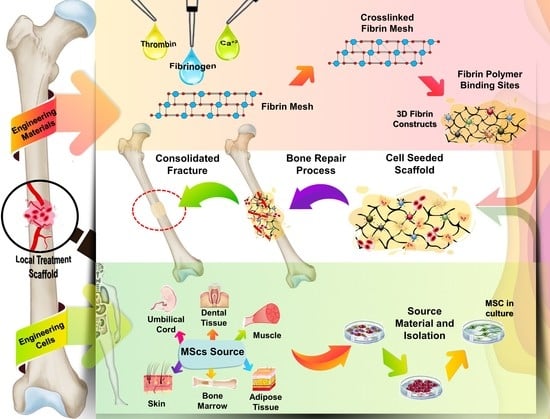
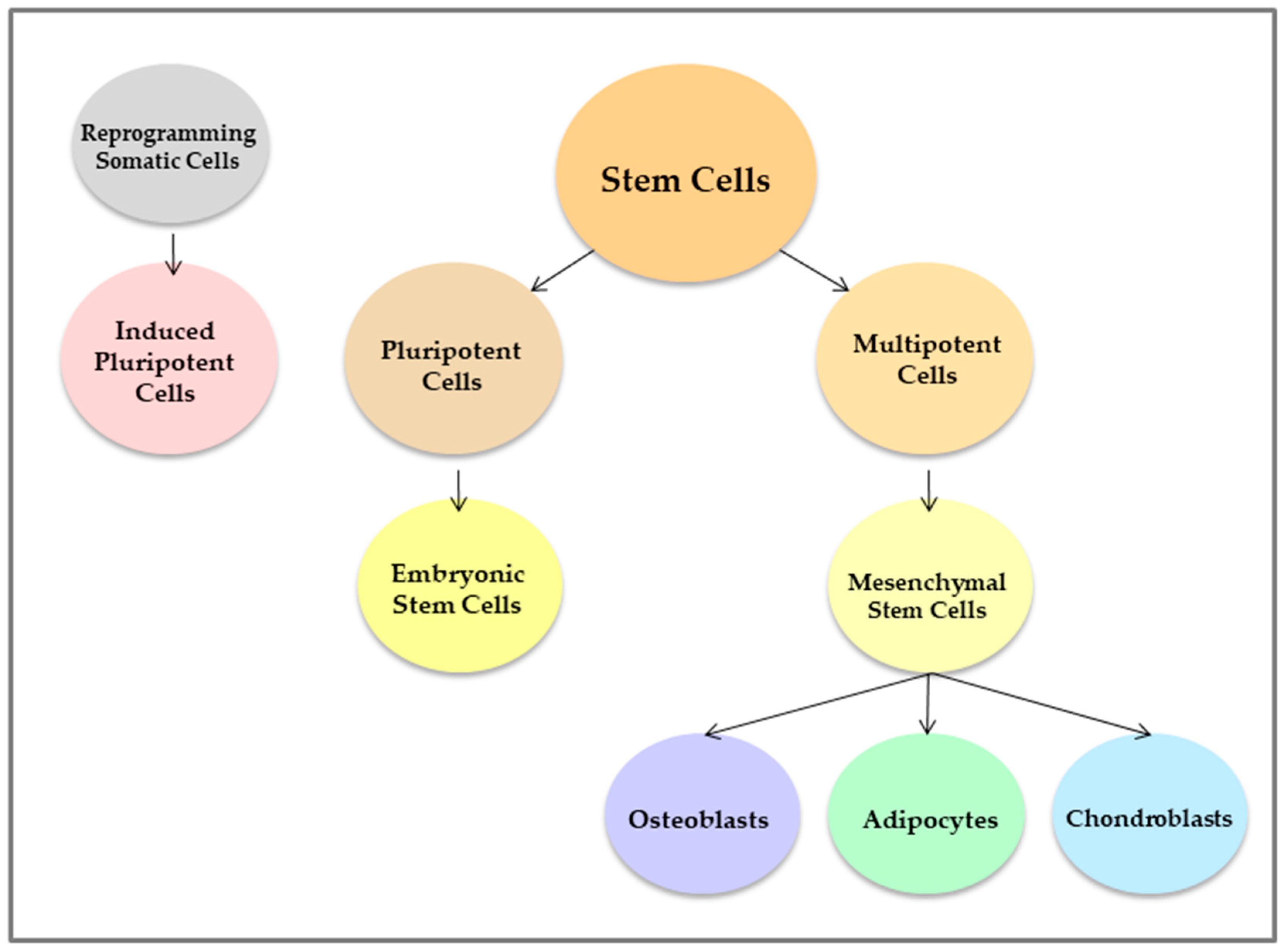
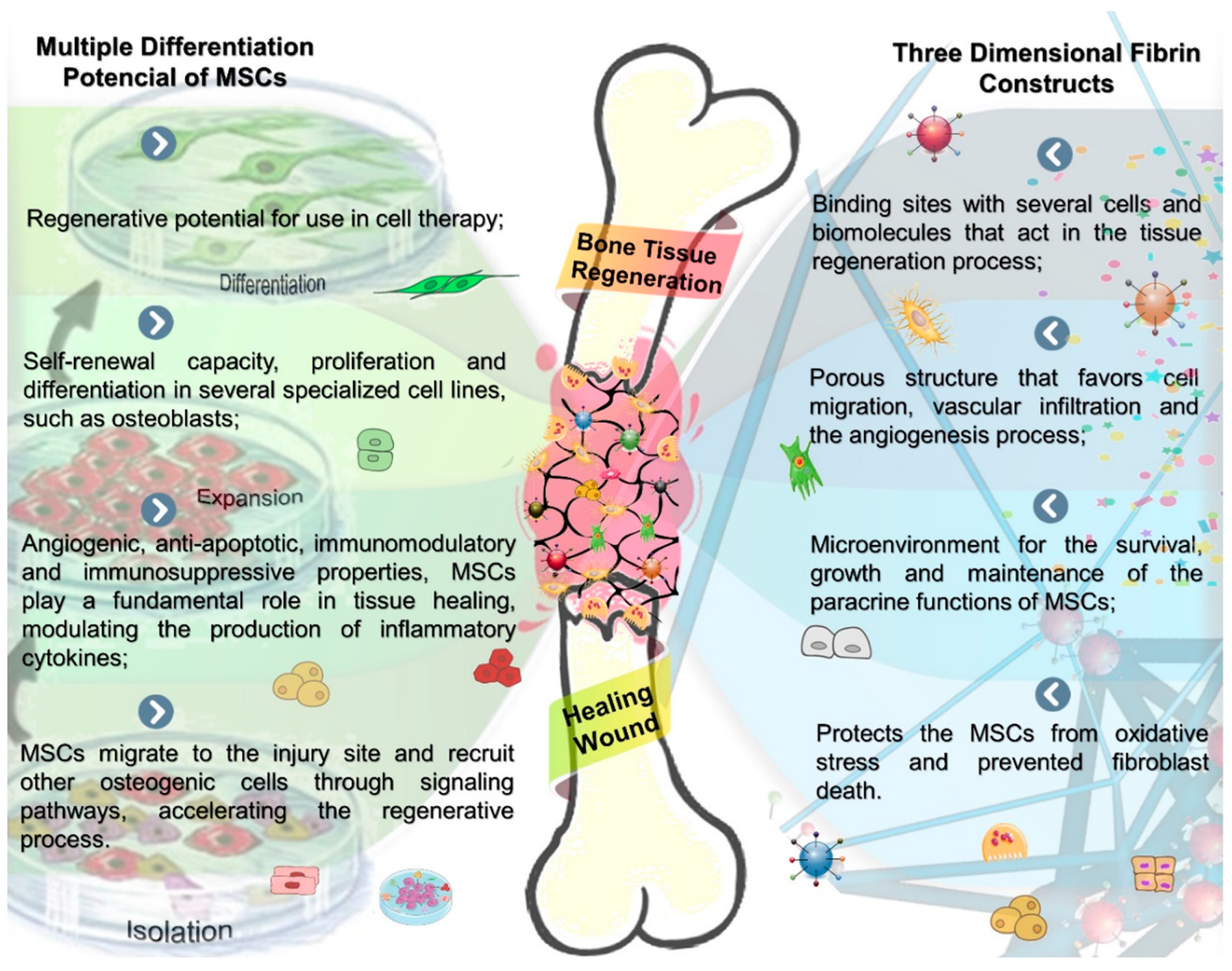
Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Bibliographic Search Strategy
2.2. Study Eligibility
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Ren, J.; Li, J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy 2012, 14, 555–562. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Doddis, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell. Reprogram. 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng.-Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Breen, A.; O’Brien, T.; Pandit, A. Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef]
- Brower, J.; Blumberg, S.; Carroll, E.; Pastar, I.; Brem, H.; Chen, W. Mesenchymal Stem Cell Therapy and Delivery Systems in Nonhealing Wounds. Adv. Skin Wound Care 2011, 24, 524–532. [Google Scholar] [CrossRef]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 1–15. [Google Scholar] [CrossRef]
- Valbonesi, M. Fibrin glues of human origin. Best Pract. Res. Clin. Haematol. 2006, 19, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Tabélé, C.; Curti, C.; Terme, T.; Rathelot, P.; Gensollen, S.; Vanelle, P. Organic glues or fibrin glues from pooled plasma: Efficacy, safety and potential as scaffold delivery systems. J. Pharm. Pharm. Sci. 2012, 15, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.; Caplice, N.M.; Clover, A.J.P. Fibrin as a delivery system in wound healing tissue engineering applications. J. Control. Release 2014, 196, 1–8. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; Shahrokhi, S.; Finnerty, C.C.; Branski, L.K.; Dibildox, M. Wound Coverage Technologies in Burn Care: Established Techniques. J. Burn Care Res. 2018, 39, 313–318. [Google Scholar] [CrossRef]
- Krug, C.; Beer, A.; Hartmann, B.; Prein, C.; Clause-Schaumann, H.; Holzbach, T.; Aszodi, A.; Giunta, R.E.; Saller, M.M.; Volkmer, E. Fibrin glue displays promising in vitro characteristics as a potential carrier of adipose progenitor cells for tissue regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Khodakaram-Tafti, A.; Mehrabani, D.; Shaterzadeh-Yazdi, H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent. Res. J. 2017, 14, 79–86. [Google Scholar] [CrossRef]
- Kim, B.S.; Sung, H.M.; You, H.K.; Lee, J. Effects of fibrinogen concentration on fibrin glue and bone powder scaffolds in bone regeneration. J. Biosci. Bioeng. 2014, 118, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.; Huss, R. Bone engineering: Combining smart biomaterials and the application of stem cells. Artif. Organs 2006, 30, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Tawil, B.; Dunn, J.C.Y.; Wu, B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.K.; Yoon, J.I.; Kim, D.E.; Kim, M.; Ha, H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Eng.