khalimonchuk, O.; Reddi, A.R. Mitochondrial-nuclear heme trafficking in budding yeast is regulated by GTPases that control mitochondrial dynamics and ER contact sites. J. Cell Sci. 2020, 133, jcs237917. [Google Scholar] [CrossRef] [PubMed]
- Kranz, R.G.; Richard-Fogal, C.; Taylor, J.-S.; Frawley, E.R. Cytochrome c biogenesis: Mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009, 73, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Babbitt, S.E.; Francisco, B.S.; Mendez, D.L.; Lukat-Rodgers, G.S.; Rodgers, K.R.; Bretsnyder, E.C.; Kranz, R.G. Mechanisms of mitochondrial holocytochrome c synthase and the key roles played by cysteines and histidine of the heme attachment site, Cys-XX-Cys-His. J. Biol. Chem. 2014, 289, 28795–28807. [Google Scholar] [CrossRef]
- Babbitt, S.E.; Sutherland, M.; Francisco, B.S.; Mendez, D.L.; Kranz, R.G. Mitochondrial cytochrome c biogenesis: No longer an enigma. Trends Biochem. Sci. 2015, 40, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.G.; Gabilly, S.T.; Dujardin, G.; Merchant, S.; Hamel, P.P. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 2003, 278, 49732–49742. [Google Scholar] [CrossRef] [PubMed]
- Khalimonchuk, O.; Bestwick, M.; Meunier, B.; Watts, T.C.; Winge, D.R. Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol. Cell. Biol. 2010, 30, 1004–1017. [Google Scholar] [CrossRef]
- Khalimonchuk, O.; Kim, H.; Watts, T.; Perez-Martinez, X.; Winge, D.R. Oligomerization of heme o Synthase in Cytochrome Oxidase Biogenesis Is Mediated by Cytochrome Oxidase Assembly Factor Coa2. J. Biol. Chem. 2012, 287, 26715–26726. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef]
- Bareth, B.; Dennerlein, S.; Mick, D.; Nikolov, M.; Urlaub, H.; Rehling, P. The heme a synthase Cox15 associates with cytochrome c oxidase assembly intermediates during Cox1 maturation. Mol. Cell. Biol. 2013, 33, 4128–4137. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, F.A.; Hannappel, A.; Anderka, O.; Ludwig, B. Surf1, associated with leigh syndrome in humans, is a heme-binding protein in bacterial oxidase biogenesis. J. Biol. Chem. 2009, 284, 25735–25741. [Google Scholar] [CrossRef] [PubMed]
- Bestwick, M.; Khalimonchuk, O.; Pierrel, F.; Winge, D.R. The role of Coa2 in hemylation of yeast Cox1 revealed by its genetic interaction with Cox10. Mol. Cell. Biol. 2010, 30, 172–185. [Google Scholar] [CrossRef][Green Version]
