Regulation of T Cell Responses by Ionic Salt Signals
Abstract
1. Introduction
2. Ionic Signals in the Tissue Microenvironment
3. The Role of Ionic Signals in Shaping Th1–Th2 Cell Dualism
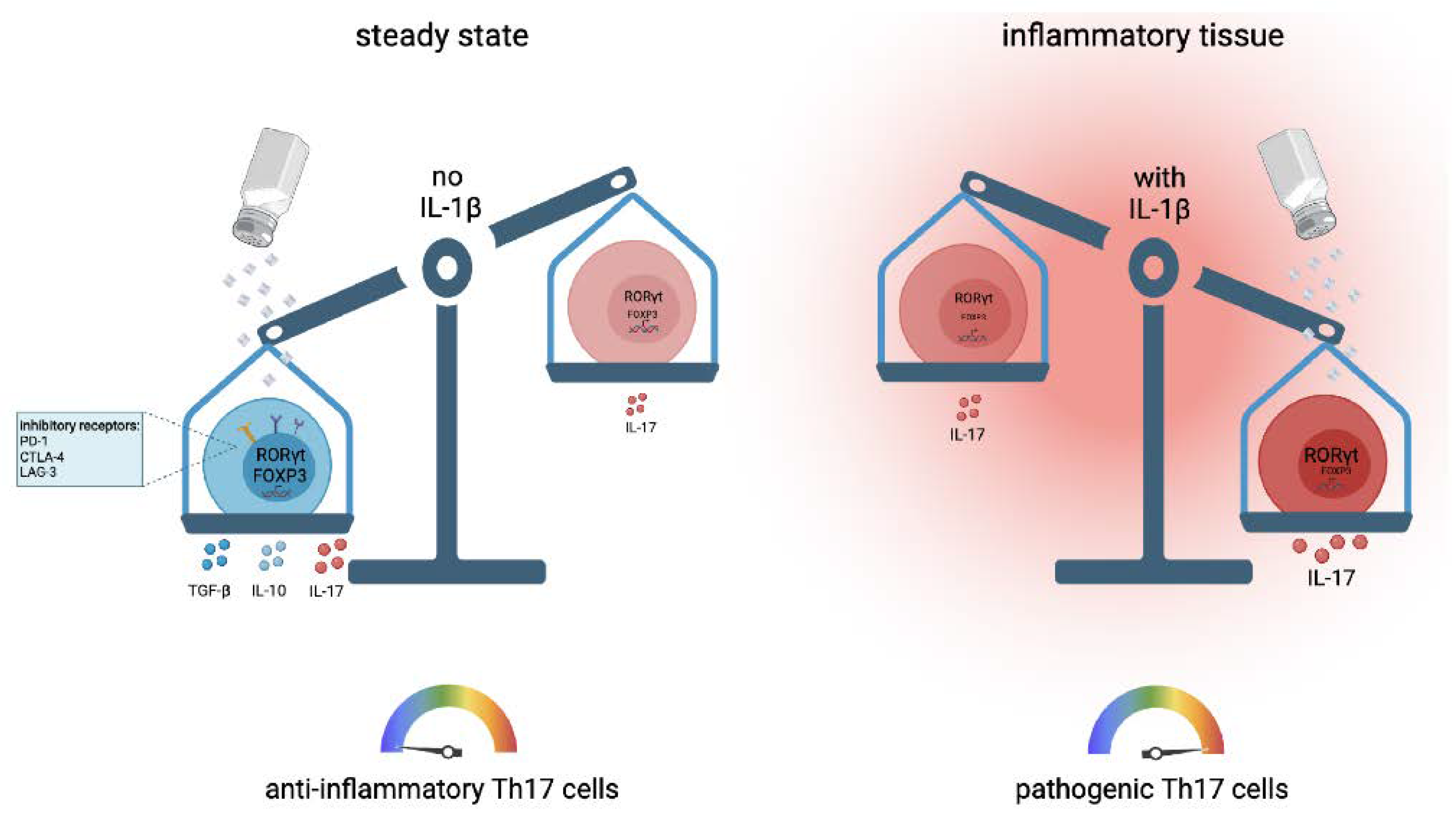
4. The++ Role of Ionic Signals in Th17 Cell Development and Th17 Cell Functions
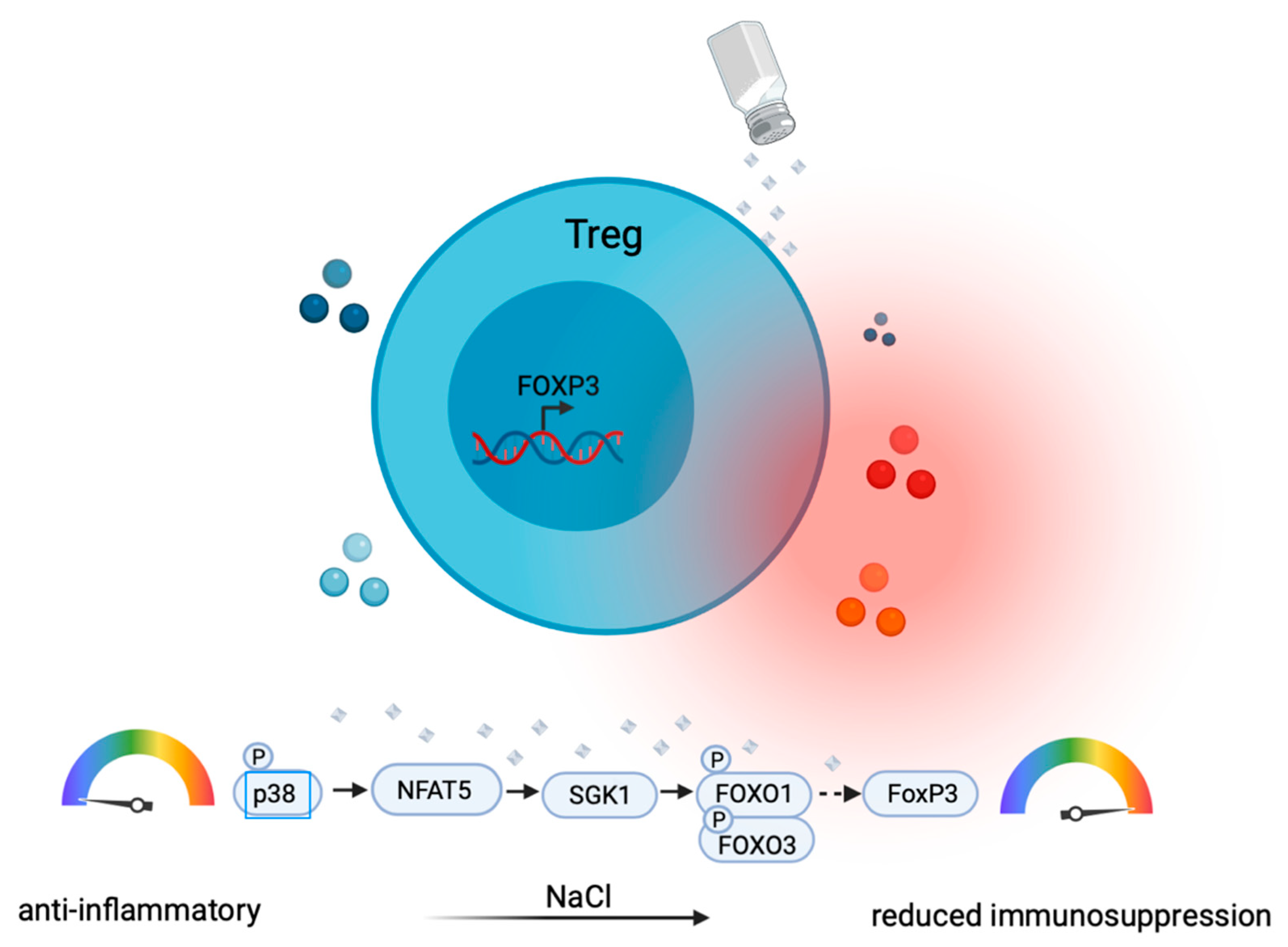
5. The Role of Ionic Signals in Treg Cells
6. Regulation of Pro- versus Anti-Inflammatory T Cell Functions by Salt
7. Therapeutic Considerations for Ionic Signaling in T Helper Cells
8. Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eyerich, S.; Zielinski, C. Defining Th-cell subsets in a classical and tissue-specific manner: Examples from the skin. Eur. J. Immunol. 2014, 44, 3475–3483. [Google Scholar] [CrossRef]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef]
- Noster, R.; Riedel, R.; Mashreghi, M.-F.; Radbruch, H.; Harms, L.; Haftmann, C.; Chang, H.-D.; Radbruch, A.; Zielinski, C.E. IL-17 and GM-CSF Expression Are Antagonistically Regulated by Human T Helper Cells. Sci. Transl. Med. 2014, 6, 241ra80. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; Van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar]
- E Zielinski, C. Autoimmunity beyond Th17: GM-CSF producing T cells. Cell Cycle 2014, 13, 2489–2490. [Google Scholar] [CrossRef][Green Version]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Reich, K.; Armstrong, A.W.; Langley, R.G.; Flavin, S.; Randazzo, B.; Li, S.; Hsu, M.-C.; Branigan, P.; Blauvelt, A. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): Results from a phase 3, randomised controlled trial. Lancet 2019, 394, 831–839. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef]
- Sitkovsky, M.V.; Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat. Rev. Immunol. 2005, 5, 712–721. [Google Scholar] [CrossRef]
- Shapiro, L.; Dinarello, C.A. Osmotic regulation of cytokine synthesis in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 12230–12234. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.N.; Wilck, N.; Haase, S.; Kleinewietfeld, M.; Linker, R.A. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat. Rev. Immunol. 2019, 19, 243–254. [Google Scholar] [CrossRef]
