Transcriptomic Response Dynamics of Human Primary and Immortalized Adrenocortical Cells to Steroidogenic Stimuli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA-Seq Library Construction
2.3. Real Time Quantitative PCR
2.4. RNA-Seq Data Analysis
2.5. Gene Set Enrichment Analysis
3. Results
3.1. Expression Responses of Known Steroidogenic Genes in Primary Human Adrenocortical Cells Stimulated with ACTH or AngII
3.2. Transcriptome-Wide Comparison of Expression Response between ACTH and AngII in Primary Adrenocortical Cells
3.3. ACTH- and AngII-Regulated Pathways in Primary Adrenocortical Cells
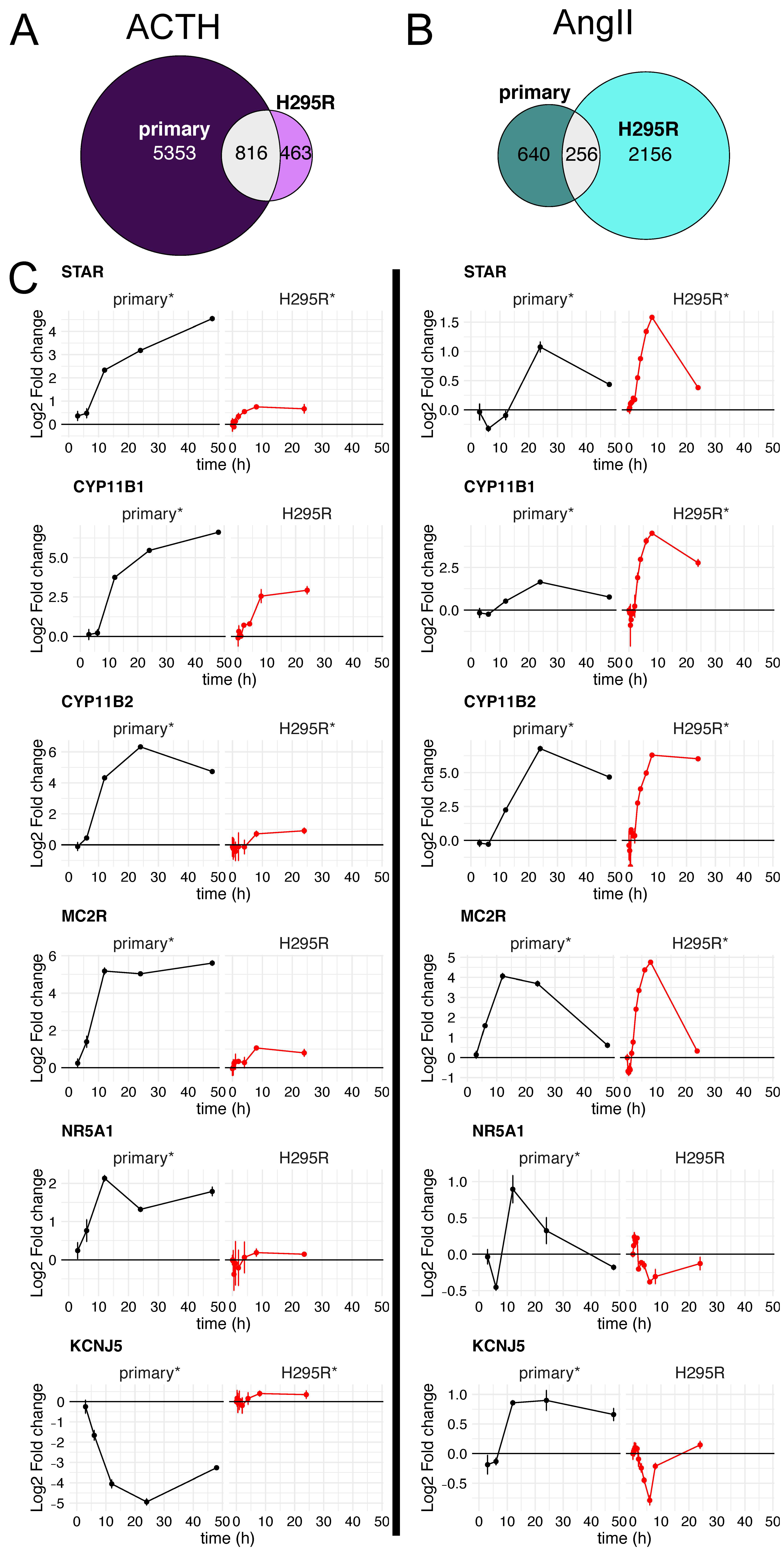
3.4. Similarity in Expression Changes and Temporal Response between Primary Adrenocortical Cells and H295R Cells
3.5. Gene-Level Comparison between Primary and H295R Cell Responses
- STAR is a key steroidogenic protein that facilitates the transport of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, which is the rate-limiting step of adrenocortical steroidogenesis [37]. STAR was significantly induced in each cell type and by both ligand pathways (Figure 5C-top). However, the ~16-fold induction in primary cells upon ACTH treatment was substantially more than ~2-fold induction by forskolin in H295R cells or either primary or H295R cells treated with AngII. Basal STAR expression was ~5-fold higher in primary cultures (~800 TPM) than H295R cells (~150 TPM).
