The Atypical Cyclin-Dependent Kinase 5 (Cdk5) Guards Podocytes from Apoptosis in Glomerular Disease While Being Dispensable for Podocyte Development
Abstract
1. Introduction
2. Materials and Methods
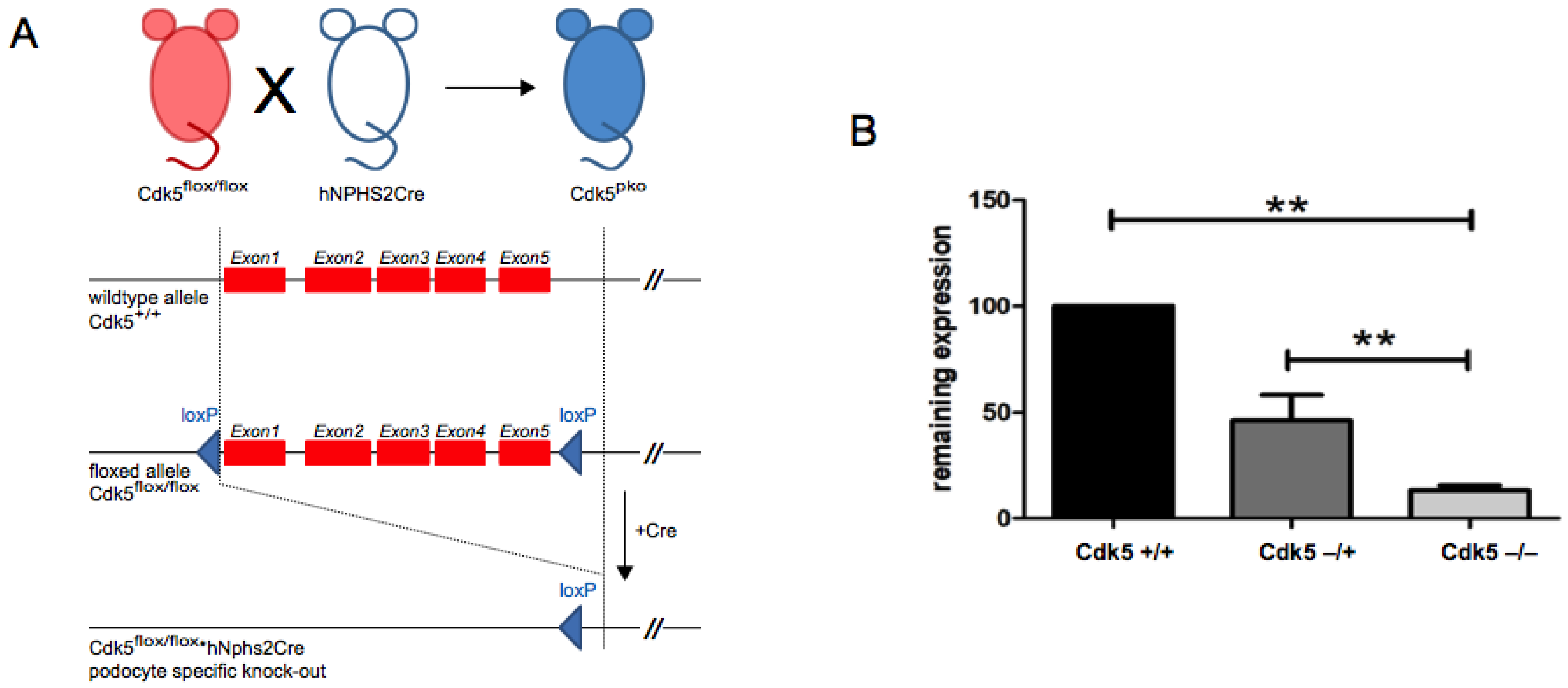
2.1. Animal Models
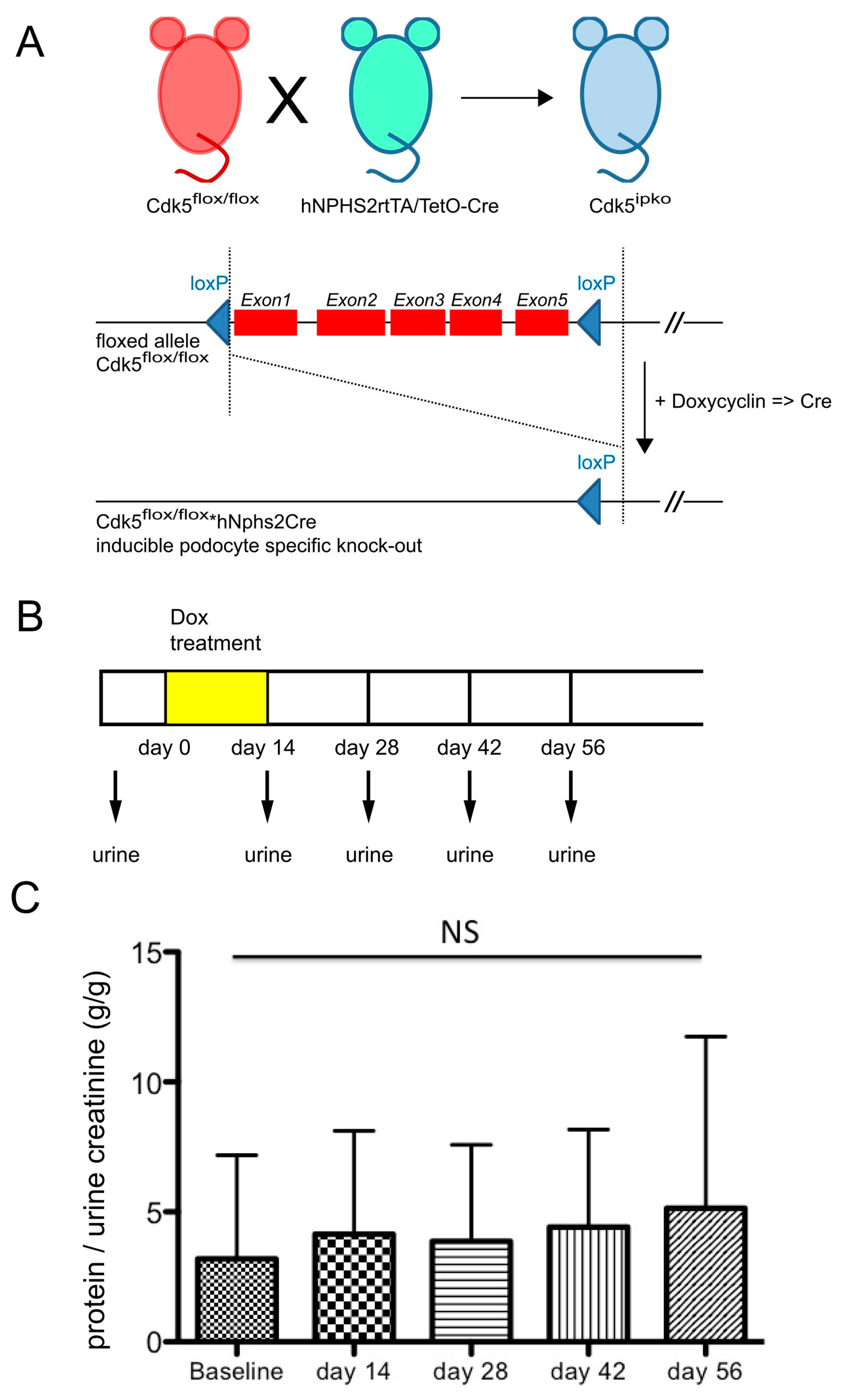
2.2. Generation of Inducible Cdk5 Knockout
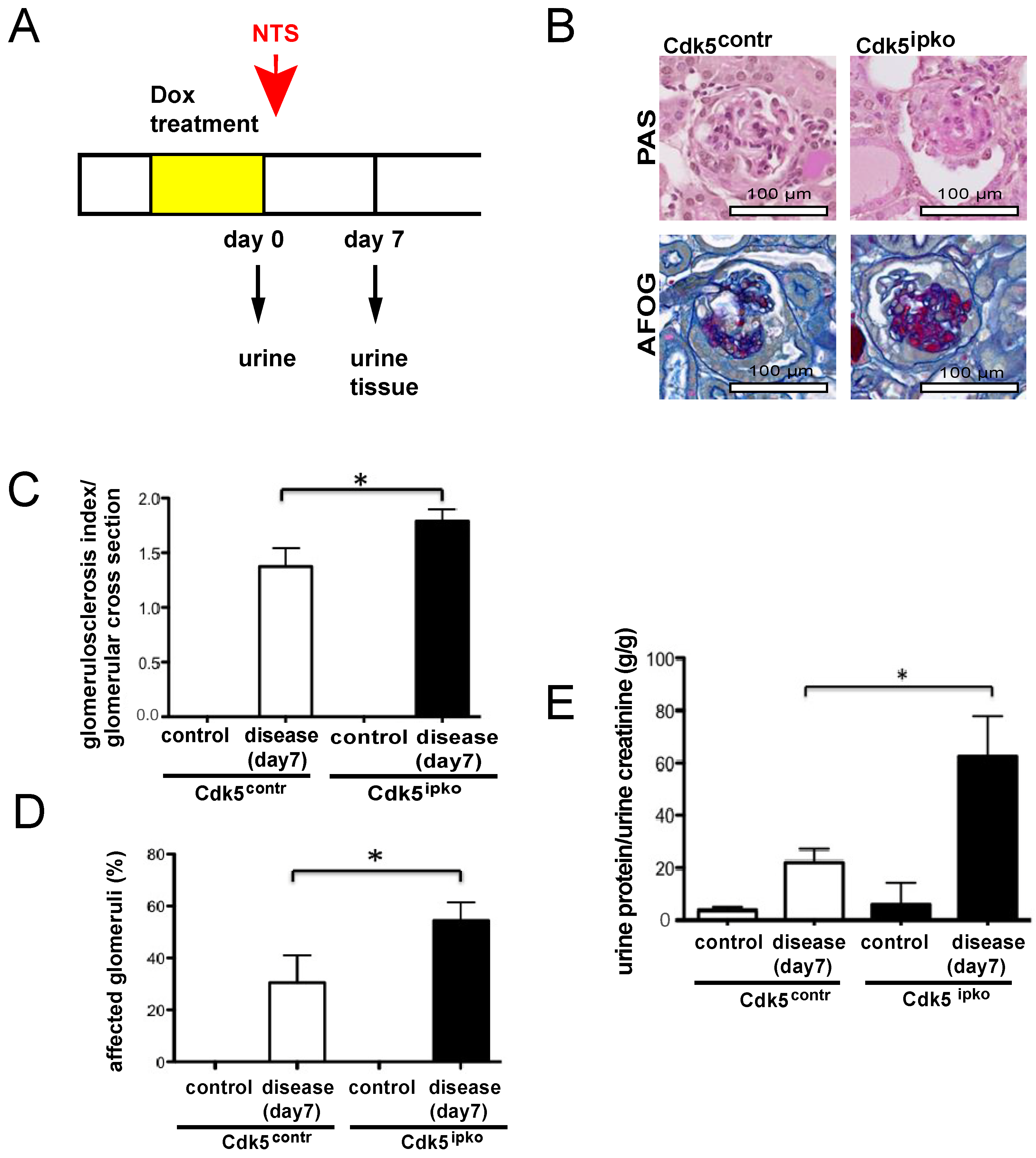
2.3. Experimental Glomerular Disease
2.4. Immunohistochemistry
2.5. Quantitative Assessment of Podocyte Number and Apoptosis
2.6. Evaluation of Glomerulosclerosis
2.7. Imaging
2.8. STED Imaging and Computed Analyses
2.9. Measurement of Proteinuria
2.10. Statistics
3. Results
3.1. Podocyte-Specific Knockout of Cdk5 Shows Regular Glomerular Histology and Function
3.2. Podocyte-Specific Knockout of Cdk5 Shows Higher Susceptibility to Glomerulosclerosis and Reduced Kidney Function in the Nephrotoxic Nephritis Model
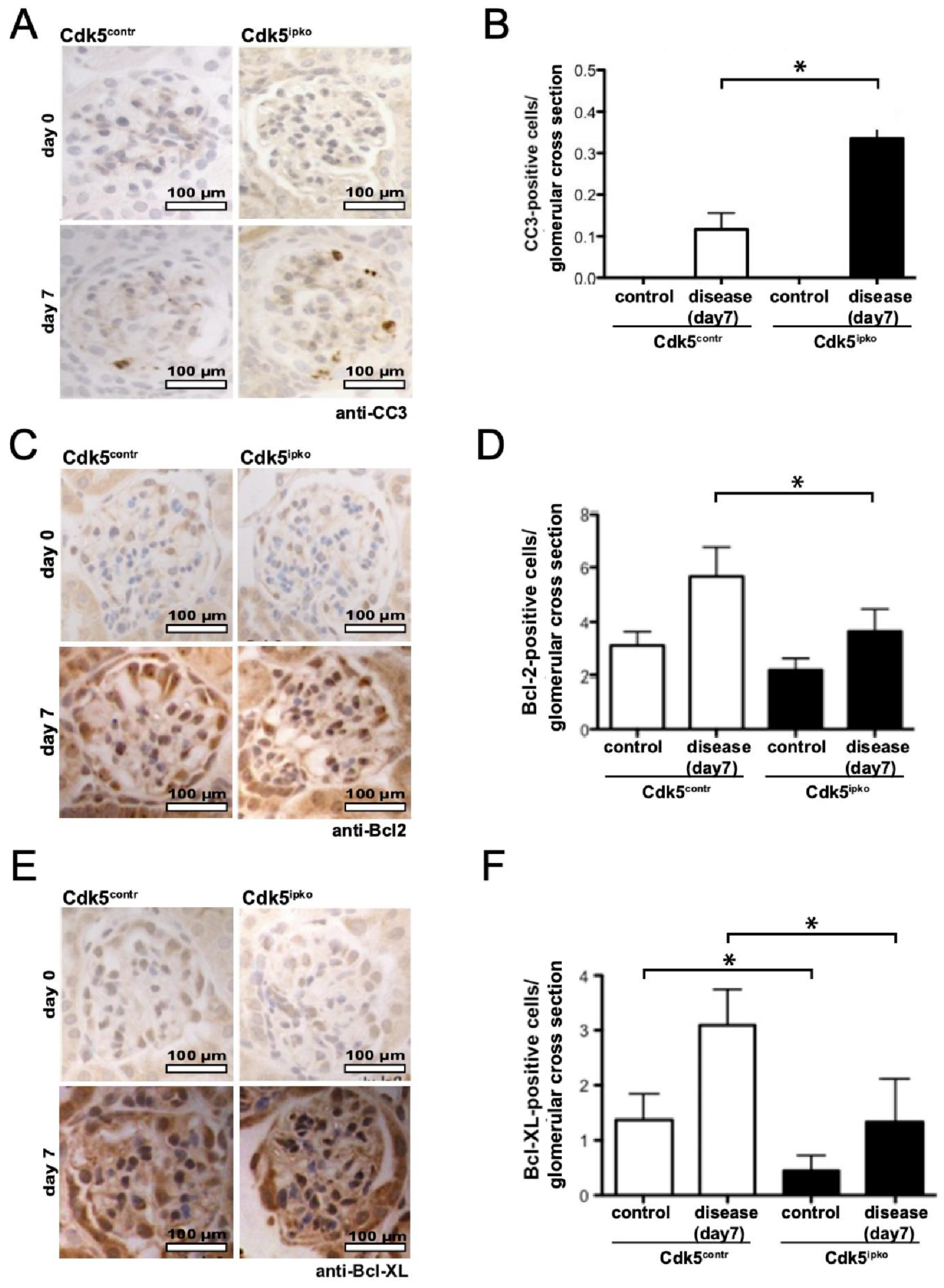
3.3. Cdk5-Deficient Mice Show Higher Rate of Apoptosis and Decreased Anti-Apoptotic Signal
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, K.; Rossie, S. Tale of the Good and the Bad Cdk5: Remodeling of the Actin Cytoskeleton in the Brain. Mol. Neurobiol. 2018, 55, 3426–3438. [Google Scholar] [CrossRef]
- Samuels, B.A.; Hsueh, Y.-P.; Shu, T.; Liang, H.; Tseng, H.-C.; Hong, C.-J.; Su, S.C.; Volker, J.; Neve, R.L.; Yue, D.T.; et al. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron 2007, 56, 823–837. [Google Scholar] [CrossRef]
- Gupta, A.; Tsai, L.-H. Cyclin-dependent kinase 5 and neuronal migration in the neocortex. Neurosignals 2003, 12, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, F.A.; Rajadhyaksha, M.S. An unusual member of the Cdk family: Cdk5. Cell. Mol. Neurobiol. 2008, 28, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Otth, C.; Concha, I.I.; Muñoz, J.P. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer’s pathology. Eur. J. Biochem. FEBS 2001, 268, 1518–1527. [Google Scholar] [CrossRef]