- Morelle, J.; Goffin, E.; Devuyst, O. Molecular Physiology of Water Balance. N. Engl. J. Med. 2015, 373, 196. [Google Scholar] [CrossRef]
- Titze, J.; Rakova, N.; Kopp, C.; Dahlmann, A.; Jantsch, J.; Luft, F.C. Balancing wobbles in the body sodium. Nephrol. Dial. Transplant. 2016, 31, 1078–1081. [Google Scholar] [CrossRef]
- Wang, P.; Deger, M.S.; Kang, H.; Ikizler, T.A.; Titze, J.; Gore, J.C. Sex differences in sodium deposition in human muscle and skin. Magn. Reson. Imaging 2017, 36, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.B.P.; De Nadai Fernandez, E. Neutron activation analysis: A primary method of measurement. Spectrochim. Acta Part B 2011, 66, 193–241. [Google Scholar] [CrossRef]
- Matthias, J.; Maul, J.; Noster, R.; Meinl, H.; Chao, Y.-Y.; Gerstenberg, H.; Jeschke, F.; Gasparoni, G.; Welle, A.; Walter, J.; et al. Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment. Sci. Transl. Med. 2019, 11, eaau0683. [Google Scholar] [CrossRef]
- Thulborn, K.R. Quantitative sodium MR imaging: A review of its evolving role in medicine. NeuroImage 2018, 168, 250–268. [Google Scholar] [CrossRef]
- Fischereder, M.; Michalke, B.; Schmoeckel, E.; Habicht, A.; Kunisch, R.; Pavelic, I.; Szabados, B.; Schönermarck, U.; Nelson, P.J.; Stangl, M. Sodium storage in human tissues is mediated by glycosaminoglycan expression. Am. J. Physiol. Physiol. 2017, 313, F319–F325. [Google Scholar] [CrossRef]
- Padtberg, J.H. Über die Bedeutung der Haut als Chlordepot. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1910, 63, 60–79. [Google Scholar] [CrossRef]
- Machnik, A.; Neuhofer, W.; Jantsch, J.; Dahlmann, A.; Tammela, T.; Machura, K.; Park, J.-K.; Beck, F.-X.; Muller, D.N.; Derer, W.; et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat. Med. 2009, 15, 545–552. [Google Scholar] [CrossRef]
- Ivanova, L.N.; Archibasova, V.K.; Shterental’, I.S. Sodium-depositing function of the skin in white rats. Fiziol. Zhurnal SSSR Im. I. M. Sechenova 1978, 64, 358–363. [Google Scholar]
