Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation
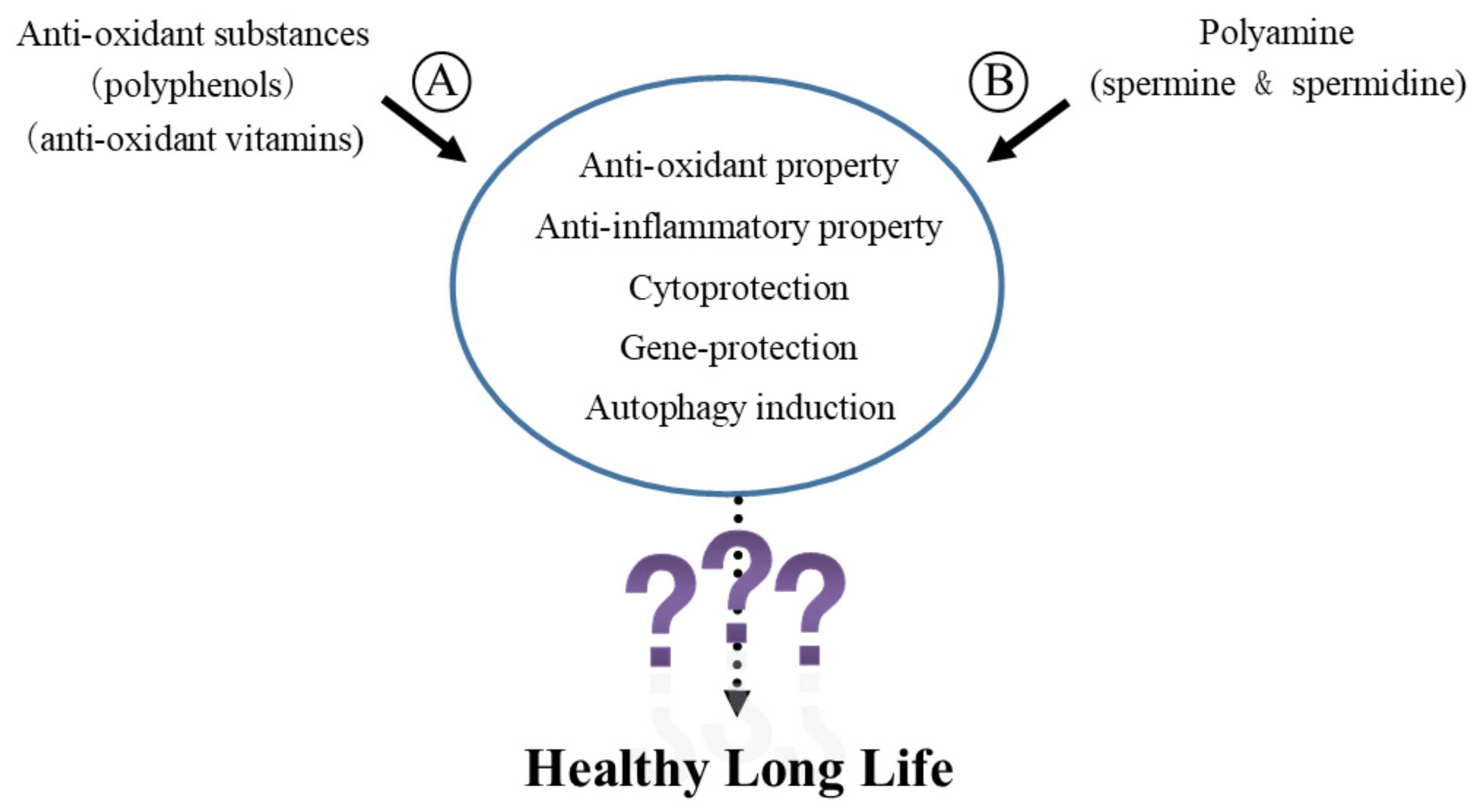
Abstract
:1. Introduction
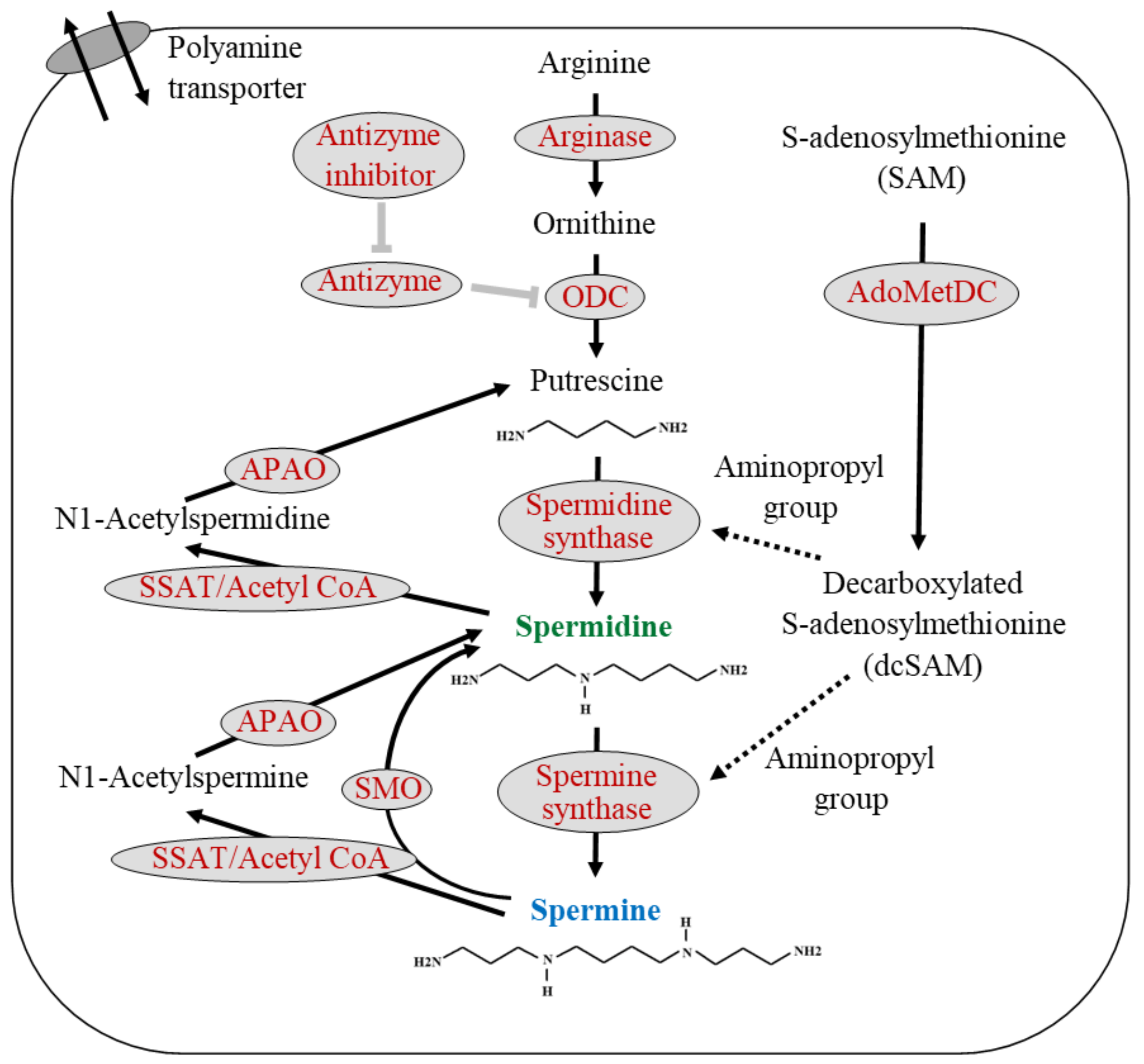
2. Polyamine
3. Aging and Polyamine
4. The Effect of Dietary Polyamines on the Body Polyamine
5. Polyamine Localization in the Body
6. Biological Activities of Polyamines
7. Aging, Proinflammatory Status, and DNA Methylation
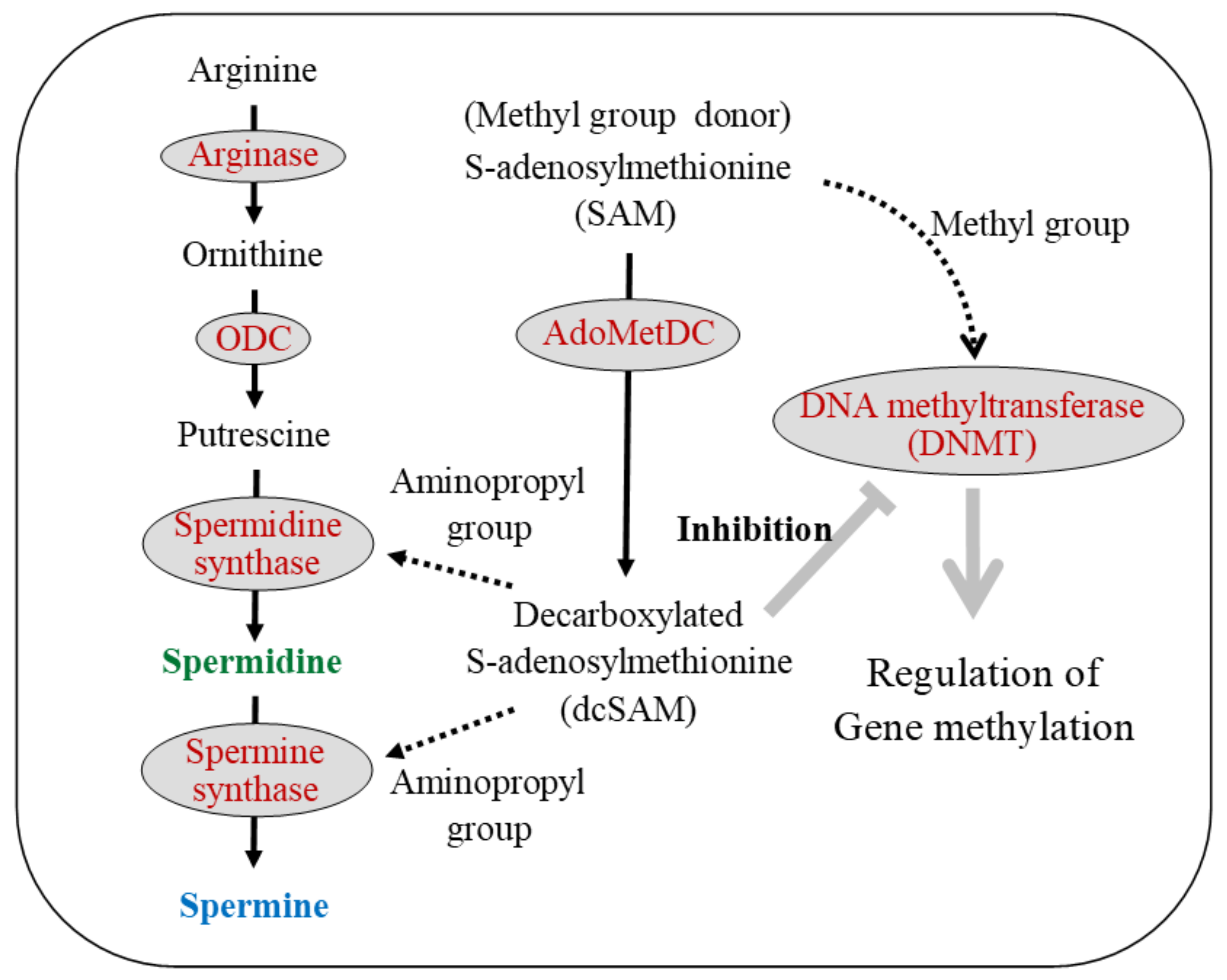
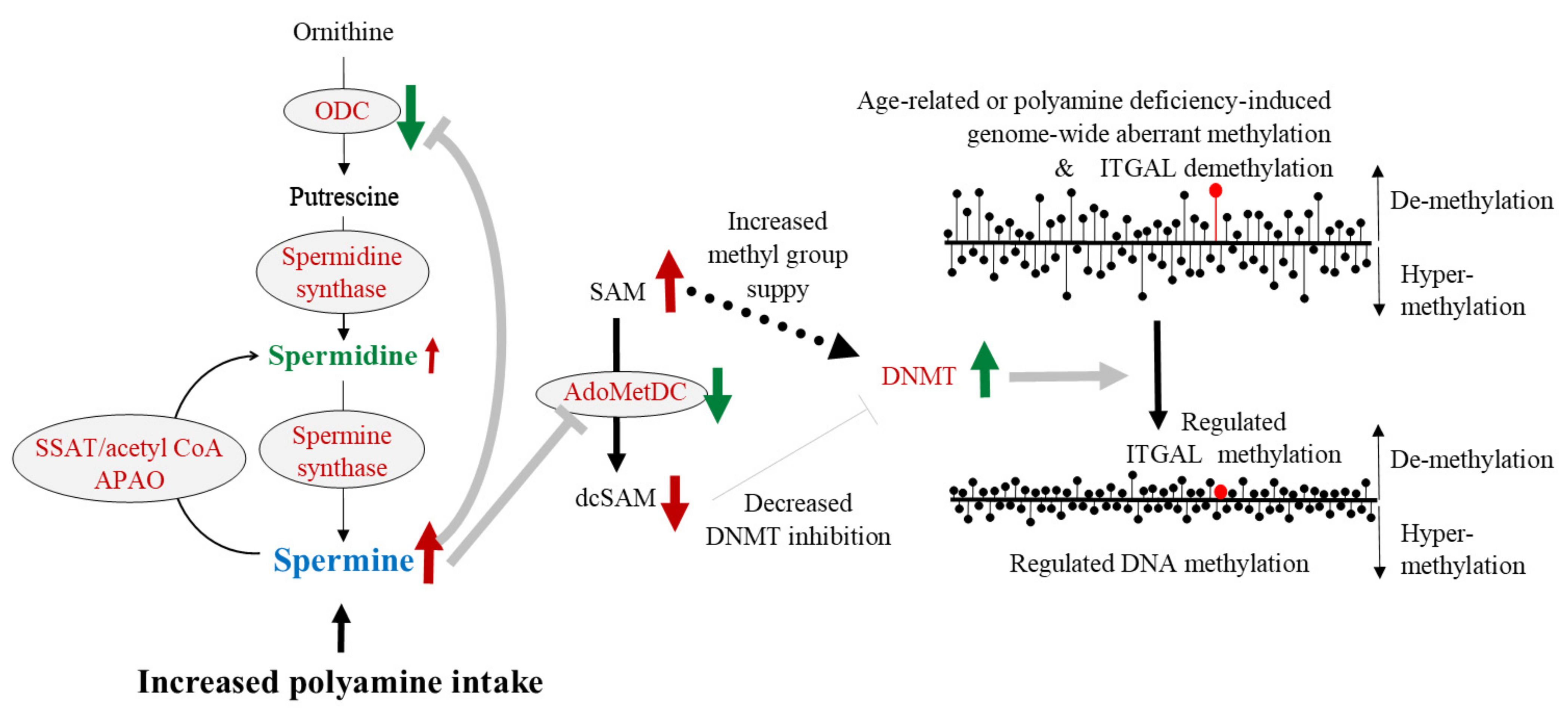
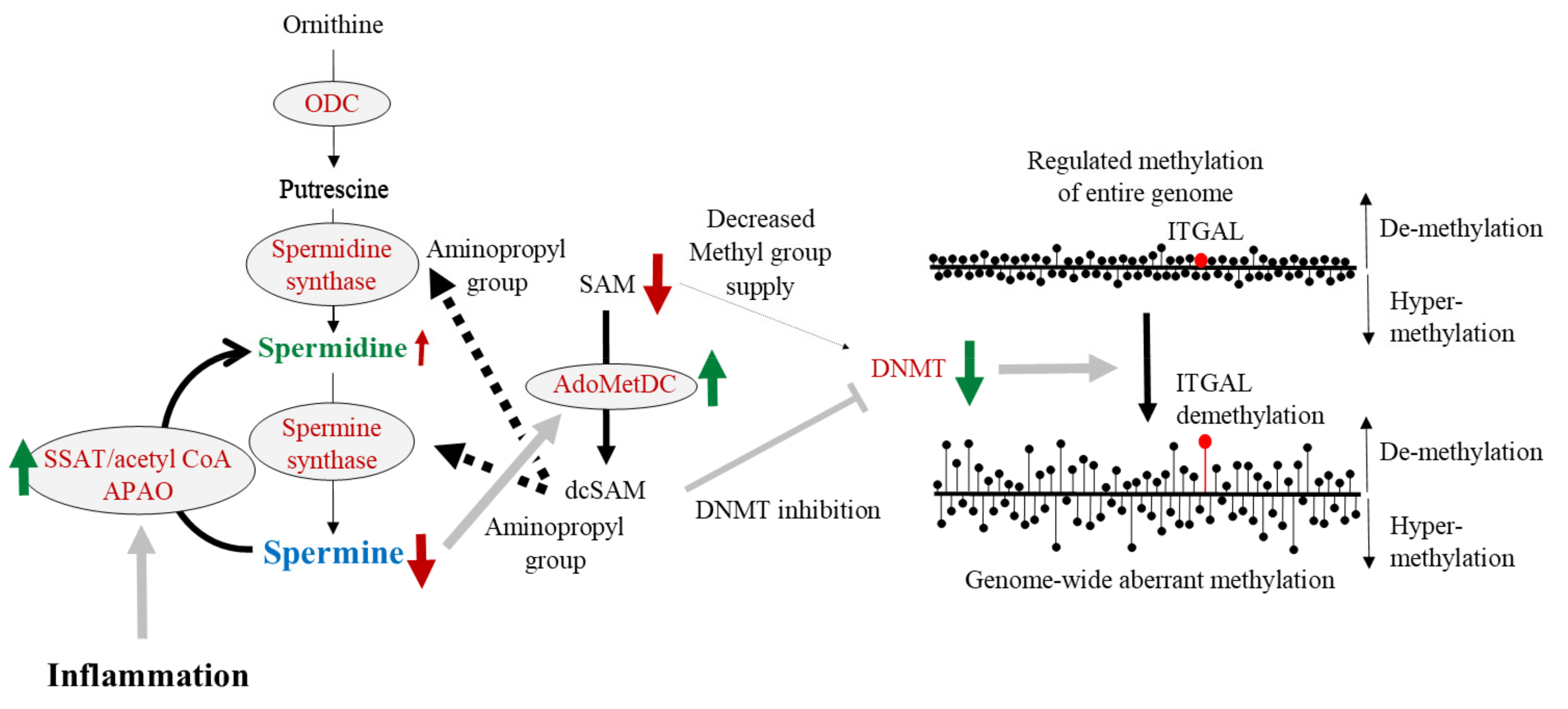
8. Polyamine, DNA Methylation, and LFA-1
9. Possible Role of Polyamine in Cognitive Function
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Erdman, J.W., Jr. AHA Science Advisory: Soy protein and cardiovascular disease: A statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation 2000, 102, 2555–2559. [Google Scholar] [CrossRef] [Green Version]
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Kim, J.H.; Nam, S.J.; Ryu, S.; Kong, G. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr. Cancer 2008, 60, 568–576. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Spitznagel, E.L.; Bosland, M.C. Soy consumption and colorectal cancer risk in humans: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Shu, X.O.; Li, H.; Chow, W.H.; Cai, H.; Zhang, X.; Gao, Y.T.; Zheng, W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am. J. Clin. Nutr. 2009, 89, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Oba, S.; Nagata, C.; Shimizu, N.; Shimizu, H.; Kametani, M.; Takeyama, N.; Ohnuma, T.; Matsushita, S. Soy product consumption and the risk of colon cancer: A prospective study in Takayama, Japan. Nutr. Cancer 2007, 57, 151–157. [Google Scholar] [CrossRef]
- Spector, D.; Anthony, M.; Alexander, D.; Arab, L. Soy consumption and colorectal cancer. Nutr. Cancer 2003, 47, 1–12. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Kris-Etherton, P.; Eckel, R.H.; Howard, B.V.; St Jeor, S.; Bazzarre, T.L.; Nutrition Committee Population Science Committee; Clinical Science Committee of the American Heart Association. AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation 2001, 103, 1823–1825. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Benetou, V.; Trichopoulou, A.; Orfanos, P.; Naska, A.; Lagiou, P.; Boffetta, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and cancer incidence: The Greek EPIC cohort. Br. J. Cancer 2008, 99, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.T.; Hu, F.B.; McCullough, M.L.; Newby, P.K.; Willett, W.C.; Holmes, M.D. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J. Nutr. 2006, 136, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Bamia, C.; Lagiou, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am. J. Clin. Nutr. 2010, 92, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Ferraresi, A.; Phadngam, S.; Morani, F.; Galetto, A.; Alabiso, O.; Chiorino, G.; Isidoro, C. Resveratrol inhibits IL-6-induced ovarian cancer cell migration through epigenetic up-regulation of autophagy. Mol. Carcinog. 2017, 56, 1164–1181. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Wisnuwardani, R.W.; De Henauw, S.; Ferrari, M.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Kafatos, A.G.; Kersting, M.; Knaze, V.; Manios, Y.; et al. Total Polyphenol Intake Is Inversely Associated with a Pro/Anti-Inflammatory Biomarker Ratio in European Adolescents of the HELENA Study. J. Nutr. 2020, 150, 1610–1618. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Alhadrami, H.A.; Amin, E.; Aly, H.F.; Othman, A.M.; Rateb, M.E.; Hetta, M.H.; Abdelmohsen, U.R.; Hassan, M.H. Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver. Molecules 2020, 25, 3116. [Google Scholar] [CrossRef]
- Guthrie, A.R.; Chow, H.S.; Martinez, J.A. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef]
- Jayasena, T.; Poljak, A.; Smythe, G.; Braidy, N.; Munch, G.; Sachdev, P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 867–883. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M.; Liu, Y. Genistein and Myd88 Activate Autophagy in High Glucose-Induced Renal Podocytes In Vitro. Med. Sci. Monit. 2018, 24, 4823–4831. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.; Sui, S. Resveratrol inhibited the progression of human hepatocellular carcinoma by inducing autophagy via regulating p53 and the phosphoinositide 3kinase/protein kinase B pathway. Oncol. Rep. 2018, 40, 2758–2765. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiang, J.; Yu, P.; Zhang, G.; Zhang, G.; Liu, X. Green tea polyphenol treatment attenuates atherosclerosis in high-fat diet-fed apolipoprotein E-knockout mice via alleviating dyslipidemia and up-regulating autophagy. PLoS ONE 2017, 12, e0181666. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Yang, F.; Fang, Z.; Hu, C. Resveratrol Ameliorates Alcoholic Fatty Liver by Inducing Autophagy. Am. J. Chin. Med. 2016, 44, 1207–1220. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Xu, J.; Lu, Y.; Jiang, J.; Wang, L.; Shen, H.M.; Xia, D. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Burnett, C.; Valentini, S.; Cabreiro, F.; Goss, M.; Somogyvari, M.; Piper, M.D.; Hoddinott, M.; Sutphin, G.L.; Leko, V.; McElwee, J.J.; et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 2011, 477, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.A.; Riehle, M.A. Resveratrol Fails to Extend Life Span in the Mosquito Anopheles stephensi. Rejuvenation Res. 2015, 18, 473–478. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Staats, S.; Wagner, A.E.; Kowalewski, B.; Rieck, F.T.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Dietary Resveratrol Does Not Affect Life Span, Body Composition, Stress Response, and Longevity-Related Gene Expression in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 223. [Google Scholar] [CrossRef] [Green Version]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Ernst, I.M.; Pallauf, K.; Bendall, J.K.; Paulsen, L.; Nikolai, S.; Huebbe, P.; Roeder, T.; Rimbach, G. Vitamin E supplementation and lifespan in model organisms. Ageing Res. Rev. 2013, 12, 365–375. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Lin, B.F. Opposite effects of low and high dose supplementation of vitamin E on survival of MRL/lpr mice. Nutrition 2005, 21, 940–948. [Google Scholar] [CrossRef]
- Karim, M.R.; Fujimura, S.; Kadowaki, M. Vitamin E as a novel enhancer of macroautophagy in rat hepatocytes and H4-II-E cells. Biochem. Biophys. Res. Commun. 2010, 394, 981–987. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Morley, A.A.; Trainor, K.J. Lack of an effect of vitamin E on lifespan of mice. Biogerontology 2001, 2, 109–112. [Google Scholar] [CrossRef]
- Selman, C.; McLaren, J.S.; Meyer, C.; Duncan, J.S.; Redman, P.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech. Ageing Dev. 2006, 127, 897–904. [Google Scholar] [CrossRef]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Nakamura, T.; Kasono, K.; Kawakami, M.; Konishi, F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J. Immunol. 2005, 175, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease—An epidemiological study. Glob. J. Health Sci. 2012, 4, 170–178. [Google Scholar] [CrossRef]