-Part A 2013, 19, 2373–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparotto, V.P.O.; Landim-Alvarenga, F.C.; Oliveira, A.L.R.; Simões, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Süloğlu, A.K.; Karacaoğlu, E.; Bilgic, H.A.; Selmanoğlu, G.; Koçkaya, E.A.; Karaaslan, C. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells on fibrin glue- or fibronectin-coated Ceraform®. J. Biomater. Appl. 2019, 34, 375–385. [Google Scholar] [CrossRef]
- Yamada, Y.; Boo, J.S.; Ozawa, R.; Nagasaka, T.; Okazaki, Y.; Hata, K.I.; Ueda, M. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J. Cranio-Maxillofac. Surg. 2003, 31, 27–33. [Google Scholar] [CrossRef]
- McDuffee, L.A.; Esparza Gonzalez, B.P.; Nino-Fong, R.; Aburto, E. Evaluation of an in vivo heterotopic model of osteogenic differentiation of equine bone marrow and muscle mesenchymal stem cells in fibrin glue scaffold. Cell Tissue Res. 2014, 355, 327–335. [Google Scholar] [CrossRef]
- Cassaro, C.V.; Justulim, L.A., Jr.; Lima, P.R.; Golim, M.A.; Biscola, N.P.; Castro, M.V.; Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S., Jr.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Bai, B.; Zhang, S.; Ye, J.; Zhai, H.; Chen, Y.; Zhang, L.; Zeng, Y. Study of a novel three-dimensional scaffold to repair bone defect in rabbit. J. Biomed. Mater. Res.-Part A 2014, 102, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Chang, H.K.; Park, J.H.; Kim, K.R.; Woo, S.M. Consideration of bone regeneration effect of stem cells: Comparison between adipose-derived stem cells and demineralized bone matrix. J. Craniofac. Surg. 2014, 25, 189–195. [Google Scholar] [CrossRef]
- Hao, C.; Wang, Y.; Shao, L.; Liu, J.; Chen, L.; Zhao, Z. Local Injection of Bone Mesenchymal Stem Cells and Fibrin Glue Promotes the Repair of Bone Atrophic Nonunion In Vivo. Adv. Ther. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Kang, E.J.; Byun, J.H.; Choi, Y.J.; Maeng, G.H.; Lee, S.L.; Kang, D.H.; Lee, J.S.; Rho, G.J.; Park, B.W. In Vitro and In Vivo Osteogenesis of Porcine Skin-Derived Mesenchymal Stem Cell–like Cells with a Demineralized Bone and Fibrin Glue Scaffold. Tissue Eng. Part A 2010, 16, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Golshahi, H.; Saffarian, Z.; Montazeri, S.; Khorasani, S.; Kazemnejad, S. Repair of Osteochondral Defects in Rabbit Knee Using Menstrual Blood Stem Cells Encapsulated in Fibrin Glue: A Good Stem Cell Candidate for the Treatment of Osteochondral Defects. Tissue Eng. Regen. Med. 2019, 16, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Ji, Y.H.; Rhee, S.C.; Dhong, E.S.; Park, S.H.; Yoon, E.S. Enhancement of Bone Regeneration Using Osteogenic-Induced Adipose- Derived Stem Cells Combined with Demineralized Bone Matrix in a Rat Critically-Sized Calvarial Defect Model. Curr. Stem Cell Res. Ther. 2012, 7, 165–172. [Google Scholar] [CrossRef]
