Endosomal Recycling Defects and Neurodevelopmental Disorders
Abstract
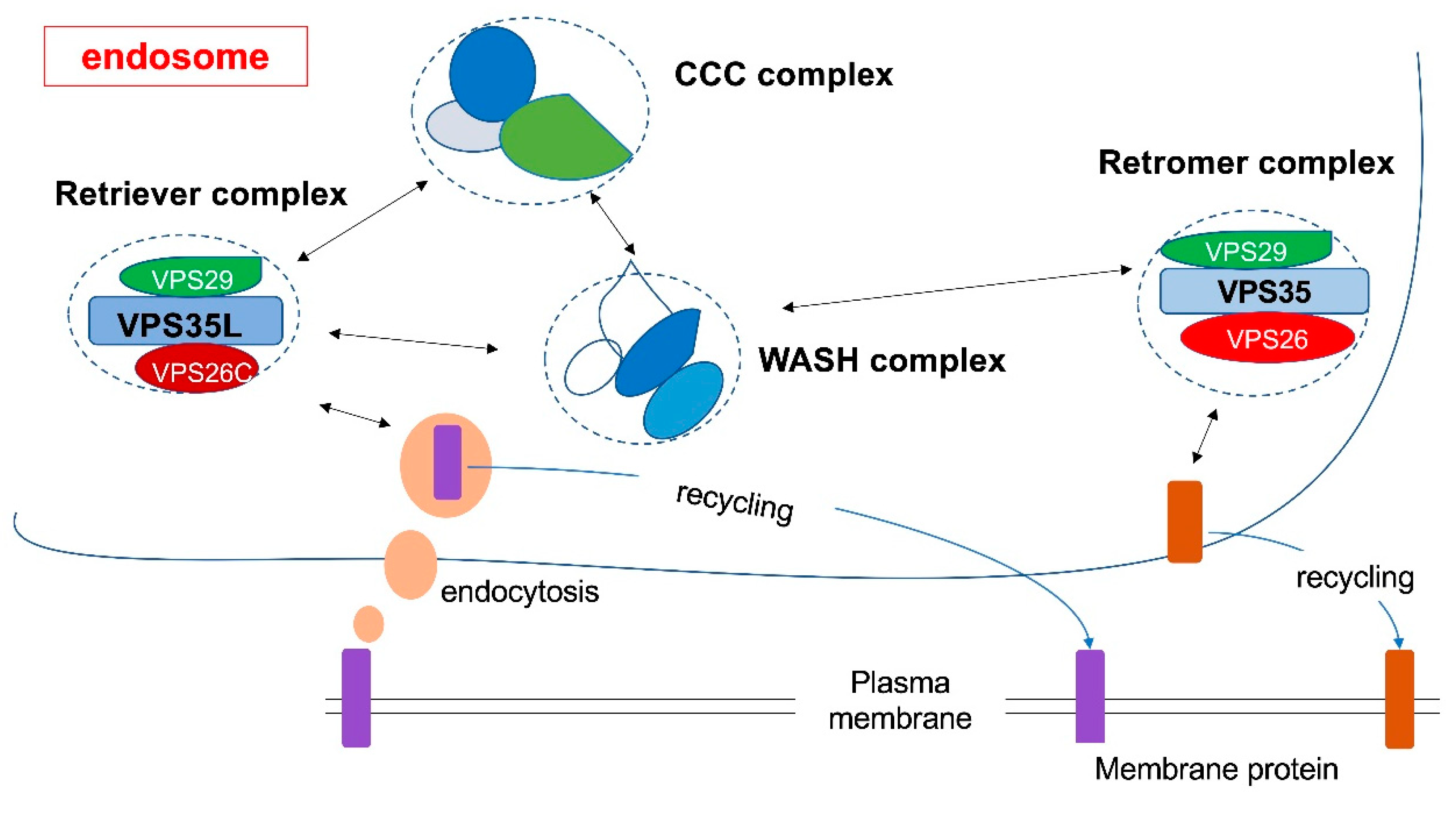
:1. Overview of the Endosomal Recycling System
2. Retromer Complex
3. Retriever Complex
4. Relationship between Retromer and Retriever
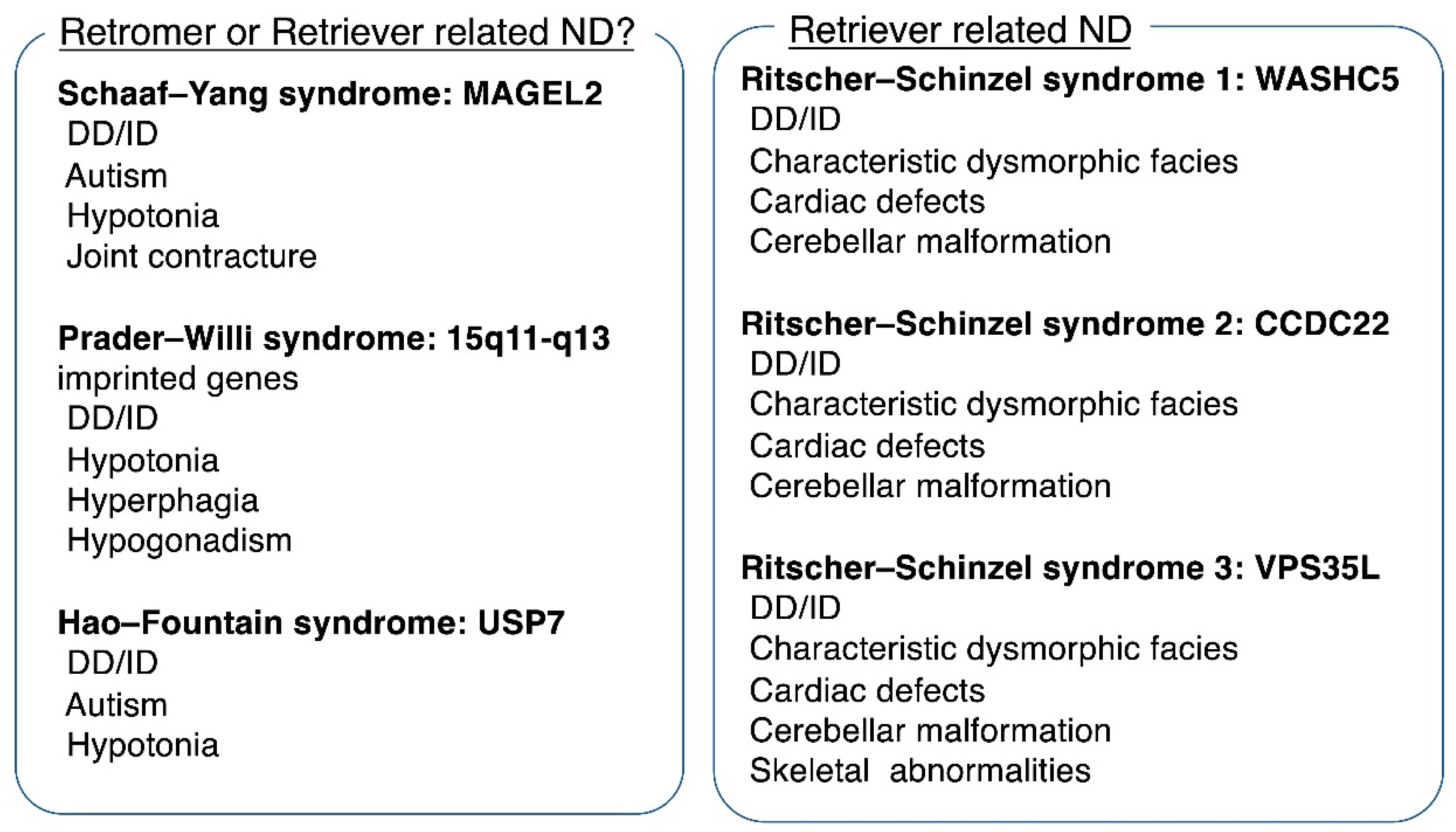
5. Human Disorders Associated with Retromer Dysfunction
6. Mouse Models of Retromer Function
7. Human Disorders Associated with Retriever Dysfunction
8. Mouse Models for Retriever Function
9. Neurodevelopment and Endosomal Recycling
10. Perspectives of Endosomal Recycle Disorders
Funding
Conflicts of Interest
References
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Chen, K.E.; Healy, M.D.; Collins, B.M. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20, 465–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012, 22, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, P.J.; Steinberg, F. To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2018, 19, 679–696. [Google Scholar] [CrossRef]
- McNally, K.E.; Faulkner, R.; Steinberg, F.; Gallon, M.; Ghai, R.; Pim, D.; Langton, P.; Pearson, N.; Danson, C.M.; Nägele, H.; et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017, 19, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.N.; McCaffery, J.M.; Emr, S.D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998, 142, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Hierro, A. Retromer. Curr. Biol. 2017, 27, R687–R689. [Google Scholar] [CrossRef] [Green Version]
- Chandra, M.; Kendall, A.K.; Jackson, L.P. Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease. Front. Cell Dev. Biol. 2021, 9, 642378. [Google Scholar] [CrossRef]
- Steinberg, F.; Gallon, M.; Winfield, M.; Thomas, E.C.; Bell, A.J.; Heesom, K.J.; Tavaré, J.M.; Cullen, P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013, 15, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Clairfeuille, T.; Mas, C.; Chan, A.S.; Yang, Z.; Tello-Lafoz, M.; Chandra, M.; Widagdo, J.; Kerr, M.C.; Paul, B.; Mérida, I. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 2016, 23, 921–932. [Google Scholar] [CrossRef]
- Ghai, R.; Bugarcic, A.; Liu, H.; Norwood, S.J.; Skeldal, S.; Coulson, E.J.; Li, S.S.; Teasdale, R.D.; Collins, B.M. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E643–E652. [Google Scholar] [CrossRef] [Green Version]