- Hammon, M.; Grossmann, S.; Linz, P.; Kopp, C.W.; Dahlmann, A.; Garlichs, C.D.; Janka, R.; Cavallaro, A.; Luft, F.C.; Uder, M.; et al. 23Na Magnetic Resonance Imaging of the Lower Leg of Acute Heart Failure Patients during Diuretic Treatment. PLoS ONE 2015, 10, e0141336. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Titze, J.; Shakibaei, M.; Schafflhuber, M.; Schulze-Tanzil, G.; Porst, M.; Schwind, K.H.; Dietsch, P.; Hilgers, K.F. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am. J. Physiol. Circ. Physiol. 2004, 287, H203–H208. [Google Scholar] [CrossRef]
- Zielinski, C.E. Human T cell immune surveillance: Phenotypic, functional and migratory heterogeneity for tailored immune responses. Immunol. Lett. 2017, 190, 125–129. [Google Scholar] [CrossRef]
- DeVore, S.B.; Gonzalez, T.; Sherenian, M.G.; Herr, A.B.; Hershey, G.K.K. On the surface. Ann. Allergy Asthma Immunol. 2020, 125, 628–638. [Google Scholar] [CrossRef]
- Parfentjev, I.A.; Catelli, A.R. Tolerance of staphylococcus aureus to sodium chloride. J. Bacteriol. 1964, 88, 1–3. [Google Scholar] [CrossRef]
- Matthias, J.; Zielinski, C.E. Shaping the diversity of Th2 cell responses in epithelial tissues and its potential for allergy treatment. Eur. J. Immunol. 2019, 49, 1321–1333. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Kopp, C.; Beyer, C.; Eckardt, K.-U.; Schett, G.; Luft, F.C.; Uder, M.; Distler, J.H.W.; Titze, J.; Linz, P.; Dahlmann, A.; et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatology 2017, 56, 556–560. [Google Scholar] [CrossRef]
- Crescenzi, R.; Marton, A.; Donahue, P.M.; Mahany, H.B.; Lants, S.K.; Wang, P.; Beckman, J.A.; Donahue, M.J.; Titze, J. Tissue Sodium Content is Elevated in the Skin and Subcutaneous Adipose Tissue in Women with Lipedema. Obesity 2018, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, J.; Schatz, V.; Friedrich, D.; Schröder, A.; Kopp, C.; Siegert, I.; Maronna, A.; Wendelborn, D.; Linz, P.; Binger, K.; et al. Cutaneous Na+ Storage Strengthens the Antimicrobial Barrier Function of the Skin and Boosts Macrophage-Driven Host Defense. Cell Metab. 2015, 21, 493–501. [Google Scholar] [CrossRef]