- Lazarini, M.; Bourdeaux-Rego, P.; Giardini-Rosa, R.; Duarte, A.S.S.; Baratti, M.O.; Zorzi, A.R.; de Miranda, J.B.; Cesar, C.L.; Luzo, A.; Saad, S.T.O. Natural Type II Collagen Hydrogel, Fibrin Sealant, and Adipose-Derived Stem Cells as a Promising Combination for Articular Cartilage Repair. Cartilage 2017, 8, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.T.; Kwan, P.C.; Chen, Y.F.; Wong, Y.K. Comparison of the effectiveness of autologous fibrin glue and macroporous biphasic calcium phosphate as carriers in the osteogenesis process with or without mesenchymal stem cells. J. Chin. Med. Assoc. 2008, 71, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani, D.; Khodakaram-Tafti, A.; Shaterzadeh-Yazdi, H.; Zamiri, B.; Omidi, M. Comparison of the regenerative effect of adipose-derived stem cells, fibrin glue scaffold, and autologous bone graft in experimental mandibular defect in rabbit. Dent. Traumatol. 2018, 34, 413–420. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Mei, S.; Li, C.; Cai, C.; Ding, Y. In vivo alveolar bone regeneration by bone marrow stem cells/fibrin glue composition. Arch. Oral Biol. 2012, 57, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A., Jr.; Kaneno, R.; Golim, M.A.; Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendeckel, S.; Jodicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrich, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Cranio-Maxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Haleem, A.M.; El Singergy, A.A.; Sabry, D.; Atta, H.M.; Rashed, L.A.; Chu, C.R.; El Shewy, M.T.; Azzam, A.; Aziz, M.T.A. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: A pilot study and preliminary results. Cartilage 2010, 1, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.S.; Koh, Y.G. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold? Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthrosc.-J. Arthrosc. Relat. Surg. 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef]
- Jung, S.N.; Rhie, J.W.; Kwon, H.; Jun, Y.J.; Seo, J.W.; Yoo, G.; Oh, D.Y.; Ahn, S.T.; Woo, J.; Oh, J. In vivo cartilage formation using chondrogenic-differentiated human adipose-derived mesenchymal stem cells mixed with fibrin glue. J. Craniofac. Surg. 2010, 21, 468–472. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Ravari, H.; Hamidi-Almadari, D.; Salimifar, M.; Bonakdaran, S.; Parizadeh, M.R.; Koliakos, G. Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy 2011, 13, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.W.; Kim, I.; et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn’s fistula. Stem Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Mizushima, T.; Takahashi, H.; Takeyama, H.; Naito, A.; Haraguchi, N.