- Binh, P.N.T.; Soda, K.; Kawakami, M. Mediterranean diet and polyamine intake: Possible contribution of increased polyamine intake to inhibition of age-associated disease. Nutr. Diet. Suppl. 2011, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef] [Green Version]
- Kano, Y.; Soda, K.; Konishi, F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS ONE 2013, 8, e56056. [Google Scholar]
- Fukui, T.; Soda, K.; Takao, K.; Rikiyama, T. Extracellular Spermine Activates DNA Methyltransferase 3A and 3B. Int. J. Mol. Sci. 2019, 20, 1254. [Google Scholar] [CrossRef] [Green Version]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-Rich Diet Elevates Blood Spermine Levels and Inhibits Pro-Inflammatory Status: An Interventional Study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Inouye, M.; Pardee, A.B. Requirement of polyamines for bacterial division. J. Bacteriol. 1970, 101, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Kashiwagi, K.; Ito, K.; Watanabe, S.; Igarashi, K. Estimation of polyamine distribution and polyamine stimulation of protein synthesis in Escherichia coli. Arch. Biochem. Biophys. 1993, 300, 63–68. [Google Scholar] [CrossRef]
- Nickerson, K.W.; Dunkle, L.D.; Van Etten, J.L. Absence of spermine in filamentous fungi. J. Bacteriol. 1977, 129, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Raina, A.; Jansen, M.; Cohen, S.S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J. Bacteriol. 1967, 94, 1684–1696. [Google Scholar] [CrossRef] [Green Version]
- Wortham, B.W.; Patel, C.N.; Oliveira, M.A. Polyamines in bacteria: Pleiotropic effects yet specific mechanisms. Adv. Exp. Med. Biol. 2007, 603, 106–115. [Google Scholar]
- Hamasaki-Katagiri, N.; Katagiri, Y.; Tabor, C.W.; Tabor, H. Spermine is not essential for growth of Saccharomyces cerevisiae: Identification of the SPE4 gene (spermine synthase) and characterization of a spe4 deletion mutant. Gene 1998, 210, 195–201. [Google Scholar] [CrossRef]
- Gordon, R.; Cornect, M.; Walters, B.M.; Hall, D.E.; Brosnan, M.E. Polyamine Synthesis by the Mermithid Nematode Romanomermis culicivorax. J. Nematol. 1989, 21, 81–86. [Google Scholar]
- Pegg, A.E.; Michael, A.J. Spermine synthase. Cell. Mol. Life Sci. 2010, 67, 113–121. [Google Scholar] [CrossRef]
- Becciolini, A.; Porciani, S.; Lanini, A.; Balzi, M.; Cionini, L.; Bandettini, L. Polyamine levels in healthy and tumor tissues of patients with colon adenocarcinoma. Dis. Colon Rectum 1991, 34, 167–173. [Google Scholar] [CrossRef]
