An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides
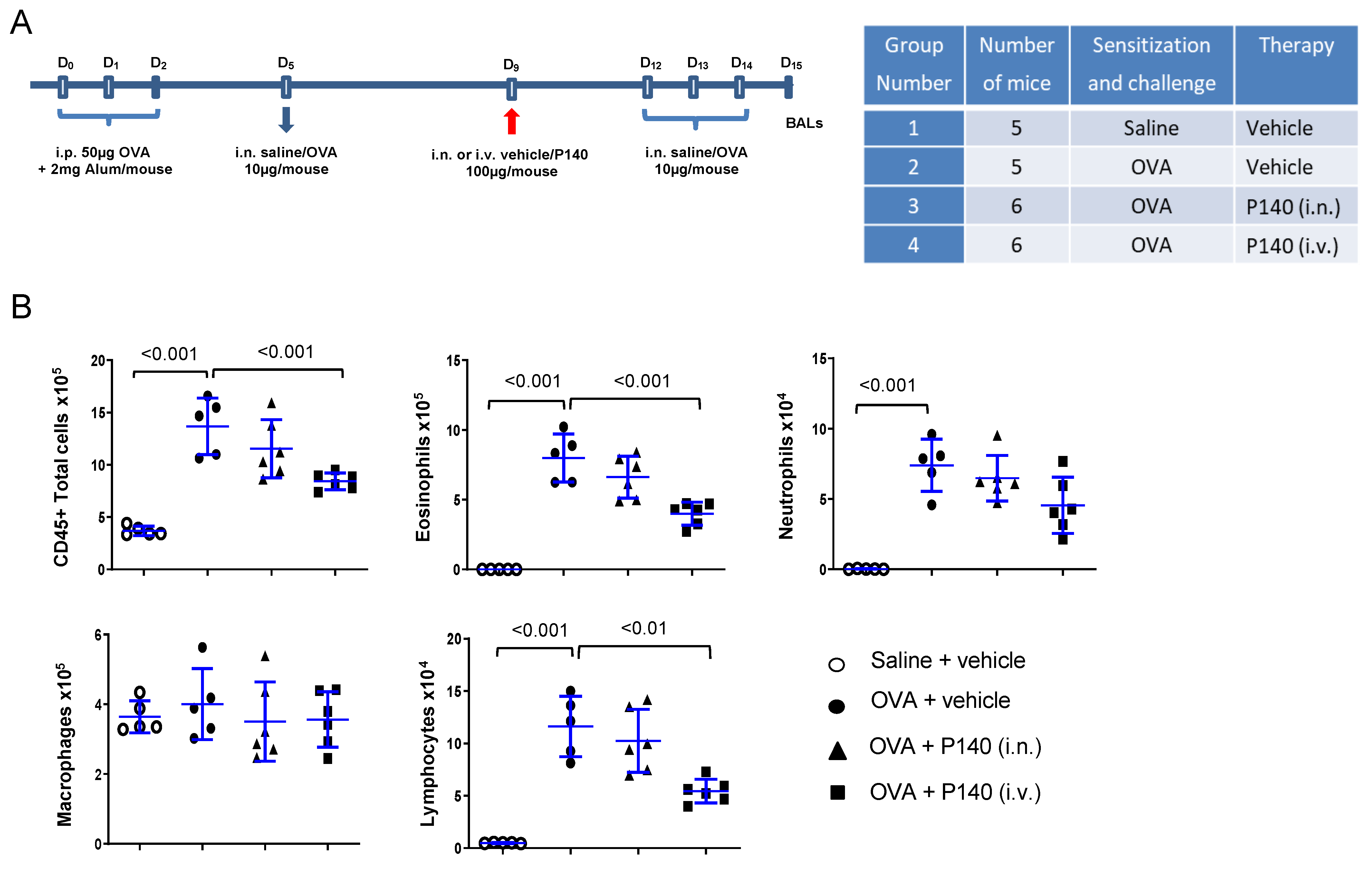
2.2. Mouse Models of Allergic Asthma
2.3. Airway Response to Methacholine (Flexivent)
2.4. Flow Cytometry
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Effect of the Autophagy Regulator P140 in an Acute Model of OVA-Induced Eosinophilic Airway Inflammation
3.2. Effect of the Autophagy Regulator P140 in a Chronic Model of HDM-Induced Airway Inflammation
3.3. Effect of P140 Treatment on the Immune Cell Accumulation in the BALF Collected from HDM-Sensitized Mice
3.4. Effect of P140 on the Levels of Circulating Anti-HDM Antibodies
3.5. Effect of P140 on the Expression Profile of Autophagy Markers in the Spleen and BAL Cells of Model Mice with HDM-Induced Allergic Asthma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón, M.A.; Linneberg, A.; Kleine-Tebbe, J.; De Blay, F.; Hernandez Fernandez de Rojas, D.; Virchow, J.C.; Demoly, P. Respiratory Allergy Caused by House Dust Mites: What Do We Really Know? J. Allergy Clin. Immunol. 2015, 136, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.D. The Role of Dust Mites in Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 312–329. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Allinne, J.; Scott, G.; Lim, W.K.; Birchard, D.; Erjefält, J.S.; Sandén, C.; Ben, L.-H.; Agrawal, A.; Kaur, N.; Kim, J.H.; et al. IL-33 Blockade Affects Mediators of Persistence and Exacerbation in a Model of Chronic Airway Inflammation. J. Allergy Clin. Immunol. 2019, 144, 1624–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, W.W.; Morgan, W.J.; Gergen, P.J.; Mitchell, H.E.; Gern, J.E.; Liu, A.H.; Gruchalla, R.S.; Kattan, M.; Teach, S.J.; Pongracic, J.A.; et al. Randomized Trial of Omalizumab (Anti-IgE) for Asthma in Inner-City Children. N. Engl. J. Med. 2011, 364, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.A.; Kleine-Tebbe, J.; Linneberg, A.; De Blay, F.; Hernandez Fernandez de Rojas, D.; Virchow, J.C.; Demoly, P. House Dust Mite Respiratory Allergy: An Overview of Current Therapeutic Strategies. J. Allergy Clin. Immunol. Pr. 2015, 3, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, G.M. Recent Developments in the Use of Biologics Targeting IL-5, IL-4, or IL-13 in Severe Refractory Asthma. Expert Rev. Respir. Med. 2018, 12, 957–963. [Google Scholar] [CrossRef]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pr. 2020, 8, 516–526. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The Basic Immunology of Asthma. Cell 2021, 184, 2521–2522. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a Therapeutic Target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular Definitions of Autophagy and Related Processes. Embo. J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The Coming of Age of Chaperone-Mediated Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Clarke, A.J.; Simon, A.K. Autophagy in the Renewal, Differentiation and Homeostasis of Immune Cells. Nat. Rev. Immunol. 