- Tsai, L.H.; Delalle, I.; Caviness, V.S., Jr.; Chae, T.; Harlow, E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 1994, 371, 419–423. [Google Scholar] [CrossRef]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef]
- Tang, D.; Yeung, J.; Lee, K.-Y.; Matsushita, M.; Matsui, H.; Tomizawa, K.; Hatase, O.; Wang, J.H. An Isoform of the Neuronal Cyclin-dependent Kinase 5 (Cdk5) Activator. J. Biol. Chem. 1995, 270, 26897–26903. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.-H.; Chang, K.-H.; Clawson, S.; Ghosh, S.; Mirzaei, H.; Regnier, F.; Shah, K. Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity: GSTP1 negatively regulates Cdk5 activity. J. Neurochem. 2011, 118, 902–914. [Google Scholar] [CrossRef]
- Modi, P.K.; Komaravelli, N.; Singh, N.; Sharma, P. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol. Biol. Cell 2012, 23, 3722–3730. [Google Scholar] [CrossRef]
- Odajima, J.; Wills, Z.P.; Ndassa, Y.M.; Terunuma, M.; Kretschmannova, K.; Deeb, T.Z.; Geng, Y.; Gawrzak, S.; Quadros, I.M.; Newman, J.; et al. Cyclin E Constrains Cdk5 Activity to Regulate Synaptic Plasticity and Memory Formation. Dev. Cell 2011, 21, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Asada, A.; Yamamoto, N.; Gohda, M.; Saito, T.; Hayashi, N.; Hisanaga, S.-I. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J. Neurochem. 2008, 106, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Humbert, S.; Dhavan, R.; Tsai, L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci. 2000, 113 Pt 6, 975–983. [Google Scholar] [CrossRef]