- The P450 cytochrome enzymes are important players in steroid hormone production. Specifically, CYP11B1 and CYP11B2 are responsible for the final step of cortisol and aldosterone synthesis, respectively. Both CYP11B1 and CYP11B2 were significantly induced by all ligands in primary cells, with CYP11B1 induction by AngII being the weakest as expected (Figure 5C). The largest outlier was the very modest ~2-fold induction of CYP11B2 by forskolin in H295R cells; all other combinations of ligands and cells resulted in at least 60-fold induction of CYP11B2. The induction of CYP11B2 by ACTH and AngII in primary cells was validated using qRT-PCR (Supplementary Figure S3).
- MC2R and MRAP are both critical components of receptor-mediated signaling by ACTH [38]. These genes are both expressed at low levels in H295R cells (avg TPM of 5, 2, respectively) and have much higher expression in primary cells (avg TPM of 40, 60, respectively). This is consistent with the insensitivity of H295R cells to ACTH. Regardless, we did indeed detect statistically significant changes in gene expression for MC2R for primary cells stimulated with ACTH or AngII as described previously [39]. Similar to CYP11B2, forskolin stimulation of H295R cells resulted in only a modest increase in MC2R, which was at least 16-fold induced in all other cell type ligand combinations.
- NR5A1, also known as SF-1, is a transcriptional activator for several P450 cytochrome enzymes and STAR [40]. This key regulator showed significant induction for the ACTH treated primary cells (~4-fold) only very modest induction in AngII treated primary cells (~1.9-fold). However, there were no significant changes in NR5A1 mRNA levels in H295R cells, even though the basal expression level of NR5A1 is similar in both primary and H295R cells (~25 TPM).
- KCNJ5 encodes an inward rectifying K+ channel crucial for the influx of Ca+ that precedes the PKC signal transduction pathway and transcriptional upregulation of pro-steroidogenic genes [41]. Both ACTH and AngII also lead to differential expression of KCNJ5 in primary cells, but not in H295R cells. However, KCNJ5 exhibited a discordant response in primary cells between ACTH and AngII stimulation. While we were not surprised to see KCNJ5 induction upon AngII stimulation in primary cells because it is predominantly expressed in glomerulosa cells, we were surprised to see this gene repressed upon ACTH stimulation in the same cell type. The repression of KCNJ5 by ACTH, but not AngII in primar cells was validated using qRT-PCR (Supplementary Figure S3)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freedman, B.D.; Kempna, P.B.; Carlone, D.L.; Shah, M.; Guagliardo, N.A.; Barrett, P.Q.; Gomez-Sanchez, C.E.; Majzoub, J.A.; Breault, D.T. Adrenocortical Zonation Results from Lineage Conversion of Differentiated Zona Glomerulosa Cells. Dev. Cell 2013, 26, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Gallo-Payet, N.; Battista, M.-C. Steroidogenesis-Adrenal Cell Signal Transduction. Compr. Physiol. 2014, 4, 889–964. [Google Scholar]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Renal Outcomes in Medically and Surgically Treated Primary Aldosteronism. Hypertension 2018, 72, 658–666. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Nishizaka, M.K.; Zaman, M.A.; Thakkar, R.B.; Weissmann, P. Hyperaldosteronism among Black and White Subjects with Resistant Hypertension. Hypertension 2002, 40, 892–896. [Google Scholar] [CrossRef]
- Connell, J.M.C.; MacKenzie, S.M.; Freel, E.M.; Fraser, R.; Davies, E. A Lifetime of Aldosterone Excess: Long-Term Consequences of Altered Regulation of Aldosterone Production for Cardiovascular Function. Endocr. Rev. 2008, 29, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaduva, P.; Bonnet, F.; Bertherat, J. Molecular Basis of Primary Aldosteronism and Adrenal Cushing Syndrome. J. Endocr. Soc. 2020, 4, bvaa075. [Google Scholar] [CrossRef]