- Gallesio, C.; Colombatto, S.; Modica, R. Free and acetylated polyamines as markers of oral cavity tumors. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 167–171. [Google Scholar] [CrossRef]
- Upp, J.R., Jr.; Saydjari, R.; Townsend, C.M., Jr.; Singh, P.; Barranco, S.C.; Thompson, J.C. Polyamine levels and gastrin receptors in colon cancers. Ann. Surg. 1988, 207, 662–669. [Google Scholar] [CrossRef]
- Loser, C.; Folsch, U.R.; Paprotny, C.; Creutzfeldt, W. Polyamines in colorectal cancer. Evaluation of polyamine concentrations in the colon tissue, serum, and urine of 50 patients with colorectal cancer. Cancer 1990, 65, 958–966. [Google Scholar] [CrossRef]
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Kanungo, M.S. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 1982, 17, 95–103. [Google Scholar] [CrossRef]
- Ferioli, M.E.; Ceruti, G.; Comolli, R. Changes in rat liver ornithine decarboxylase activity during ageing and effect of stimulation by dexamethasone. Exp. Gerontol. 1976, 11, 153–156. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Ishizuka, J.; Evers, B.M.; Townsend, C.M., Jr.; Thompson, J.C. Age-related changes in polyamine biosynthesis after fasting and refeeding. Exp. Gerontol. 1993, 28, 565–572. [Google Scholar] [CrossRef]
- Janne, J.; Raina, A. On the stimulation of ornithine decarboxylase and RNA polymerase activity in rat liver after treatment with growth hormone. Biochim. Biophys. Acta 1969, 174, 769–772. [Google Scholar] [CrossRef]
- Bedford, M.R.; Smith, T.K.; Summers, J.D. Effect of dietary ornithine on renal and hepatic polyamine synthesis. Ann. Nutr. Metab. 1988, 32, 265–270. [Google Scholar] [CrossRef]
- Schleiffer, R.; Duranton, B.; Gosse, F.; Hasselmann, M.; Raul, F. Blood polyamine levels after oral ornithine load, a diagnostic marker of hyperproliferative premalignant and malignant stages in a model of colon carcinogenesis. Cancer Detect. Prev. 2000, 24, 542–548. [Google Scholar]
- Teixeira, D.; Santaolaria, M.L.; Meneu, V.; Alonso, E. Dietary arginine slightly and variably affects tissue polyamine levels in male swiss albino mice. J. Nutr. 2002, 132, 3715–3720. [Google Scholar] [CrossRef] [Green Version]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef]