- Bestwick, M.; Jeong, M.-Y.; Khalimonchuk, O.; Kim, H.; Winge, D.R. Analysis of leigh syndrome mutations in the yeast SURF1 homolog reveals a new member of the cytochrome oxidase assembly factor family. Mol. Cell. Biol. 2010, 30, 4480–4491. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, J.; Johns, T.; Fu, K.; De Bie, I.; Macmillan, C.; Cuthbert, A.P.; Newbold, R.F.; Wang, J.-C.; Chevrette, M.; et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998, 20, 337–343. [Google Scholar] [CrossRef]
- Pierrel, F.; Khalimonchuk, O.; Cobine, P.; Bestwick, M.; Winge, D.R. Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis that facilitates the maturation of Cox1. Mol. Cell. Biol. 2008, 28, 4927–4939. [Google Scholar] [CrossRef]
- Hannappel, A.; Bundschuh, F.A.; Ludwig, B. Role of Surf1 in heme recruitment for bacterial COX biogenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kaplan, J. Characterization of two homologous yeast genes that encode mitochondrial iron transporters. J. Biol. Chem. 1997, 272, 28485–28493. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miao, R.; Jia, X.; Ward, D.M.; Kaplan, J. Expression of the yeast cation diffusion facilitators Mmt1 and Mmt2 affects mitochondrial and cellular iron homeostasis: Evidence for mitochondrial iron export. J. Biol. Chem. 2014, 289, 17132–17141. [Google Scholar] [CrossRef]
- Luk, E.; Carroll, M.; Baker, M.; Culotta, V.C. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 2003, 100, 10353–10357. [Google Scholar] [CrossRef]
- Naranuntarat, A.; Jensen, A.; Pazicni, S.; Penner-Hahn, J.E.; Culotta, V.C. The interaction of mitochondrial iron with manganese superoxide dismutase. J. Biol. Chem. 2009, 284, 22633–22640. [Google Scholar] [CrossRef]
- Yang, M.; Cobine, P.; Molik, S.; Naranuntarat, A.; Lill, R.; Winge, D.R.; Culotta, V.C. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 2006, 25, 1775–1783. [Google Scholar] [CrossRef]
- Nilsson, R.; Schultz, I.J.; Pierce, E.L.; Soltis, K.A.; Naranuntarat, A.; Ward, D.M.; Baughman, J.M.; Paradkar, P.N.; Kingsley, P.D.; Culotta, V.C.; et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009, 10, 119–130. [Google Scholar] [CrossRef]
- Galmozzi, A.; Kok, B.; Kim, A.; Montenegro-Burke, J.R.; Lee, J.; Spreafico, R.; Mosure, S.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef]