2019, 19, 170–183. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in Immunity and Inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Zhang, H. Autophagy in Immunity: Implications in Etiology of Autoimmune/Autoinflammatory Diseases. Autophagy 2012, 8, 1286–1299. [Google Scholar] [CrossRef] [Green Version]
- Gros, F.; Arnold, J.; Page, N.; Décossas, M.; Korganow, A.-S.; Martin, T.; Muller, S. Macroautophagy Is Deregulated in Murine and Human Lupus T Lymphocytes. Autophagy 2012, 8, 1113–1123. [Google Scholar] [CrossRef] [Green Version]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of Autophagy in Immunity and Autoimmunity, with a Special Focus on Systemic Lupus Erythematosus. Faseb. J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Mills, K.H.G.; Harris, J. Autophagy and Inflammatory Diseases. Immunol. Cell Biol. 2013, 91, 250–258. [Google Scholar] [CrossRef] [PubMed]
- van Beek, N.; Klionsky, D.J.; Reggiori, F. Genetic Aberrations in Macroautophagy Genes Leading to Diseases. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 803–816. [Google Scholar] [CrossRef]
- Fleming, A.; Rubinsztein, D.C. Autophagy in Neuronal Development and Plasticity. Trends Neurosci. 2020, 43, 767–779. [Google Scholar] [CrossRef]
- Keller, C.W.; Münz, C.; Lünemann, J.D. Autophagy Pathways in CNS Myeloid Cell Immune Functions. Trends Neurosci. 2020, 43, 1024–1033. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in Inflammation, Infection, and Immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Filali-Mouncef, Y.; Hunter, C.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The Ménage à Trois of Autophagy, Lipid Droplets and Liver Disease. Autophagy 2021, 1–24. [Google Scholar] [CrossRef]
- Ban, G.-Y.; Pham, D.L.; Trinh, T.H.K.; Lee, S.-I.; Suh, D.-H.; Yang, E.-M.; Ye, Y.-M.; Shin, Y.S.; Chwae, Y.-J.; Park, H.-S. Autophagy Mechanisms in Sputum and Peripheral Blood Cells of Patients with Severe Asthma: A New Therapeutic Target. Clin. Exp. Allergy 2016, 46, 48–59. [Google Scholar] [CrossRef]
- Poon, A.H.; Choy, D.F.; Chouiali, F.; Ramakrishnan, R.K.; Mahboub, B.; Audusseau, S.; Mogas, A.; Harris, J.M.; Arron, J.R.; Laprise, C.; et al. Increased Autophagy-Related 5 Gene Expression Is Associated with Collagen Expression in the Airways of Refractory Asthmatics. Front. Immunol. 2017, 8, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlinden, K.D.; Deshpande, D.A.; Ghavami, S.; Xenaki, D.; Sohal, S.S.; Oliver, B.G.; Haghi, M.; Sharma, P. Autophagy Activation in Asthma Airways Remodeling. Am. J. Respir. Cell Mol. Biol. 2019, 60, 541–553. [Google Scholar] [CrossRef]