- Neubert, P.; Weichselbaum, A.; Reitinger, C.; Schatz, V.; Schröder, A.; Ferdinand, J.R.; Simon, M.; Bär, A.-L.; Brochhausen, C.; Gerlach, R.G.; et al. HIF1A and NFAT5 coordinate Na+-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting. Autophagy 2019, 15, 1899–1916. [Google Scholar] [CrossRef]
- Paling, D.; Solanky, B.S.; Riemer, F.; Tozer, D.J.; Wheeler-Kingshott, C.A.M.; Kapoor, R.; Golay, X.; Miller, D.H. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain 2013, 136, 2305–2317. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zhong, A.; Xie, P.; Jia, S.; Xie, Z.; Zeitchek, M.; Niknam-Bienia, S.; Zhao, J.; Porterfield, D.M.; et al. Sodium channel Na x is a regulator in epithelial sodium homeostasis. Sci. Transl. Med. 2015, 7, 312ra177. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, S.; Xie, P.; Friedrich, E.; Galiano, R.D.; Qi, S.; Mao, R.; Mustoe, T.A.; Hong, S.J. Knockdown of sodium channel Nax reduces dermatitis symptoms in rabbit skin. Lab. Investig. 2020, 100, 751–761. [Google Scholar] [CrossRef]
- Dolivo, D.; Rodrigues, A.; Sun, L.; Li, Y.; Hou, C.; Galiano, R.; Hong, S.J.; Mustoe, T. The Nax (SCN7A) channel: An atypical regulator of tissue homeostasis and disease. Cell. Mol. Life Sci. 2021, 78, 5469–5488. [Google Scholar] [CrossRef]
- Chae, W.-J.; Ehrlich, A.; Maher, S.E.; Goldsmith-Pestana, K.; Shan, P.; Hwa, J.; Lee, P.J.; Krause, D.S.; Rothlin, C.V.; McMahon-Pratt, D.; et al. The Wnt Antagonist Dickkopf-1 Promotes Pathological Type 2 Cell-Mediated Inflammation. Immunity 2016, 44, 246–258. [Google Scholar] [CrossRef]
- Heikamp, E.; Patel, C.H.; Collins, S.L.; Waickman, A.; Oh, M.-H.; Sun, I.-H.; Illei, P.B.; Sharma, A.; Naray-Fejes-Toth, A.; Fejes-Toth, G.; et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat. Immunol. 2014, 15, 457–464. [Google Scholar] [CrossRef]
- Zielinski, C.; Corti, D.; Mele, F.; Pinto, D.; Lanzavecchia, A.; Sallusto, F. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 2011, 240, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Zielinski, C.E.; Lanzavecchia, A. Human Th17 subsets. Eur. J. Immunol. 2012, 42, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nat. Cell Biol. 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Matthias, J.; Heink, S.; Picard, F.S.R.; Zeiträg, J.; Kolz, A.; Chao, Y.