- Nikolic, M.; Dudek, H.; Kwon, Y.T.; Ramos, Y.F.; Tsai, L.H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996, 10, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Ward, J.M.; Huh, C.G.; Longenecker, G.; Veeranna; Pant, H.C.; Brady, R.O.; Martin, L.J.; Kulkarni, A.B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 1996, 93, 11173–11178. [Google Scholar] [CrossRef]
- Griffin, S.V.; Hiromura, K.; Pippin, J.; Petermann, A.T.; Blonski, M.J.; Krofft, R.; Takahashi, S.; Kulkarni, A.B.; Shankland, S.J. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am. J. Pathol. 2004, 165, 1175–1185. [Google Scholar] [CrossRef]
- Butt, L.; Unnersjö-Jess, D.; Höhne, M.; Edwards, A.; Binz-Lotter, J.; Reilly, D.; Hahnfeldt, R.; Ziegler, V.; Fremter, K.; Rinschen, M.M.; et al. A molecular mechanism explaining albuminuria in kidney disease. Nat. Metab. 2020, 2, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, H.; Taniguchi, Y.; Pippin, J.W.; Kauerz, H.-M.; Benzing, T.; Shankland, S.J.; Brinkkoetter, P.T. Cyclin I and p35 determine the subcellular distribution of Cdk5. Am. J. Physiol. Cell Physiol. 2015, 308, C339–C347. [Google Scholar] [CrossRef]
- Brinkkoetter, P.T.; Wu, J.S.; Ohse, T.; Krofft, R.D.; Schermer, B.; Benzing, T.; Pippin, J.W.; Shankland, S.J. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int. 2010, 77, 690–699. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Pippin, J.W.; Hagmann, H.; Krofft, R.D.; Chang, A.M.; Zhang, J.; Terada, Y.; Brinkkoetter, P.; Shankland, S.J. Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am. J. Physiol. Renal Physiol. 2012, 302, F1161–F1171. [Google Scholar] [CrossRef][Green Version]
- Griffin, S.V. Cyclin I Protects Podocytes from Apoptosis. J. Biol. Chem. 2006, 281, 28048–28057. [Google Scholar] [CrossRef]