- Jaenne, J.; Raina, A.; Siimes, M. Spermidine and Spermine in Rat Tissues at Different Ages. Acta Physiol. Scand. 1964, 62, 352–358. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Morrison, L.D.; Becker, L.; Ang, L.C.; Kish, S.J. Polyamines in human brain: Regional distribution and influence of aging. J. Neurochem. 1995, 65, 636–642. [Google Scholar] [CrossRef]
- Dezortova, M.; Jiru, F.; Skoch, A.; Capek, V.; Ryznarova, Z.; Vik, V.; Hajek, M. The aging effect on prostate metabolite concentrations measured by (1)H MR spectroscopy. MAGMA 2017, 30, 65–74. [Google Scholar] [CrossRef]
- Rui, H.; Thomassen, Y.; Oldereid, N.B.; Purvis, K. Accessory sex gland function in normal young (20–25 years) and middle-aged (50–55 years) men. J. Androl. 1986, 7, 93–99. [Google Scholar] [CrossRef]
- Elworthy, P.; Hitchcock, E. Polyamine levels in red blood cells from patient groups of different sex and age. Biochim. Biophys. Acta 1989, 993, 212–216. [Google Scholar] [CrossRef]
- Chaisiri, P.; Harper, M.E.; Blamey, R.W.; Peeling, W.B.; Griffiths, K. Plasma spermidine concentrations in patients with tumours of the breast or prostate or testis. Clin. Chim. Acta 1980, 104, 367–375. [Google Scholar] [CrossRef]
- Van den Berg, G.A.; Muskiet, F.A.; Kingma, A.W.; van der Slik, W.; Halie, M.R. Simultaneous gas-chromatographic determination of free and acetyl-conjugated polyamines in urine. Clin. Chem. 1986, 32, 1930–1937. [Google Scholar] [CrossRef]
- Yodfat, Y.; Weiser, M.; Kreisel, M.; Bachrach, U. Diamine and polyamine levels in the urine of healthy adults. Clin. Chim. Acta 1988, 176, 107–113. [Google Scholar] [CrossRef]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Pekar, T.; Wendzel, A.; Flak, W.; Kremer, A.; Pauschenwein-Frantsich, S.; Gschaider, A.; Wantke, F.; Jarisch, R. Spermidine in dementia : Relation to age and memory performance. Wien. Klin. Wochenschr. 2020, 132, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, Z.; Podolsky, R.; Nir, A.; Yu, J.; Nir, R.; Halvorsen, S.W.; Quinn, J.F.; Kaye, J.; Kolb, C. The Utility of Spermidine Serum Levels as a Biomarker of Alzheimer’s Disease a Pilot Study. Alzheimers Dis. Dement. 2021, 5, 119–125. [Google Scholar]
- Cipolla, B.; Guilli, F.; Moulinoux, J.P. Polyamine-reduced diet in metastatic hormone-refractory prostate cancer (HRPC) patients. Biochem. Soc. Trans. 2003, 31, 384–387. [Google Scholar] [CrossRef]