- Gallo-Payet, N.; Payet, M.D. Mechanism of Action of ACTH: Beyond cAMP. Microsc. Res. Tech. 2003, 61, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Spät, A.; Hunyady, L. Control of Aldosterone Secretion: A Model for Convergence in Cellular Signaling Pathways. Physiol. Rev. 2004, 84, 489–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, B.J.; Pezzi, V.; Stocco, D.M.; Rainey, W.E. The Steroidogenic Acute Regulatory Protein Is Induced by Angiotensin II and K+ in H295R Adrenocortical Cells. Mol. Cell. Endocrinol. 1995, 115, 215–219. [Google Scholar] [CrossRef]
- Giacchetti, G.; Opocher, G.; Sarzani, R.; Rappelli, A.; Mantero, F. Angiotensin II and the Adrenal. Clin. Exp. Pharmacol. Physiol. Suppl. 1996, 3, S119–S124. [Google Scholar] [CrossRef]
- Sewer, M.B.; Waterman, M.R. ACTH Modulation of Transcription Factors Responsible for Steroid Hydroxylase Gene Expression in the Adrenal Cortex. Microsc. Res. Tech. 2003, 61, 300–307. [Google Scholar] [CrossRef]
- Hattangady, N.G.; Olala, L.O.; Bollag, W.B.; Rainey, W.E. Acute and Chronic Regulation of Aldosterone Production. Mol. Cell. Endocrinol. 2012, 350, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, I.M.; Hanley, N.A.; Word, R.A.; Mathis, J.M.; McCarthy, J.L.; Mason, J.I.; Rainey, W.E. Human NCI-H295 Adrenocortical Carcinoma Cells: A Model for Angiotensin-II-Responsive Aldosterone Secretion. Endocrinology 1993, 133, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and Characterization of a Human Adrenocortical Carcinoma Cell Line That Expresses Multiple Pathways of Steroid Biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar] [PubMed]
- Rainey, W.E.; Bird, I.M.; Mason, J.I. The NCI-H295 Cell Line: A Pluripotent Model for Human Adrenocortical Studies. Mol. Cell. Endocrinol. 1994, 100, 45–50. [Google Scholar] [CrossRef]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical Cell Lines. Mol. Cell. Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Nanba, K.; Chen, A.X.; Turcu, A.F.; Rainey, W.E. H295R Expression of Melanocortin 2 Receptor Accessory Protein Results in ACTH Responsiveness. J. Mol. Endocrinol. 2016, 56, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Hornsby, P.J.; McAllister, J.M. Culturing Steroidogenic Cells. Methods Enzymol. 1991, 206, 371–380. [Google Scholar]
- Cheng, C.Y.; Flasch, M.V.; Hornsby, P.J. Expression of 17 -Hydroxylase and 3 -Hydroxysteroid Dehydrogenase in Fetal Human Adrenocortical Cells Transfected with SV40 T Antigen. J. Mol. Endocrinol. 1992, 9, 7–17. [Google Scholar] [CrossRef]
- Mason, J.I.; Carr, B.R.; Rainey, W.E. The Action of Phorbol Ester on Steroidogenesis in Cultured Human Fetal Adrenal Cells. Endocr. Res. 1986, 12, 447–467. [Google Scholar] [CrossRef]
- Xing, Y.; Edwards, M.A.; Ahlem, C.; Kennedy, M.; Cohen, A.; Gomez-Sanchez, C.E.; Rainey, W.E. The Effects of ACTH on Steroid Metabolomic Profiles in Human Adrenal Cells. J. Endocrinol. 2011, 209, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Nanba, K.; Blinder, A.R.; Rainey, W.E. Primary Cultures and Cell Lines for In Vitro Modeling of the Human Adrenal Cortex. Tohoku J. Exp. Med. 2021, 253, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Kurlbaum, M.; Sbiera, S.; Kendl, S.; Martin Fassnacht, M.; Kroiss, M. Steroidogenesis in the NCI-H295 Cell Line Model Is Strongly Affected By Culture Conditions and Substrain. Exp. Clin. Endocrinol. Diabetes 2020, 128, 672–680. [Google Scholar] [PubMed]
- Wang, T.; Rowland, J.G.; Parmar, J.; Nesterova, M.; Seki, T.; Rainey, W.E. Comparison of Aldosterone Production among Human Adrenocortical Cell Lines. Horm. Metab. Res. 2012, 44, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.; Wellman, K.; Baldwin, A.; Rege, J.; Walters, K.; Hirsekorn, A.; Riemondy, K.; Rainey, W.E.; Mukherjee, N. RNA-Binding Proteins Regulate Aldosterone Homeostasis in Human Steroidogenic Cells. RNA 2021. [Google Scholar] [CrossRef]