- Hagmann, H.; Mangold, N.; Rinschen, M.M.; Koenig, T.; Kunzelmann, K.; Schermer, B.; Benzing, T.; Brinkkoetter, P.T. Proline-dependent and basophilic kinases phosphorylate human TRPC6 at serine 14 to control channel activity through increased membrane expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. A Mutation in the TRPC6 Cation Channel Causes Familial Focal Segmental Glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.C.; Wei, C.; Altintas, M.M.; Li, J.; Greka, A.; Ohse, T.; Pippin, J.W.; Rastaldi, M.P.; Wawersik, S.; Schiavi, S.; et al. Induction of TRPC6 Channel in Acquired Forms of Proteinuric Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Polu, K.R.; Moller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef]
- Moeller, M.J.; Sanden, S.K.; Soofi, A.; Wiggins, R.C.; Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 2003, 35, 39–42. [Google Scholar] [CrossRef]
- Koehler, S.; Brähler, S.; Braun, F.; Hagmann, H.; Rinschen, M.M.; Späth, M.R.; Höhne, M.; Wunderlich, F.T.; Schermer, B.; Benzing, T.; et al. Construction of a viral T2A-peptide based knock-in mouse model for enhanced Cre recombinase activity and fluorescent labeling of podocytes. Kidney Int. 2017, 91, 1510–1517. [Google Scholar] [CrossRef]
- Takemoto, M.; Asker, N.; Gerhardt, H.; Lundkvist, A.; Johansson, B.R.; Saito, Y.; Betsholtz, C. A New Method for Large Scale Isolation of Kidney Glomeruli from Mice. Am. J. Pathol. 2002, 161, 799–805. [Google Scholar] [CrossRef]
- Wanner, N.; Noutsou, F.; Baumeister, R.; Walz, G.; Huber, T.B.; Neumann-Haefelin, E. Functional and spatial analysis of C. elegans SYG-1 and SYG-2, orthologs of the Neph/nephrin cell adhesion module directing selective synaptogenesis. PLoS ONE 2011, 6, e23598. [Google Scholar] [CrossRef]
- Juhila, J.; Roozendaal, R.; Lassila, M.; Verbeek, S.J.; Holthofer, H. Podocyte Cell–Specific Expression of Doxycycline Inducible Cre Recombinase in Mice. J. Am. Soc. Nephrol. 2006, 17, 648–654. [Google Scholar] [CrossRef]