- Mallam, A.L.; Marcotte, E.M. Systems-Wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development. Cell Syst. 2017, 4, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Small, S.A.; Kent, K.; Pierce, A.; Leung, C.; Kang, M.S.; Okada, H.; Honig, L.; Vonsattel, J.P.; Kim, T.W. Model-Guided microarray implicates the retromer complex in Alzheimer’s disease. Ann. Neurol. 2005, 58, 909–919. [Google Scholar] [CrossRef]
- Small, S.A.; Simoes-Spassov, S.; Mayeux, R.; Petsko, G.A. Endosomal Traffic Jams Represent a Pathogenic Hub and Therapeutic Target in Alzheimer’s Disease. Trends Neurosci. 2017, 40, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Vilariño-Güell, C.; Wider, C.; Ross, O.A.; Dachsel, J.C.; Kachergus, J.M.; Lincoln, S.J.; Soto-Ortolaza, A.I.; Cobb, S.A.; Wilhoite, G.J.; Bacon, J.A.; et al. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 2011, 89, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimprich, A.; Benet-Pagès, A.; Struhal, W.; Graf, E.; Eck, S.H.; Offman, M.N.; Haubenberger, D.; Spielberger, S.; Schulte, E.C.; Lichtner, P.; et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 2011, 89, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavodszky, E.; Seaman, M.N.; Moreau, K.; Jimenez-Sanchez, M.; Breusegem, S.Y.; Harbour, M.E.; Rubinsztein, D.C. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 2014, 5, 3828. [Google Scholar] [CrossRef] [PubMed]
- McGough, I.J.; Steinberg, F.; Jia, D.; Barbuti, P.A.; McMillan, K.J.; Heesom, K.J.; Whone, A.L.; Caldwell, M.A.; Billadeau, D.D.; Rosen, M.K.; et al. Retromer Binding to FAM21 and the WASH Complex Is Perturbed by the Parkinson Disease-Linked VPS35(D620N) Mutation. Curr. Biol. 2014, 24, 1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippone, A.; Praticò, D. Endosome Dysregulation in Down Syndrome: A Potential Contributor to Alzheimer Disease Pathology. Ann. Neurol. 2021, 90, 4–14. [Google Scholar] [CrossRef]
- Hithersay, R.; Startin, C.M.; Hamburg, S.; Mok, K.Y.; Hardy, J.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Nizetic, D.; Strydom, A. Association of Dementia with Mortality Among Adults with Down Syndrome Older Than 35 Years. JAMA Neurol. 2019, 76, 152–160. [Google Scholar] [CrossRef]
- Wiseman, F.K.; Pulford, L.J.; Barkus, C.; Liao, F.; Portelius, E.; Webb, R.; Chávez- Gutiérrez, L.; Cleverley, K.; Noy, S.; Sheppard, O.; et al. Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain 2018, 141, 2457–2474. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.E.; Yu, D.; Praticò, D. Dysregulation of the Retromer Complex System in Down Syndrome. Ann. Neurol. 2020, 88, 137–147. [Google Scholar] [CrossRef]