- Nishimura, K.; Araki, N.; Ohnishi, Y.; Kozaki, S. Effects of dietary polyamine deficiency on Trypanosoma gambiense infection in rats. Exp. Parasitol. 2001, 97, 95–101. [Google Scholar] [CrossRef]
- Sarhan, S.; Knodgen, B.; Seiler, N. The gastrointestinal tract as polyamine source for tumor growth. Anticancer Res. 1989, 9, 215–223. [Google Scholar]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2006, 100, 491–497. [Google Scholar] [CrossRef]
- Soda, K.; Mogi, S.; Shiina, M.; Kawabata, N. The Polyamine Content in Various Foods on a Calorie Basis. Jacobs J. Food Nutr. 2017, 4, 029. [Google Scholar]
- Watanabe, S.; Sato, S.; Nagase, S.; Shimosato, K.; Ohkuma, S. Effects of methotrexate and cyclophosphamide on polyamine levels in various tissues of rats. J. Drug Target. 1999, 7, 197–205. [Google Scholar] [CrossRef]
- Brodal, B.P.; Eliassen, K.A.; Ronning, H.; Osmundsen, H. Effects of dietary polyamines and clofibrate on metabolism of polyamines in the rat. J. Nutr. Biochem. 1999, 10, 700–708. [Google Scholar] [CrossRef]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging Albany NY 2018, 10, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Wirth, M.; Benson, G.; Schwarz, C.; Kobe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef]
- Wirth, M.; Schwarz, C.; Benson, G.; Horn, N.; Buchert, R.; Lange, C.; Kobe, T.; Hetzer, S.; Maglione, M.; Michael, E.; et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial. Alzheimers Res. Ther. 2019, 11, 36. [Google Scholar] [CrossRef]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ohishi, H.; Benno, Y. Impact of LKM512 yogurt on improvement of intestinal environment of the elderly. FEMS Immunol. Med. Microbiol. 2001, 31, 181–186. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef]
- Watanabe, S.; Kusama-Eguchi, K.; Kobayashi, H.; Igarashi, K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 1991, 266, 20803–20809. [Google Scholar] [CrossRef]
- Cooper, K.D.; Shukla, J.B.; Rennert, O.M. Polyamine distribution in cellular compartments of blood and in aging erythrocytes. Clin. Chim. Acta 1976, 73, 71–88. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef] [Green Version]
- Kremzner, L.T. Metabolism of polyamines in the nervous system. Fed. Proc. 1970, 29, 1583–1588. [Google Scholar]
- Shin, W.W.; Fong, W.F.; Pang, S.F.; Wong, P.C. Limited blood-brain barrier transport of polyamines. J. Neurochem. 1985, 44, 1056–1059. [Google Scholar] [CrossRef]