- Ophascharoensuk, V.; Pippin, J.W.; Gordon, K.L.; Shankland, S.J.; Couser, W.G.; Johnson, R.J. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int. 1998, 54, 416–425. [Google Scholar] [CrossRef]
- Unnersjö-Jess, D.; Scott, L.; Blom, H.; Brismar, H. Super-resolution stimulated emission depletion imaging of slit diaphragm proteins in optically cleared kidney tissue. Kidney Int. 2016, 89, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Bradley, G.; Benson, E. Todd-Sanford Clinical Diagnostics by Laboratory Methods—Examination of the Urine, 15th ed.; WB Sanders: Philadelphia, PA, USA, 1974. [Google Scholar]
- Weishaupt, J.H.; Neusch, C.; Bähr, M. Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res. 2003, 312, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sicinski, P. A kinase of many talents: Non-neuronal functions of CDK5 in development and disease. Open Biol. 2020, 10, 190287. [Google Scholar] [CrossRef]
- Ko, J.; Humbert, S.; Bronson, R.T.; Takahashi, S.; Kulkarni, A.B.; Li, E.; Tsai, L.-H. p35 and p39 Are Essential for Cyclin-Dependent Kinase 5 Function during Neurodevelopment. J. Neurosci. 2001, 21, 6758–6771. [Google Scholar] [CrossRef]
- Hirasawa, M.; Ohshima, T.; Takahashi, S.; Longenecker, G.; Honjo, Y.; Veeranna; Pant, H.C.; Mikoshiba, K.; Brady, R.O.; Kulkarni, A.B. Perinatal abrogation of Cdk5 expression in brain results in neuronal migration defects. Proc. Natl. Acad. Sci. USA 2004, 101, 6249–6254. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, A.; Mita, N.; Hirasawa, M.; Adachi, T.; Suzuki, H.; Shafeghat, N.; Kulkarni, A.B.; Mikoshiba, K.; Inoue, T.; Ohshima, T. Cyclin-dependent kinase 5 is required for normal cerebellar development. Mol. Cell. Neurosci. 2013, 52, 97–105. [Google Scholar] [CrossRef]