- Poston, C.N.; Duong, E.; Cao, Y.; Bazemore-Walker, C.R. Proteomic analysis of lipid raft-enriched membranes isolated from internal organelles. Biochem. Biophys. Res. Commun. 2011, 415, 355–360. [Google Scholar] [CrossRef]
- Cho, I.; Adelmant, G.; Lim, Y.; Marto, J.A.; Cho, G.; Golden, J.A. Ascorbate peroxidase proximity labeling coupled with biochemical fractionation identifies promoters of ER-mitochondrial contacts. J. Biol. Chem. 2017, 292, 16382–16392. [Google Scholar] [CrossRef] [PubMed]
- Schumann, U.; Subramani, S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008, 18, 253–256. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.-L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Hughes, C.E.; Henderson, K.A.; Yazvenko, N.; Gottschling, D.E. Author response: Selective sorting and destruction of mitochondrial membrane proteins in aged yeast. eLife 2016, 5, e13943. [Google Scholar] [CrossRef]
- Neuspiel, M.; Schauss, A.C.; Braschi, E.; Zunino, R.; Rippstein, P.; Rachubinski, R.A.; Andrade, M.; McBride, H.M. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr. Biol. 2008, 18, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Vasam, G.; Nadeau, R.; Cadete, V.J.J.; Lavallée-Adam, M.; Menzies, K.J.; Burelle, Y. Proteomics characterization of mitochondrial-derived vesicles under oxidative stress. FASEB J. 2021, 35, e21278. [Google Scholar] [CrossRef]
- Chiabrando, D.; Marro, S.; Mercurio, S.; Giorgi, C.; Petrillo, S.; Vinchi, F.; Fiorito, V.; Fagoonee, S.; Camporeale, A.; Turco, E.; et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Investig. 2012, 122, 4569–4579. [Google Scholar] [CrossRef]
- Khan, A.A.; Quigley, J.G. Heme and FLVCR-related transporter families SLC48 and SLC49. Mol. Asp. Med. 2013, 34, 669–682. [Google Scholar] [CrossRef]
- Korolnek, T.; Zhang, J.; Beardsley, S.; Scheffer, G.L.; Hamza, I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014, 19, 1008–1019. [Google Scholar] [CrossRef]