-Y.; Soll, D.; De Almeida, G.P.; Glasmacher, E.; Jacobsen, I.D.; et al. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J. Clin. Investig. 2020, 130, 4587–4600. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Na, S.-Y.; Janakiraman, M.; Leliavski, A.; Krishnamoorthy, G. High-salt diet suppresses autoimmune demyelination by regulating the blood–brain barrier permeability. Proc. Natl. Acad. Sci. USA 2021, 118, e2025944118. [Google Scholar] [CrossRef]
- Wenstedt, E.F.E.; Remmerswaal, E.B.M.; Van Der Bom-Baylon, N.D.; Schrooten, E.M.; Bemelman, F.J.; Vogt, L. The effect of high-salt diet on t-lymphocyte subpopulations in healthy males—A pilot study. J. Clin. Hypertens. 2020, 22, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Hoeppli, R.E.; Wu, D.; Cook, L.; Levings, M.K. The Environment of Regulatory T Cell Biology: Cytokines, Metabolites, and the Microbiome. Front. Immunol. 2015, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Hori, S. Lineage stability and phenotypic plasticity of Foxp3+regulatory T cells. Immunol. Rev. 2014, 259, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Xiao, S.; Thalhamer, T.; Madi, A.; Han, T.; Kuchroo, V. SGK1 Governs the Reciprocal Development of Th17 and Regulatory T Cells. Cell Rep. 2018, 22, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef]
- Yang, Y.H.; Istomine, R.; Alvarez, F.; Al-Aubodah, T.-A.; Shi, X.Q.; Takano, T.; Thornton, A.M.; Shevach, E.M.; Zhang, J.; Piccirillo, C.A. Salt Sensing by Serum/Glucocorticoid-Regulated Kinase 1 Promotes Th17-like Inflammatory Adaptation of Foxp3+ Regulatory T Cells. Cell Rep. 2020, 30, 1515–1529.e4. [Google Scholar] [CrossRef]
- Alvarez, F.; Istomine, R.; Shourian, M.; Pavey, N.; Al-Aubodah, T.-A.; Qureshi, S.; Fritz, J.H.; Piccirillo, C.A. The alarmins IL-1 and IL-33 differentially regulate the functional specialisation of Foxp3+ regulatory T cells during mucosal inflammation. Mucosal Immunol. 2019, 12, 746–760. [Google Scholar] [CrossRef]