- Drenckhahn, D.; Franke, R.P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab. Investig. J. Tech. Methods Pathol. 1988, 59, 673–682. [Google Scholar]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef]
- Kawauchi, T.; Chihama, K.; Nabeshima, Y.; Hoshino, M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006, 8, 17–26. [Google Scholar] [CrossRef]
- Besson, A. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004, 18, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Sahlgren, C.M.; Pallari, H.-M.; He, T.; Chou, Y.-H.; Goldman, R.D.; Eriksson, J.E. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006, 25, 4808–4819. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Hao, J.; Liu, S.; Liu, Q.; Zhao, S.; Shi, Y.; Duan, H. Nestin protects mouse podocytes against high glucose-induced apoptosis by a Cdk5-dependent mechanism. J. Cell. Biochem. 2012, 113, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, E.; Regoli, M.; Fonzi, L.; Occhini, R.; Mannucci, S.; Ermini, L.; Toti, P. Nestin Expression in Adult and Developing Human Kidney. J. Histochem. Cytochem. 2007, 55, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; He, W.; Zhou, D.; Tan, R.J.; Nie, J.; Hou, F.F.; Liu, Y. Mutual Antagonism of Wilms’ Tumor 1 and β -Catenin Dictates Podocyte Health and Disease. J. Am. Soc. Nephrol. 2015, 26, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Saleem, M.A.; Holzman, L.B.; Mathieson, P.; Liu, Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010, 77, 962–973. [Google Scholar] [CrossRef]
- Sánchez-Alcázar, J.A.; Rodríguez-Hernández, Á.; Cordero, M.D.; Fernández-Ayala, D.J.M.; Brea-Calvo, G.; Garcia, K.; Navas, P. The apoptotic microtubule network preserves plasma membrane integrity during the execution phase of apoptosis. Apoptosis 2007, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Brancolini, C.; Lazarevic, D.; Rodriguez, J.; Schneider, C. Dismantling Cell–Cell Contacts during Apoptosis Is Coupled to a Caspase-dependent Proteolytic Cleavage of β-Catenin. J. Cell Biol. 1997, 139, 759–771. [Google Scholar] [CrossRef]