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Yamamoto, H.; et al. A clinical trial of autologous adipose-derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg. Today 2016, 46, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Frisbie, D.; Kisiday, J.; McIlwraith, C.W. In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.A.E.; Giulivi, A.; Griffith, M.; Hincke, M. Fibrin Glues in Combination with Mesenchymal Stem Cells to Develop a Tissue-Engineered Cartilage Substitute. Tissue Eng. Part A 2011, 17, 323–335. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Wronski, T.J.; Turner, R.T. Histological analysis of bone. Methods Mol. Biol. 2008, 447, 325–341. [Google Scholar] [PubMed]
- Varela, A.; Jolette, J. Bone Toolbox: Biomarkers, Imaging Tools, Biomechanics, and Histomorphometry. Toxicol. Pathol. 2018, 46, 511–529. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Dou, W.; Jansen, J.A.; Walboomers, X.F. Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-substitutes. Mol. Imaging Biol. 2019, 21, 1003–1019. [Google Scholar] [CrossRef] [Green Version]
- Wehrli, F.W. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J. Magn. Reson. Imaging 2007, 25, 390–409. [Google Scholar] [CrossRef]


| Reference | Stem Cells Source | Treatment Groups Delivery System | Intervention Implantation Site | Analysis | Main Outcomes Conclusions |
|---|---|---|---|---|---|
| Cassaro et al., 2019 [31] | Allogeneic rats BM-MSCs (femurs/tibias) | G1: No filling G2: Biphasic calcium phosphate (BCP) G3: Fibrin biopolymer (FBP) + BCP G4: FBP + MSCs G5: FBP + BCP + MSCs | Implantation of the scaffold in a 5 mm bone defect in the right femur of the rats (n = 8). Analyzes were performed after 30 and 60 days of the procedures. | Computed tomography, scanning electron microscopy and histological analysis. | All groups exhibited bone matrix formation, with significantly higher bone formation in FBP + MSCs. FBP proved to be an excellent scaffold for bone repair therapies. |
| Chen et al., 2014 [32] | Allogeneic rabbits BM-MSCs (femurs) | G1: Decalcified bone matrix (DBM) + fibrin gel (FG) + MSCs G2: DBM + FG G3: No filling | Implantation of the scaffold in a 10 mm bone defect in the left femur of the rabbits (n = 10). Analyzes were performed after 12 weeks of the procedures. | Serum proteomics (2D-PAGE and MALDI-TOF-TOF-MS), hematoxylin–eosin (HE) staining, ALP staining and osteopontin immunofluorescence detection. | DBM + FG + MSCs exhibited better bone regeneration than other groups. The combination of DBM + FG + MSCs can result in successful bone formation and can be used as a scaffold for bone tissue engineering. |
| Han et al., 2014 [33] | Human AD-MSCs (human abdominal fat) | G1: Decalcified bone matrix (DBM) + fibrin glue (FG) G2: MSCs + FG G3: MSCs + DBM + FG G4: No filling | Implantation of the scaffold in a 10 mm cranial defects in rabbits (n = 5). Analyzes were performed after 6 weeks of the procedures. | Computed tomography and histological analysis. | MSCs + DBM + FG presented better bone formation than others groups. Scaffold containing MSCs could be helpful in the correction of extensive bone defects. |