- Rege, J.; Nishimoto, H.K.; Nishimoto, K.; Rodgers, R.J.; Auchus, R.J.; Rainey, W.E. Bone Morphogenetic Protein-4 (BMP4): A Paracrine Regulator of Human Adrenal C19 Steroid Synthesis. Endocrinology 2015, 156, 2530–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, M.H.; White, P.C.; Rainey, W.E. The Regulation of Aldosterone Synthase Expression. Mol. Cell. Endocrinol. 2004, 217, 67–74. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, N.; Calviello, L.; Hirsekorn, A.; de Pretis, S.; Pelizzola, M.; Ohler, U. Integrative Classification of Human Coding and Noncoding Genes through RNA Metabolism Profiles. Nat. Struct. Mol. Biol. 2017, 24, 86–96. [Google Scholar] [CrossRef]
- Xing, Y.; Parker, C.R.; Edwards, M.; Rainey, W.E. ACTH Is a Potent Regulator of Gene Expression in Human Adrenal Cells. J. Mol. Endocrinol. 2010, 45, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Cherradi, N.; Bideau, M.; Arnaudeau, S.; Demaurex, N.; James, R.W.; Azhar, S.; Capponi, A.M. Angiotensin II Promotes Selective Uptake of High Density Lipoprotein Cholesterol Esters in Bovine Adrenal Glomerulosa and Human Adrenocortical Carcinoma Cells through Induction of Scavenger Receptor Class B Type I. Endocrinology 2001, 142, 4540–4549. [Google Scholar] [CrossRef]
- Helfenberger, K.E.; Castillo, A.F.; Mele, P.G.; Fiore, A.; Herrera, L.; Finocchietto, P.; Podestá, E.J.; Poderoso, C. Angiotensin II Stimulation Promotes Mitochondrial Fusion as a Novel Mechanism Involved in Protein Kinase Compartmentalization and Cholesterol Transport in Human Adrenocortical Cells. J. Steroid Biochem. Mol. Biol. 2019, 192, 105413. [Google Scholar] [CrossRef]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. ACTH Regulation of Adrenal SR-B1. Front. Endocrinol. 2016, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Rainey, W.E.; Shay, J.W.; Mason, J.I. ACTH Induction of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase, Cholesterol Biosynthesis, and Steroidogenesis in Primary Cultures of Bovine Adrenocortical Cells. J. Biol. Chem. 1986, 261, 7322–7326. [Google Scholar] [CrossRef]
- Infante, R.E.; Wang, M.L.; Radhakrishnan, A.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. NPC2 Facilitates Bidirectional Transfer of Cholesterol between NPC1 and Lipid Bilayers, a Step in Cholesterol Egress from Lysosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 15287–15292. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Sugawara, T.; Strauss, J.F., 3rd; Clark, B.J.; Stocco, D.M.; Saenger, P.; Rogol, A.; Miller, W.L. Role of Steroidogenic Acute Regulatory Protein in Adrenal and Gonadal Steroidogenesis. Science 1995, 267, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Metherell, L.A.; Chapple, J.P.; Cooray, S.; David, A.; Becker, C.; Rüschendorf, F.; Naville, D.; Begeot, M.; Khoo, B.; Nürnberg, P.; et al. Mutations in MRAP, Encoding a New Interacting Partner of the ACTH Receptor, Cause Familial Glucocorticoid Deficiency Type 2. Nat. Genet. 2005, 37, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Lebrethon, M.C.; Naville, D.; Begeot, M.; Saez, J.M. Regulation of Corticotropin Receptor Number and Messenger RNA in Cultured Human Adrenocortical Cells by Corticotropin and Angiotensin II. J. Clin. Investig. 1994, 93, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B.P.; White, P.C. Minireview: Steroidogenic Factor 1: Its Roles in Differentiation, Development, and Disease. Mol. Endocrinol. 2010, 24, 1322–1337. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breault, L.; Lehoux, J.G.; Gallo-Payet, N. Angiotensin II Receptors in the Human Adrenal Gland. Endocr. Res. 1996, 22, 355–361. [Google Scholar] [CrossRef]
- Omata, K.; Tomlins, S.A.; Rainey, W.E. Aldosterone-Producing Cell Clusters in Normal and Pathological States. Horm. Metab. Res. 2017, 49, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Payet, N.; Escher, E. Adrenocorticotropin Receptors in Rat Adrenal Glomerulosa Cells. Endocrinology 1985, 117, 38–46. [Google Scholar] [CrossRef]