- Schaaf, C.P.; Gonzalez-Garay, M.L.; Xia, F.; Potocki, L.; Gripp, K.W.; Zhang, B.; Peters, B.A.; McElwain, M.A.; Drmanac, R.; Beaudet, A.L.; et al. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat. Genet. 2013, 45, 1405–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fountain, M.D.; Aten, E.; Cho, M.T.; Juusola, J.; Walkiewicz, M.A.; Ray, J.W.; Xia, F.; Yang, Y.; Graham, B.H.; Bacino, C.A.; et al. The phenotypic spectrum of Schaaf-Yang syndrome: 18 new affected individuals from 14 families. Genet. Med. 2017, 19, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi, Y.; Ieda, D.; Hori, I.; Nozaki, Y.; Yamagata, T.; Komaki, H.; Tohyama, J.; Nagasaki, K.; Tada, H.; Saitoh, S. Schaaf-Yang syndrome shows a Prader-Willi syndrome-like phenotype during infancy. Orphanet J. Rare Dis. 2019, 14, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.H.; Doyle, J.M.; Ramanathan, S.; Gomez, T.S.; Jia, D.; Xu, M.; Chen, Z.J.; Billadeau, D.D.; Rosen, M.K.; Potts, P.R. Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell 2013, 152, 1051–1064. [Google Scholar] [CrossRef] [Green Version]
- Kanber, D.; Giltay, J.; Wieczorek, D.; Zogel, C.; Hochstenbach, R.; Caliebe, A.; Kuechler, A.; Horsthemke, B.; Buiting, K. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader-Willi syndrome. Eur. J. Hum. Genet. 2009, 17, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Buiting, K.; Di Donato, N.; Beygo, J.; Bens, S.; von der Hagen, M.; Hackmann, K.; Horsthemke, B. Clinical phenotypes of MAGEL2 mutations and deletions. Orphanet J. Rare Dis. 2014, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.H.; Fountain, M.D.; Fon Tacer, K.; Xia, F.; Bi, W.; Kang, S.H.; Patel, A.; Rosenfeld, J.A.; Le Caignec, C.; Isidor, B.; et al. USP7 Acts as a Molecular Rheostat to Promote WASH-Dependent Endosomal Protein Recycling and Is Mutated in a Human Neurodevelopmental Disorder. Mol. Cell 2015, 59, 956–969. [Google Scholar] [CrossRef] [Green Version]
- Fountain, M.D.; Oleson, D.S.; Rech, M.E.; Segebrecht, L.; Hunter, J.V.; McCarthy, J.M.; Lupo, P.J.; Holtgrewe, M.; Moran, R.; Rosenfeld, J.A.; et al. Pathogenic variants in USP7 cause a neurodevelopmental disorder with speech delays, altered behavior, and neurologic anomalies. Genet. Med. 2019, 21, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Fountain, M.D.; Schaaf, C.P. Prader-Willi syndrome and Schaaf-Yang syndrome: Neurodevelopmental diseases intersecting at the MAGEL2 gene. Diseases 2016, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Tang, F.L.; Hong, Y.; Luo, S.W.; Wang, C.L.; He, W.; Shen, C.; Jung, J.U.; Xiong, F.; Lee, D.H.; et al. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J. Cell Biol. 2011, 195, 765–779. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kordich, J.K.; Williams, E.T.; Levine, N.; Cole-Strauss, A.; Marshall, L.; Labrie, V.; Ma, J.; Lipton, J.W.; Moore, D.J. Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 5765–5774. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Zhao, F.; Bondelid, K.; Siedlak, S.L.; Torres, S.; Fujioka, H.; Wang, W.; Liu, J.; Zhu, X. VPS35 D620N knockin mice recapitulate cardinal features of Parkinson’s disease. Aging Cell 2021, 20, e13347. [Google Scholar] [CrossRef]
- Tang, F.L.; Erion, J.R.; Tian, Y.; Liu, W.; Yin, D.M.; Ye, J.; Tang, B.; Mei, L.; Xiong, W.C. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for α-Synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. J. Neurosci. 2015, 35, 10613–10628. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.L.; Liu, W.; Hu, J.X.; Erion, J.R.; Ye, J.; Mei, L.; Xiong, W.C. VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep. 2015, 12, 1631–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischof, J.M.; Stewart, C.L.; Wevrick, R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum. Mol. Genet. 2007, 16, 2713–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, F.; Watrin, F.; Sturny, R.; Massacrier, A.; Szepetowski, P.; Muscatelli, F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 2010, 19, 4895–4905. [Google Scholar] [CrossRef]
- Ieda, D.; Negishi, Y.; Miyamoto, T.; Johmura, Y.; Kumamoto, N.; Kato, K.; Miyoshi, I.; Nakanishi, M.; Ugawa, S.; Oishi, H.; et al. Two mouse models carrying truncating mutations in Magel2 show distinct phenotypes. PLoS ONE 2020, 15, e0237814. [Google Scholar] [CrossRef]
- Kosaki, K.; Curry, C.J.; Roeder, E.; Jones, K.L. Ritscher-Schinzel (3C) syndrome: Documentation of the phenotype. Am. J. Med. Genet. 1997, 68, 421–427. [Google Scholar] [CrossRef]
- Elliott, A.M.; Simard, L.R.; Coghlan, G.; Chudley, A.E.; Chodirker, B.N.; Greenberg, C.R.; Burch, T.; Ly, V.; Hatch, G.M.; Zelinski, T. A novel mutation in KIAA0196: Identification of a gene involved in Ritscher-Schinzel/3C syndrome in a First Nations cohort. J. Med. Genet. 2013, 50, 819–822. [Google Scholar] [CrossRef]