| Hao et al., 2014 [34] | Allogeneic rats BM-MSCs (femurs/tibias) | G1: control group G2: atrophic nonunion Group—fibrin glue (FG) G3: experimental group (MSCs + FG) | Injection of FG and MSCs + FG into the osteotomized right rat femur (with a length of 1 mm) of G2 and G3, respectively (n = 12). Analyzes were performed after 8 weeks of the procedures. | Radiographic and histological analysis. Biomechanical test. | MSCs + FG presented complete bony bridging of the osteotomy gap, with the formation of plenty of woven bone. Local injection of MSCs seeded fibrin glue promoted atrophic nonunion repair. |
| Kang et al., 2010, [35] | Autogenous pigs skin-derived MSCs (SD-MSCs) (ears) | G1: Demineralized bone (DMB) + fibrin glue (FG) + MSCs G2: Demineralized bone (DMB) + fibrin glue (FG) | Implantation of the scaffold in a lateral window (1 cm) in maxillary sinus of 4 pigs. In each pig, one maxillary sinus received only the scaffold and the other sinus received the scaffold with MSCs. Analyzes were performed after 2 and 4 weeks of the procedures. | Histological analysis. | Trabecular bone formation were more pronounced in DMB + FG + MSCs, Autogenous MSCs grafting with a DMB + FG scaffold can serve as a predictable alternative to bone grafting in the maxillary sinus floor. |
| Khanmoha-mmadi et al., 2019 [36] | Human menstrual blood-derived stem cells (MenSCs) | G1: Right defect: fibrin glue (FG) + MenSCs Left defect: FG G2: Left defect: No filling | Implantation of the scaffold in a knees osteochondral defects (3 × 4 mm2) of the rabbits (n = 12). Analyzes were performed after 3 and 6 months of the procedures. | Gross morphological and histological analysis. | The most regenerated tissue in FG + MenSCs was similar to hyaline cartilage and it was higher than other experimental groups. MenSCs encapsulated in FG was more effective in defect repair compared to FG alone. |
| Kim et al., 2012 [37] | Autogenous Rats AD-MSCs (inguinal adipose tissue) | G1: Fibrin glue (FG) G2: FG + Demineralized Bone Matrix (DBX) G3: FG + DBX + MSCs G4: FG + DBX + iMSCs (osteogenic induced) | Implantation of the scaffold in a 8 mm critical calvarial bone defect in rats (n = 10). Analyzes were performed after 8 weeks of the procedures. | Radiographic, histological and radiodensitometric analysis. | The mean radiodensity of the FG, FG + DBX, FG + DBX + MSCs and FG + DBX + iMSCs groups was 16.78%, 39.94%, 25.58% and 51.31%, respectively. FG + DBX + iMSCs group showed the better potential to regenerate bone defects. |