- Luo, Y.; Xue, Y.; Wang, J.; Dang, J.; Fang, Q.; Huang, G.; Olsen, N.; Zheng, S.G. Negligible Effect of Sodium Chloride on the Development and Function of TGF-β-Induced CD4+ Foxp3+ Regulatory T Cells. Cell Rep. 2019, 26, 1869–1879.e3. [Google Scholar] [CrossRef]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nat. Cell Biol. 2012, 484, 514–518. [Google Scholar] [CrossRef]
- Noster, R.; De Koning, H.D.; Maier, E.; Prelog, M.; Lainka, E.; Zielinski, C.E. Dysregulation of proinflammatory versus anti-inflammatory human T H 17 cell functionalities in the autoinflammatory Schnitzler syndrome. J. Allergy Clin. Immunol. 2016, 138, 1161–1169.e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braun, J.M.; Zielinski, C.E. In Vitro Generation of Microbe-Specific Human Th17 Cells. Adv. Struct. Saf. Stud. 2014, 1193, 97–104. [Google Scholar] [CrossRef]
- Malcova, H.; Milota, T.; Strizova, Z.; Cebecauerova, D.; Striz, I.; Sediva, A.; Horvath, R. Interleukin-1 Blockade in Polygenic Autoinflammatory Disorders: Where Are We now? Front. Pharmacol. 2021, 11, 619273. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Jobin, K.; Stumpf, N.E.; Schwab, S.; Eichler, M.; Neubert, P.; Rauh, M.; Adamowski, M.; Babyak, O.; Hinze, D.; Sivalingam, S.; et al. A high-salt diet compromises antibacterial neutrophil responses through hormonal perturbation. Sci. Transl. Med. 2020, 12, eaay3850. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Bhargava, A.; Mastroberardino, L.; Meijer, O.; Wang, J.; Buse, P.; Firestone, G.L.; Verrey, F.; Pearce, D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl. Acad. Sci. USA 1999, 96, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol. Metab. 2001, 12, 341–347. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinski, C.E. Regulation of T Cell Responses by Ionic Salt Signals. Cells 2021, 10, 2365. https://doi.org/10.3390/cells10092365
Zielinski CE. Regulation of T Cell Responses by Ionic Salt Signals. Cells. 2021; 10(9):2365. https://doi.org/10.3390/cells10092365
Chicago/Turabian StyleZielinski, Christina E. 2021. "Regulation of T Cell Responses by Ionic Salt Signals" Cells 10, no. 9: 2365. https://doi.org/10.3390/cells10092365
APA StyleZielinski, C. E. (2021). Regulation of T Cell Responses by Ionic Salt Signals. Cells, 10(9), 2365. https://doi.org/10.3390/cells10092365