- Taylor, E.B.; Rutter, J. Mitochondrial quality control by the ubiquitin–proteasome system. Biochem. Soc. Trans. 2011, 39, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.B.; Doty, R.T.; Yang, Z.; Quigley, J.G.; Chen, J.; Knoblaugh, S.; Kingsley, P.D.; De Domenico, I.; Vaughn, M.B.; Kaplan, J.; et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 2008, 319, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.; Yang, Z.; Worthington, M.T.; Phillips, J.D.; Sabo, K.M.; Sabath, D.E.; Berg, C.L.; Sassa, S.; Wood, B.L.; Abkowitz, J.L. Identification of a human heme exporter that is essential for erythropoiesis. Cell 2004, 118, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Rao, A.U.; Amigo, J.; Tian, M.; Upadhyay, S.K.; Hall, C.; Uhm, S.; Mathew, M.K.; Fleming, M.; Paw, B.; et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 2008, 453, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Yuan, X.; Schmidt, P.J.; Bresciani, E.; Samuel, T.K.; Campagna, D.; Hall, C.; Bishop, K.; Calicchio, M.L.; Lapierre, A.; et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Freibert, S.A. Mechanisms of mitochondrial iron-sulfur protein biogenesis. Ann. Rev. Biochem. 2020, 89, 471–499. [Google Scholar] [CrossRef] [PubMed]
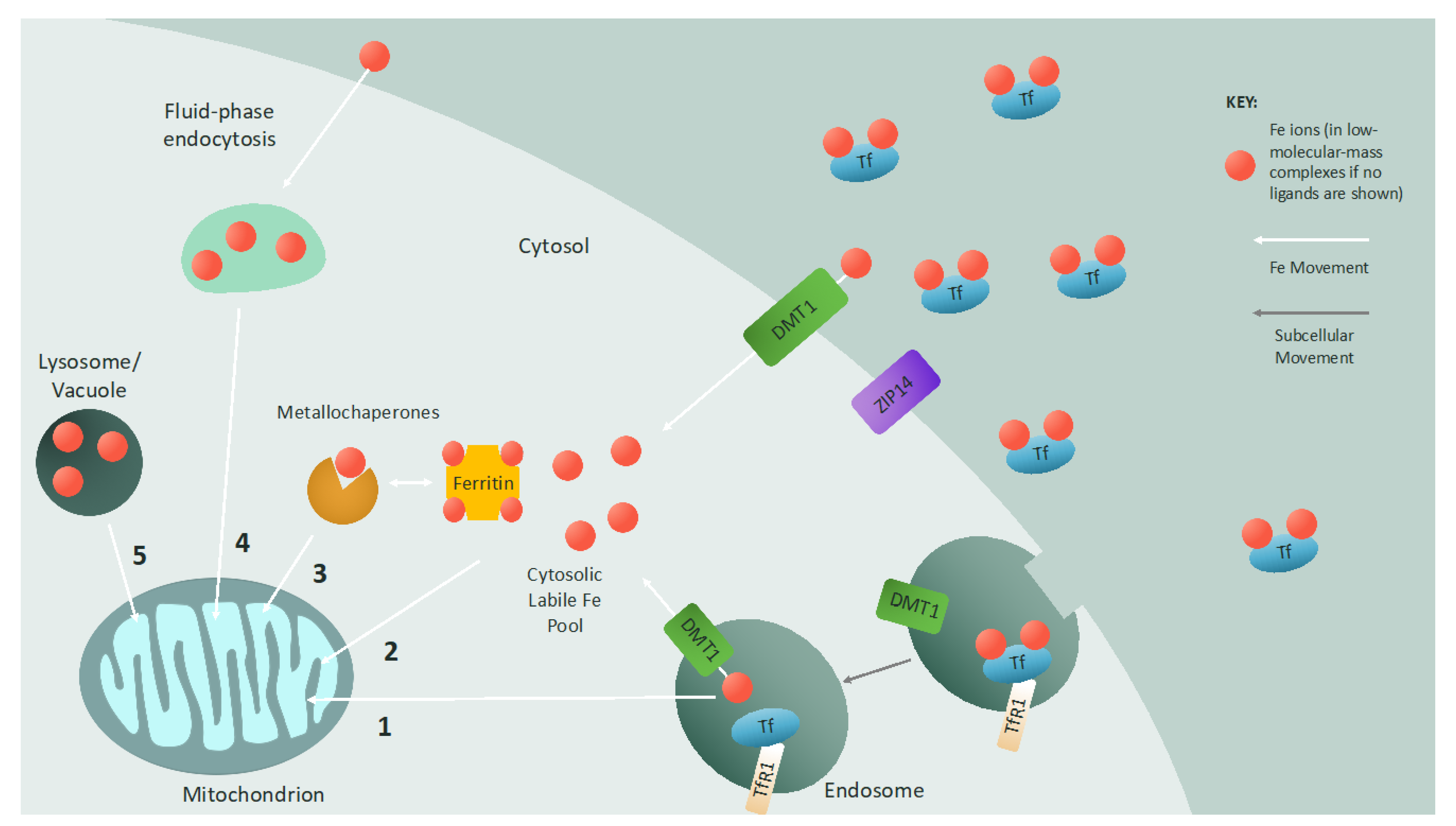

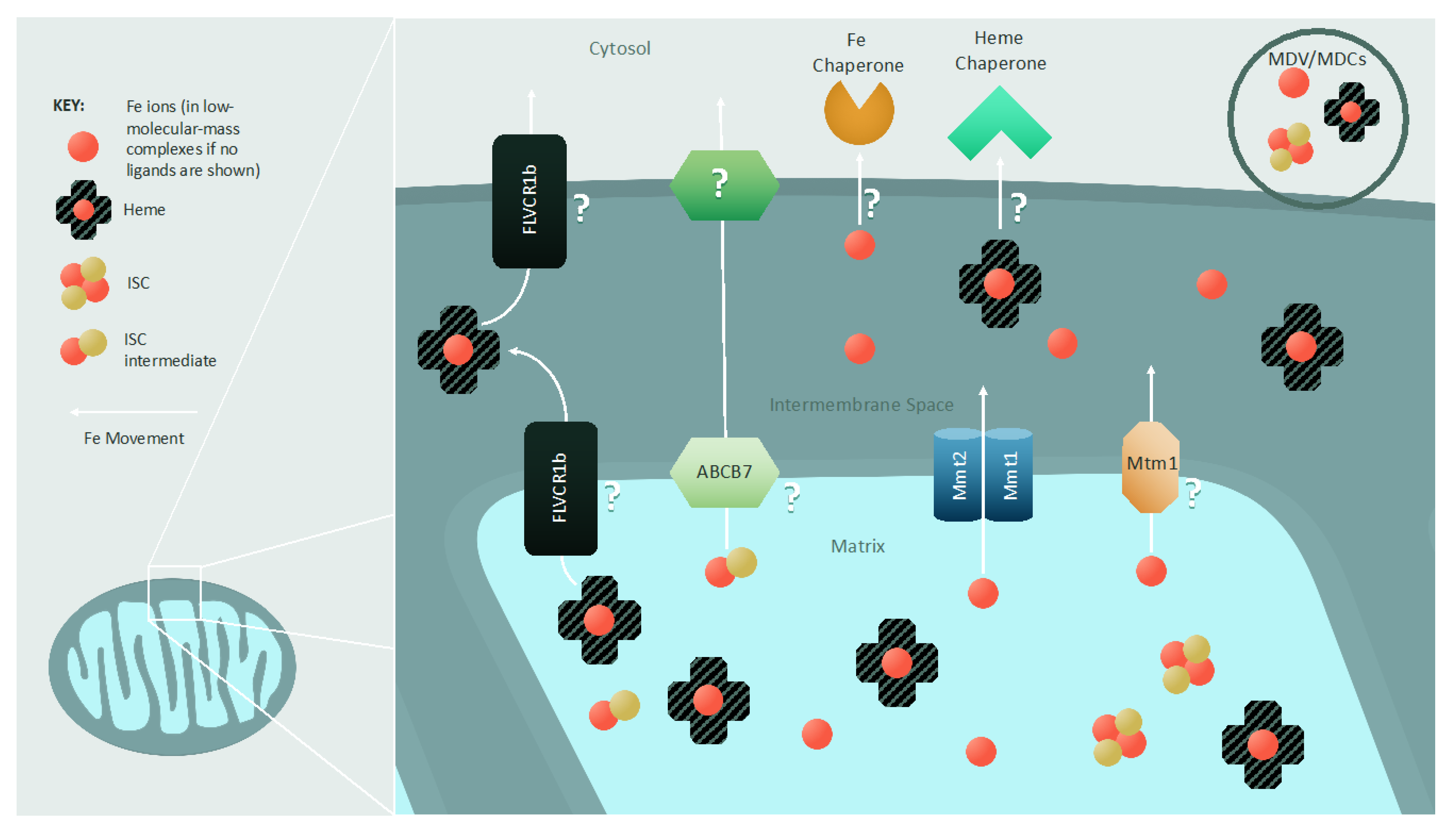
| Pathway | S. cerevisiae | Mammalian | Function | Pathology |
|---|---|---|---|---|
| Mitochondrial Fe Import | Por1/Por2 | VDAC | Mitochondrial Fe import? | - |
| - | DMT1 | Mitochondrial Fe import | Hypochromic microcytic anemia [70,71,72] | |
| - | PCBP2 | Fe transporter | - | |
| Mrs3/Mrs4 | MFRN1/ MFRN2 | IMM Fe importer | - | |
| Rim2 | - | IMM low-affinity Fe importer | - | |
| Mdl1 (peptide transporter) | ABCB10 | Stabilization of MFRN1/IMM biliverdin transporter in mammals? | - | |
| - | MCU | IMM Ca (Fe?) import | - | |
| - | SFXN1-5 | Similar and differing functions in serine transport and Fe transport across IMM | Mitochondriopathy; macrocytic anemia [73] | |
| Mitochondrial Iron Storage | - | FTMT | Mitochondrial Fe storage | - |
| ISC Biogenesis and Maturation of ISC Proteins | Nfs1 | NFS1 | Cysteine desulfurase/sulfur donation | Combined oxidative phosphorylation deficiency [74,75] |
| Isd11 | ISD11 | Nfs1 stabilization/regulation | Combined oxidative phosphorylation deficiency [76] | |
| Acp1 | ACP1 | Nfs1 stabilization/regulation | - | |
| Yfh1 | FXN | Iron donation?/ISC biogenesis regulator | Friedreich’s ataxia [77,78,79,80,81,82,83] | |
| Isu1/Isu2 | ISCU | Core ISC biogenesis scaffold | Hereditary myopathy with lactic acidosis [84,85,86,87] | |
| Yah1 | FDX2 | Electrons for ISC synthesis | Episodic mitochondrial myopathy [88,89] | |