| Lazarini et al., 2017 [38] | Human AD-MSCs (abdominal liposuction) | Type II collagen hydrogel and fibrin sealant implant with (right knee) or without AD-MSCs (left knee) | Implantation of the scaffold in a 3 mm knees defects in 4 rabbits. Analyzes were performed after 12 weeks of the procedures. | Histological analysis. | Scaffold containing MSCs induced greater repair of chondral lesions and better cell organization and alignment of collagen fibers compared to the isolated use of the scaffold, being effective for articular cartilage repair. |
| Lee et al., 2008 [39] | Autogenous Rabbits BM-MSCs (iliac bone) | Group 1 (15 rabbits): Defect 1: MSCs + autologous fibrin glue (AFG) Defect 2: MSCs + macroporous biphasic calcium phosphate (MBCP) Defect 3: No filling Group 2 (3 rabbits): Defect 1: AFG Defect 2: MBCP Defect 3: No filling | Implantation of the scaffold in a 6 mm cranial defects (3 defects/rabbit). Analyzes were performed after 1, 2 and 3 months of the procedures. | Radiographic and histological analysis. | MSCs + AFG induced more bone formation 2 months post operation and more mature bone was found 3 months post operation compared with the other groups. MSCs + AFG resulted in earlier and more mature new bone formation. |
| Mehrabani et al., 2018 [40] | Allogeneic Rabbits AD-MSCs (subcutaneous adipose tissue) | G1: (n = 10) Right defect: autologous fibrin glue + MSCs Left defect: No filling (control) G2: (n = 10) Right defect: autologous fibrin glue Left defect: autologous bone graft (iliac crest) | Implantation of the scaffold in a bilateral 1.5 × 0.5 cm uni-cortical mandibular osteotomies in 20 rabbits. Analyzes were performed after 28 and 56 days of the procedures. | Computed tomography and histopathological analysis | There was accelerated osteogenesis in the treated defects, with better bone formation in FG + MSCs and autologous bone graft groups, which showed a significant and similar increase in the thickness of new cortical bone. FG + MSCs presented a remarkable reconstruction of cortical bone. |
| Zhang et al., 2012 [41] | Allogeneic rats BM-MSCs (femurs) | G1: blank (no filling) G2: fibrin glue (FG) G3: FG + MSCs | Implantation of the scaffold in a bilateral maxillar defects (3 mm) in rats (n = 5). Analyzes were performed after 6 weeks of the procedures. | Histological analysis and micro-CT. | The amount of new bone formed in the FG + MSCs was significantly greater than the others groups. The strategy of combing MSCs with FG is effective in the repair of alveolar bone defects. |
| Orsi et al., 2017 [42] | Allogeneic rats BM-MSCs (femurs) | Non-ovariectomized (NOVX): G1: Fibrin sealant (FS) G2: FS + MSCs G3: FS + iMSCs (differentiated in osteogenic lineage) G4: No filling G5: No injury Ovariectomized (OVX): G1: FS G2: FS + MSCs G3: FS + iMSCs G4: No filling G5: No injury | Implantation of the scaffold in a femur defects (5 mm) in rats (n = 4). Analyzes were performed after 14 and 28 days of the procedures. | Microcomputed