- Pham, D.L.; Ban, G.-Y.; Kim, S.-H.; Shin, Y.S.; Ye, Y.-M.; Chwae, Y.-J.; Park, H.-S. Neutrophil Autophagy and Extracellular DNA Traps Contribute to Airway Inflammation in Severe Asthma. Clin. Exp. Allergy 2017, 47, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Ferreira, F.S.; Marques, E.P.; Schmitz, F.; Gassen, R.B.; Breda, R.V.; Wyse, A.T.S.; et al. Autophagy Induces Eosinophil Extracellular Traps Formation and Allergic Airway Inflammation in a Murine Asthma Model. J. Cell Physiol. 2020, 235, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Hosseini, A.; Yousefi, S.; Karaulov, A.; Simon, H.-U. Regulation of Eosinophil Functions by Autophagy. Semin. Immunopathol. 2021, 43, 347–362. [Google Scholar] [CrossRef]
- Page, N.; Gros, F.; Schall, N.; Décossas, M.; Bagnard, D.; Briand, J.-P.; Muller, S. HSC70 Blockade by the Therapeutic Peptide P140 Affects Autophagic Processes and Endogenous MHCII Presentation in Murine Lupus. Ann. Rheum. Dis. 2011, 70, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Tasset, I.; Cuervo, A.M.; Muller, S. In Vivo Remodeling of Altered Autophagy-Lysosomal Pathway by a Phosphopeptide in Lupus. Cells 2020, 9, 2328. [Google Scholar] [CrossRef]
- Macri, C.; Wang, F.; Tasset, I.; Schall, N.; Page, N.; Briand, J.-P.; Cuervo, A.M.; Muller, S. Modulation of Deregulated Chaperone-Mediated Autophagy by a Phosphopeptide. Autophagy 2015, 11, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, R.; Scherbarth, H.R.; Rillo, O.L.; Gomez-Reino, J.J.; Muller, S. Lupuzor/P140 Peptide in Patients with Systemic Lupus Erythematosus: A Randomised, Double-Blind, Placebo-Controlled Phase IIb Clinical Trial. Ann. Rheum. Dis. 2013, 72, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Schall, N.; Muller, S. Rescue of Autophagy and Lysosome Defects in Salivary Glands of MRL/Lpr Mice by a Therapeutic Phosphopeptide. J. Autoimmun. 2018, 90, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Wang, F.; Schall, N.; Kleinmann, J.-F.; Faludi, M.; Nashi, E.P.; Sibilia, J.; Martin, T.; Schaeffer, E.; Muller, S. Lupus Regulator Peptide P140 Represses B Cell Differentiation by Reducing HLA Class II Molecule Overexpression. Arthritis. Rheumatol. 2018, 70, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Bendorius, M.; Neeli, I.; Wang, F.; Bonam, S.R.; Dombi, E.; Buron, N.; Borgne-Sanchez, A.; Poulton, J.; Radic, M.; Muller, S. The Mitochondrion-Lysosome Axis in Adaptive and Innate Immunity: Effect of Lupus Regulator Peptide P140 on Mitochondria Autophagy and NETosis. Front. Immunol. 2018, 9, 2158. [Google Scholar] [CrossRef]
- Voynova, E.; Lefebvre, F.; Qadri, A.; Muller, S. Correction of Autophagy Impairment Inhibits Pathology in the NOD.H-2h4 Mouse Model of Primary Sjögren’s Syndrome. J. Autoimmun. 2020, 108, 102418. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.; Schall, N.; Bonam, S.R.; Bigaut, K.; Mensah-Nyagan, A.-G.; de Sèze, J.; Muller, S. An Autophagy-Targeting Peptide to Treat Chronic Inflammatory Demyelinating Polyneuropathies. J. Autoimmun. 2018, 92, 114–125. [Google Scholar] [CrossRef]