- Gorrigan, R.J.; Guasti, L.; King, P.; Clark, A.J.; Chan, L.F. Localisation of the Melanocortin-2-Receptor and Its Accessory Proteins in the Developing and Adult Adrenal Gland. J. Mol. Endocrinol. 2011, 46, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seccia, T.M.; Caroccia, B.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Rossi, G.P. The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications. Endocr. Rev. 2018, 39, 1029–1056. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, R.; Yamada, M.; Nakajima, Y.; Satoh, T.; Hashimoto, K.; Shibusawa, N.; Ozawa, A.; Okada, S.; Rokutanda, N.; Takata, D.; et al. Expression and Mutations of KCNJ5 mRNA in Japanese Patients with Aldosterone-Producing Adenomas. J. Clin. Endocrinol. Metab. 2012, 97, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Nishikido, A.; Okamura, T.; Nakajima, Y.; Ishida, E.; Miyamoto, T.; Toki, A.-K.; Matsumoto, S.; Yoshino, S.; Horiguchi, K.; Saito, T.; et al. Regulation of the KCNJ5 Gene by SF-1 in the Adrenal Cortex: Complete Genomic Organization and Promoter Function. Mol. Cell. Endocrinol. 2020, 501, 110657. [Google Scholar] [CrossRef]
- Inoue, K.; Yamazaki, Y.; Kitamoto, T.; Hirose, R.; Saito, J.; Omura, M.; Sasano, H.; Nishikawa, T. Aldosterone Suppression by Dexamethasone in Patients with KCNJ5-Mutated Aldosterone-Producing Adenoma. J. Clin. Endocrinol. Metab. 2018, 103, 3477–3485. [Google Scholar] [CrossRef]
- Ito, R.; Shima, H.; Masuda, K.; Sato, I.; Shimada, H.; Yokoyama, A.; Shirahige, K.; Igarashi, K.; Sugawara, A. Comparative Proteomic Analysis to Identify the Novel Target Gene of Angiotensin II in Adrenocortical H295R Cells. Endocr. J. 2021, 68, 441–450. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of Glucocorticoid Negative Feedback in the Regulation of HPA Axis Pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrhart-Bornstein, M.; Hinson, J.P.; Bornstein, S.R.; Scherbaum, W.A.; Vinson, G.P. Intraadrenal Interactions in the Regulation of Adrenocortical Steroidogenesis. Endocr. Rev. 1998, 19, 101–143. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, H.; Thomas, M.; Duparc, C.; Bertherat, J.; Louiset, E. Role of ACTH in the Interactive/Paracrine Regulation of Adrenal Steroid Secretion in Physiological and Pathophysiological Conditions. Front. Endocrinol. 2016, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, H.; Prévost, G.; Louiset, E. Autocrine/paracrine Regulatory Mechanisms in Adrenocortical Neoplasms Responsible for Primary Adrenal Hypercorticism. Eur. J. Endocrinol. 2013, 169, R115–R138. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellman, K.; Fu, R.; Baldwin, A.; Rege, J.; Murphy, E.; Rainey, W.E.; Mukherjee, N. Transcriptomic Response Dynamics of Human Primary and Immortalized Adrenocortical Cells to Steroidogenic Stimuli. Cells 2021, 10, 2376. https://doi.org/10.3390/cells10092376
Wellman K, Fu R, Baldwin A, Rege J, Murphy E, Rainey WE, Mukherjee N. Transcriptomic Response Dynamics of Human Primary and Immortalized Adrenocortical Cells to Steroidogenic Stimuli. Cells. 2021; 10(9):2376. https://doi.org/10.3390/cells10092376
Chicago/Turabian StyleWellman, Kimberly, Rui Fu, Amber Baldwin, Juilee Rege, Elisabeth Murphy, William E. Rainey, and Neelanjan Mukherjee. 2021. "Transcriptomic Response Dynamics of Human Primary and Immortalized Adrenocortical Cells to Steroidogenic Stimuli" Cells 10, no. 9: 2376. https://doi.org/10.3390/cells10092376
APA StyleWellman, K., Fu, R., Baldwin, A., Rege, J., Murphy, E., Rainey, W. E., & Mukherjee, N. (2021). Transcriptomic Response Dynamics of Human Primary and Immortalized Adrenocortical Cells to Steroidogenic Stimuli. Cells, 10(9), 2376. https://doi.org/10.3390/cells10092376