tomography, biochemical analysis, radiographic and histology analysis, scanning electron microscopy. | After 14 days, in both the OVX and NOVX animals, FS + MSCs and FS + iMSCs showed a higher formation of bone cells in relation to the control group. Bone neoformation was observed in all treated and control groups. No morphological differences in the femurs of the NOVX and OVX animals were observed after the surgery. |
| Reference | Stem Cells Source | Treatment Groups Delivery System | Intervention Implantation Site | Analysis | Main Outcomes Conclusions |
|---|---|---|---|---|---|
| Lendeckel et al., 2004 [43] | Autologous human AD-MSCs (gluteal area) | Autologous cancellous bone (iliac crest) + autologous fibrin glue (FG) + autologous MSCs | Implantation of bone + FG + MSCs in multifragment cranial fracture in a 7-years-old girl. Analysis were performed after 3 months of the surgery. | Computed tomography (CT) and ultrasound analysis. | Postoperative healing was uneventful and without neurological deficits. CT-scans 3 months post-operatively showed new bone formation and near complete cranial continuity. There is no way to determine how much of the effect was due to the grafted bone or the FG + MSCs. |
| Haleem et al., 2010 [44] | Autologous human BM-MSCs (iliac crest) | platelet-rich fibrin glue (PR-FG) + MSCs | Implantation of the scaffold in 5 patients with cartilage lesion in the femoral condyle. Analyzes were performed after 6 and 12 months of the procedures. | Radiographic and magnetic resonance imaging analysis. | Complete defect fill and complete surface congruity with native cartilage was found in 3 patients, while 2 patients presented incomplete congruity. BM-MSCs transplantation on PR-FG as a scaffold may be an effective approach to promote the repair of articular cartilage defects of the knee in human patients. |
| Kim et al., 2015 [45] | Autologous human AD-MSCs (buttock liposuction) | G1: MSCs G2: MSCs + fibrin glue (FG) | Injection of MSCs (n = 37) or scaffold containing MSCs (n = 17) in patients with osteoarthritis in the knees. Analyzes were performed after approximately 29 months of the procedures. | Evaluation by International Knee Documentation Committee (IKDC) score, Tegner Activity scale and International Cartilage Repair Society (ICRS) grade. | There were no significant differences in outcome scores between groups However, at second-look arthroscopy, there were better ICRS grades in G2 (23% of lesions in G1 and 58% in G2 achieved grade I and II). Fibrin glue has proven to be an effective scaffold in MSC implantation for osteoarthritis knees treatment. |