- Hammad, H.; Chieppa, M.; Perros, F.; Willart, M.A.; Germain, R.N.; Lambrecht, B.N. House Dust Mite Allergen Induces Asthma via Toll-like Receptor 4 Triggering of Airway Structural Cells. Nat. Med. 2009, 15, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Delayre-Orthez, C.; de Blay, F.; Frossard, N.; Pons, F. Dose-Dependent Effects of Endotoxins on Allergen Sensitization and Challenge in the Mouse. Clin. Exp. Allergy 2004, 34, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Monneaux, F.; Lozano, J.M.; Patarroyo, M.E.; Briand, J.-P.; Muller, S. T Cell Recognition and Therapeutic Effect of a Phosphorylated Synthetic Peptide of the 70K SnRNP Protein Administered in MR/Lpr Mice. Eur. J. Immunol. 2003, 33, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, F.; Frossard, N. Eosinophils and the Ovalbumin Mouse Model of Asthma. Methods Mol. Biol. 2014, 1178, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, F.; Frossard, N. Eosinophils in Asthma Models to House Dust Mite for Drug Development. Methods Mol. Biol. 2021, 2241, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, F.; Frossard, N. Performing Bronchoalveolar Lavage in the Mouse. Curr. Protoc. Mouse Biol. 2012, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, F.; Becker, J.; Aguilar-Pimentel, J.A.; Ebel, C.; Hrabě de Angelis, M.; Hérault, Y.; Frossard, N. A Fast, Easy, and Customizable Eight-Color Flow Cytometric Method for Analysis of the Cellular Content of Bronchoalveolar Lavage Fluid in the Mouse. Curr. Protoc. Mouse Biol. 2017, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Monneaux, F.; Parietti, V.; Briand, J.-P.; Muller, S. Importance of Spliceosomal RNP1 Motif for Intermolecular T-B Cell Spreading and Tolerance Restoration in Lupus. Arthritis. Res. 2007, 9, R111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanicolaou, A.; Wang, H.; Satzke, C.; Vlahos, R.; Wilson, N.; Bozinovski, S. Novel Therapies for Pneumonia-Associated Severe Asthma Phenotypes. Trends Mol. Med. 2020, 26, 1047–1058. [Google Scholar] [CrossRef]
- McKee, A.S.; Burchill, M.A.; Munks, M.W.; Jin, L.; Kappler, J.W.; Friedman, R.S.; Jacobelli, J.; Marrack, P. Host DNA Released in Response to Aluminum Adjuvant Enhances MHC Class II-Mediated Antigen Presentation and Prolongs CD4 T-Cell Interactions with Dendritic Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Karacs, J.; Reithofer, M.; Kitzmüller, C.; Kraller, M.; Schmalz, S.; Bleichert, S.; Huppa, J.B.; Stockinger, H.; Bohle, B.; Jahn-Schmid, B. Adjuvants and Vaccines Used in Allergen-Specific Immunotherapy Induce Neutrophil Extracellular Traps. Vaccines 2021, 9, 321. [Google Scholar] [CrossRef]