- Kolanczyk, M.; Krawitz, P.; Hecht, J.; Hupalowska, A.; Miaczynska, M.; Marschner, K.; Schlack, C.; Emmerich, D.; Kobus, K.; Kornak, U.; et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher-Schinzel/3C syndrome. Eur. J. Hum. Genet. 2015, 23, 720. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Oka, Y.; Muramatsu, H.; Vasilev, F.F.; Otomo, T.; Oishi, H.; Kawano, Y.; Kidokoro, H.; Nakazawa, Y.; Ogi, T.; et al. Biallelic VPS35L pathogenic variants cause 3C/Ritscher-Schinzel-like syndrome through dysfunction of retriever complex. J. Med. Genet. 2020, 57, 245–253. [Google Scholar] [CrossRef]
- Jeanne, M.; Demory, H.; Moutal, A.; Vuillaume, M.L.; Blesson, S.; Thépault, R.A.; Marouillat, S.; Halewa, J.; Maas, S.M.; Motazacker, M.M.; et al. Missense variants in DPYSL5 cause a neurodevelopmental disorder with corpus callosum agenesis and cerebellar abnormalities. Am. J. Hum. Genet. 2021, 108, 951–961. [Google Scholar] [CrossRef]
- Ritscher, D.; Schinzel, A.; Boltshauser, E.; Briner, J.; Arbenz, U.; Sigg, P. Dandy- Walker(like) malformation, atrio-ventricular septal defect and a similar pattern of minor anomalies in 2 sisters: A new syndrome? Am. J. Med. Genet. 1987, 26, 481–491. [Google Scholar] [CrossRef]
- Parente, D.J.; Morris, S.M.; McKinstry, R.C.; Brandt, T.; Gabau, E.; Ruiz, A.; Shinawi, M. Sorting nexin 27 (SNX27) variants associated with seizures, developmental delay, behavioral disturbance, and subcortical brain abnormalities. Clin. Genet. 2020, 97, 437–446. [Google Scholar] [CrossRef]
- Damseh, N.; Danson, C.M.; Al-Ashhab, M.; Abu-Libdeh, B.; Gallon, M.; Sharma, K.; Yaacov, B.; Coulthard, E.; Caldwell, M.A.; Edvardson, S.; et al. A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration. Neurogenetics 2015, 16, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Li, J.G.; Chiu, J.; Praticò, D. Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35. Mol. Psychiatry 2020, 25, 2630–2640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitoh, S. Endosomal Recycling Defects and Neurodevelopmental Disorders. Cells 2022, 11, 148. https://doi.org/10.3390/cells11010148
Saitoh S. Endosomal Recycling Defects and Neurodevelopmental Disorders. Cells. 2022; 11(1):148. https://doi.org/10.3390/cells11010148
Chicago/Turabian StyleSaitoh, Shinji. 2022. "Endosomal Recycling Defects and Neurodevelopmental Disorders" Cells 11, no. 1: 148. https://doi.org/10.3390/cells11010148
APA StyleSaitoh, S. (2022). Endosomal Recycling Defects and Neurodevelopmental Disorders. Cells, 11(1), 148. https://doi.org/10.3390/cells11010148