| Koh et al., 2016 [46] | Autologous human AD-MSCs (buttock liposuction) | G1: microfracture (MFX) + fibrin glue (FG) + MSCs G2: MFX alone | Injection of scaffoldin patients with symptomatic knee cartilage defects (> 3 cm2) on the femoral condyle (n = 40). Analysis were performed after 24 months of the procedures. | Evaluation by magnetic resonance imaging, Lysholm score, Knee Injury and Osteoarthritis Outcome Score (KOOS) and a 10-point visual analog scale for pain. | G1 included 26 patients (65%) who had complete cartilage coverage of the lesion at follow-up compared with 18 patients (45%) in G2. The improvements in the mean KOOS pain and symptom subscores were significantly greater at follow-up in G1 than in G2. Compared with MFX alone, MFX + FG + MSCs provided an improved radiologic appearance of lesions and KOOS pain/symptom subscore improvements. |
| Kim et al., 2017 [47] | Autologous human AD-MSCs (buttock liposuction) | G1: arthroscopic rotator cuff repair G2: arthroscopic rotator cuff repair + injection of AD-MSCs in fibrin glue (FG) | Injection of scaffols containing MSCs in patients with osteoarthritis in the knees (n = 35/per group). Analysis were performed after approximately 28 months of the procedures. | Evaluation by magnetic resonance imaging (MRI), visual analog scale (VAS), range of motion (ROM), functional measures of Constant score and University of California, Los Angeles (UCLA) shoulder rating scale. | MRI indicated a retear rate of 28.5% in G1 and 14.3% in G2. There was no significant difference between groups in the other parameters analyzed. The injection of FG + MSCs during rotator cuff repair could significantly improve structural outcomes in terms of the retear rate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.d.C.; Fideles, S.O.M.; Pomini, K.T.; Reis, C.H.B.; Bueno, C.R.d.S.; Pereira, E.d.S.B.M.; Rossi, J.d.O.; Novais, P.C.; Pilon, J.P.G.; Rosa Junior, G.M.; et al. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells 2021, 10, 2323. https://doi.org/10.3390/cells10092323
Ortiz AdC, Fideles SOM, Pomini KT, Reis CHB, Bueno CRdS, Pereira EdSBM, Rossi JdO, Novais PC, Pilon JPG, Rosa Junior GM, et al. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells. 2021; 10(9):2323. https://doi.org/10.3390/cells10092323
Chicago/Turabian StyleOrtiz, Adriana de Cássia, Simone Ortiz Moura Fideles, Karina Torres Pomini, Carlos Henrique Bertoni Reis, Cleuber Rodrigo de Souza Bueno, Eliana de Souza Bastos Mazuqueli Pereira, Jéssica de Oliveira Rossi, Paulo Cezar Novais, João Paulo Galletti Pilon, Geraldo Marco Rosa Junior, and et al. 2021. "Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review" Cells 10, no. 9: 2323. https://doi.org/10.3390/cells10092323
APA StyleOrtiz, A. d. C., Fideles, S. O. M., Pomini, K. T., Reis, C. H. B., Bueno, C. R. d. S., Pereira, E. d. S. B. M., Rossi, J. d. O., Novais, P. C., Pilon, J. P. G., Rosa Junior, G. M., Buchaim, D. V., & Buchaim, R. L. (2021). Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells, 10(9), 2323. https://doi.org/10.3390/cells10092323