- Bonam, S.R.; Bayry, J.; Tschan, M.P.; Muller, S. Progress and Challenges in The Use of MAP1LC3 as a Legitimate Marker for Measuring Dynamic Autophagy In Vivo. Cells 2020, 9, 1321. [Google Scholar] [CrossRef]
- Lin, A.; Loré, K. Granulocytes: New Members of the Antigen-Presenting Cell Family. Front. Immunol. 2017, 8, 1781. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Lee, J.M.; Hong, C.W. Autophagy in neutrophils. Korean J. Physiol. Pharmacol. 2020, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- El Kebir, D.; Filep, J.G. Modulation of neutrophil apoptosis and the resolution of inflammation through beta2 integrins. Front. Immunol. 2013, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, N.; Schall, N.; Strub, J.-M.; Quinternet, M.; Chaloin, O.; Décossas, M.; Cung, M.T.; Van Dorsselaer, A.; Briand, J.-P.; Muller, S. The Spliceosomal Phosphopeptide P140 Controls the Lupus Disease by Interacting with the HSC70 Protein and via a Mechanism Mediated by Gammadelta T Cells. PLoS ONE 2009, 4, e5273. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.; Kampinga, H.H. Computational Analysis of the Human HSPH/HSPA/DNAJ Family and Cloning of a Human HSPH/HSPA/DNAJ Expression Library. Cell Stress Chaperones 2009, 14, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske, R.F.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma Endotypes: A New Approach to Classification of Disease Entities within the Asthma Syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Emergence of Biomolecular Pathways to Define Novel Asthma Phenotypes. Type-2 Immunity and Beyond. Am. J. Respir. Cell Mol. Biol. 2016, 55, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.K.; Radic, M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front. Pharm. 2020, 11, 870. [Google Scholar] [CrossRef]
- Bonam, S.R.; Muller, S.; Bayry, J.; Klionsky, D.J. Autophagy as an Emerging Target for COVID-19: Lessons from an Old Friend, Chloroquine. Autophagy 2020, 16, 2260–2266. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daubeuf, F.; Schall, N.; Petit-Demoulière, N.; Frossard, N.; Muller, S. An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy. Cells 2021, 10, 2468. https://doi.org/10.3390/cells10092468
Daubeuf F, Schall N, Petit-Demoulière N, Frossard N, Muller S. An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy. Cells. 2021; 10(9):2468. https://doi.org/10.3390/cells10092468
Chicago/Turabian StyleDaubeuf, François, Nicolas Schall, Nathalie Petit-Demoulière, Nelly Frossard, and Sylviane Muller. 2021. "An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy" Cells 10, no. 9: 2468. https://doi.org/10.3390/cells10092468
APA StyleDaubeuf, F., Schall, N., Petit-Demoulière, N., Frossard, N., & Muller, S. (2021). An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy. Cells, 10(9), 2468. https://doi.org/10.3390/cells10092468