| Arh1 | FDXR | Electrons for ISC synthesis | Auditory neuropathy; optic atrophy [90] | |
| Ssq1 | HSPA9 | ISC transfer | SA [91,92] | |
| Jac1 | HSC20 | ISC transfer | - | |
| Mge1 | GRPEL1 | Nucleotide release factor for Ssq1/HSPA9 | - | |
| Grx5 | GLRX5 | ISC transfer | SA [93,94,95] | |
| Isa1 | ISCA1 | 4Fe-4S synthesis | MMDS [96,97] | |
| Isa2 | ISCA2 | 4Fe-4S synthesis | MMDS [98,99,100,101] | |
| Iba57 | IBA57 | 4Fe-4S synthesis | MMDS [102,103,104,105,106,107] | |
| Nfu1 | NFU1 | Maturation of ISC proteins | MMDS [108,109] | |
| - | NUBPL | Maturation of ISC proteins | Mitochondrial complex I deficiency [110,111,112,113] | |
| Bol3 | BOLA3 | Maturation of ISC proteins | MMDS 2 [108,114,115] | |
| Bol1 | BOLA1 | Maturation of ISC proteins | - | |
| Atm1 | ABCB7 | Fe or ISC intermediate export | SA with ataxia [116,117,118] | |
| - | ABCB8 | Fe or ISC intermediate export? | - | |
| Heme Biosynthesis and Modification | Hem15 | FECH | Final step in formation of heme b | Erythropoietic protoporphyria [119,120,121,122,123,124,125] |
| Cox10 | COX10 | Formation of heme o intermediate from heme b | Mitochondrial complex IV deficiency [126,127,128] | |
| Cox15 | COX15 | Formation of heme a from heme o intermediate | Mitochondrial complex IV deficiency [129,130,131,132] | |
| Cyc3/Cyt2 | HCCS | Heme installation in cytochrome c and cytochrome c1 | - | |
| Mitochondrial Fe Export | Mmt1/Mmt2 | - | Mitochondrial Fe export | - |
| Mtm1 | Slc25a39 | IMM Fe export | - | |
| - | FLVCR1b | Mitochondrial heme export? | - | |
| Dap1 | PGRMC1 | Cytosolic acceptor of mitochondrial heme? | - | |
| - | HBP-1 | Cytosolic acceptor of mitochondrial heme? | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. https://doi.org/10.3390/cells10092198
Dietz JV, Fox JL, Khalimonchuk O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells. 2021; 10(9):2198. https://doi.org/10.3390/cells10092198
Chicago/Turabian StyleDietz, Jonathan V., Jennifer L. Fox, and Oleh Khalimonchuk. 2021. "Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond" Cells 10, no. 9: 2198. https://doi.org/10.3390/cells10092198
APA StyleDietz, J. V., Fox, J. L., & Khalimonchuk, O. (2021). Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells, 10(9), 2198. https://doi.org/10.3390/cells10092198