- Levkau, B.; Herren, B.; Koyama, H.; Ross, R.; Raines, E.W. Caspase-mediated Cleavage of Focal Adhesion Kinase pp125FAK and Disassembly of Focal Adhesions in Human Endothelial Cell Apoptosis. J. Exp. Med. 1998, 187, 579–586. [Google Scholar] [CrossRef]
- Mills, J.C.; Stone, N.L.; Pittman, R.N. Extranuclear Apoptosis. J. Cell Biol. 1999, 146, 703–708. [Google Scholar] [CrossRef]
- Kraupp, B.G.; Ruttkay-Nedecky, B.; Koudelka, H.; Bukowska, K.; Bursch, W.; Schulte-Hermann, R. In situ detection of fragmented dna (tunel assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatology 1995, 21, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhang, T.; Michowski, W.; Rebecca, V.W.; Xiao, M.; Ferretti, R.; Suski, J.M.; Bronson, R.T.; Paulo, J.A.; Frederick, D.; et al. Targeting the cyclin-dependent kinase 5 in metastatic melanoma. Proc. Natl. Acad. Sci. USA 2020, 117, 8001–8012. [Google Scholar] [CrossRef] [PubMed]
- Allnutt, A.B.; Waters, A.K.; Kesari, S.; Yenugonda, V.M. Physiological and Pathological Roles of Cdk5: Potential Directions for Therapeutic Targeting in Neurodegenerative Disease. ACS Chem. Neurosci. 2020, 11, 1218–1230. [Google Scholar] [CrossRef] [PubMed]

| Mouse Model | Forward | Reverse |
|---|---|---|
| Cdk5pko | CAGTTTCTAGCACCCAACTGATGTA | GCTGTCCTGGAACTCCATCTATAGA |
| Cdk5mRNA (qPCR) | CAGTTTCTAGCACCCAACTGATGTA | GTCGTCCTGGAACTCCATCTATAGA |
| Cdk5ipko | GACCAGGTTCGTTCACTCA | TAGCGCCGTAAATCAAT |
| Name | Company | ID | Dilution |
|---|---|---|---|
| Bcl-2 | Santa Cruz Biotechnology, Dallas, TX, USA | Sc-492 | 1:200 |
| Bcl-XL | Cell Signaling Tech., Danvers, MA, USA | CST-2762 | 1:150 |
| CC3 | Cell Signaling Tech., Danvers, MA, USA | CST-9661 | 1:200 |
| Podocin | Sigma, St. Louis, MO, USA | P0372 | 1:100 |
| WT-1 | Santa Cruz Biotechnology, Dallas, TX, USA | sc-393498 | 1:100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangold, N.; Pippin, J.; Unnersjoe-Jess, D.; Koehler, S.; Shankland, S.; Brähler, S.; Schermer, B.; Benzing, T.; Brinkkoetter, P.T.; Hagmann, H. The Atypical Cyclin-Dependent Kinase 5 (Cdk5) Guards Podocytes from Apoptosis in Glomerular Disease While Being Dispensable for Podocyte Development. Cells 2021, 10, 2464. https://doi.org/10.3390/cells10092464
Mangold N, Pippin J, Unnersjoe-Jess D, Koehler S, Shankland S, Brähler S, Schermer B, Benzing T, Brinkkoetter PT, Hagmann H. The Atypical Cyclin-Dependent Kinase 5 (Cdk5) Guards Podocytes from Apoptosis in Glomerular Disease While Being Dispensable for Podocyte Development. Cells. 2021; 10(9):2464. https://doi.org/10.3390/cells10092464
Chicago/Turabian StyleMangold, Nicole, Jeffrey Pippin, David Unnersjoe-Jess, Sybille Koehler, Stuart Shankland, Sebastian Brähler, Bernhard Schermer, Thomas Benzing, Paul T. Brinkkoetter, and Henning Hagmann. 2021. "The Atypical Cyclin-Dependent Kinase 5 (Cdk5) Guards Podocytes from Apoptosis in Glomerular Disease While Being Dispensable for Podocyte Development" Cells 10, no. 9: 2464. https://doi.org/10.3390/cells10092464
APA StyleMangold, N., Pippin, J., Unnersjoe-Jess, D., Koehler, S., Shankland, S., Brähler, S., Schermer, B., Benzing, T., Brinkkoetter, P. T., & Hagmann, H. (2021). The Atypical Cyclin-Dependent Kinase 5 (Cdk5) Guards Podocytes from Apoptosis in Glomerular Disease While Being Dispensable for Podocyte Development. Cells, 10(9), 2464. https://doi.org/10.3390/cells10092464