- Koenig, H.; Goldstone, A.D.; Lu, C.Y. Blood brain barrier breakdown in brain edema following cold injury is mediated by microvascular polyamines. Biochem. Biophys. Res. Commun. 1983, 116, 1039–1048. [Google Scholar] [CrossRef]
- Paschen, W. Polyamine metabolism in different pathological states of the brain. Mol. Chem. Neuropathol. 1992, 16, 241–271. [Google Scholar] [CrossRef]
- Schmitz, M.P.; Combs, D.J.; Dempsey, R.J. Difluoromethylornithine decreases postischemic brain edema and blood-brain barrier breakdown. Neurosurgery 1993, 33, 882–887; discussion 887–888. [Google Scholar]
- Sears, E.S.; McCandless, D.W.; Chandler, M.D. Disruption of the blood-brain barrier in hyperammonemic coma and the pharmacologic effects of dexamethasone and difluoromethyl ornithine. J. Neurosci. Res. 1985, 14, 255–261. [Google Scholar] [CrossRef]
- Trout, J.J.; Koenig, H.; Goldstone, A.D.; Lu, C.Y. Blood-brain barrier breakdown by cold injury. Polyamine signals mediate acute stimulation of endocytosis, vesicular transport, and microvillus formation in rat cerebral capillaries. Lab. Investig. 1986, 55, 622–631. [Google Scholar]
- Diler, A.S.; Ziylan, Y.Z.; Uzum, G.; Lefauconnier, J.M.; Seylaz, J.; Pinard, E. Passage of spermidine across the blood-brain barrier in short recirculation periods following global cerebral ischemia: Effects of mild hyperthermia. Neurosci. Res. 2002, 43, 335–342. [Google Scholar] [CrossRef]
- Winter, C.; Bell, C.; Whyte, T.; Cardinal, J.; Macfarlane, D.; Rose, S. Blood-brain barrier dysfunction following traumatic brain injury: Correlation of K(trans) (DCE-MRI) and SUVR (99mTc-DTPA SPECT) but not serum S100B. Neurol. Res. 2015, 37, 599–606. [Google Scholar] [CrossRef]
- Baskaya, M.K.; Rao, A.M.; Dogan, A.; Donaldson, D.; Gellin, G.; Dempsey, R.J. Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res. 1997, 744, 302–308. [Google Scholar] [CrossRef]
- Uda, K.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Rapid absorption of luminal polyamines in a rat small intestine ex vivo model. J. Gastroenterol. Hepatol. 2003, 18, 554–559. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef] [Green Version]
- Bardocz, S.; Brown, D.S.; Grant, G.; Pusztai, A. Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by Phaseolus vulgaris lectin phytohaemagglutinin in vivo. Biochim. Biophys. Acta 1990, 1034, 46–52. [Google Scholar] [CrossRef]
- Yuan, Q.; Ray, R.M.; Viar, M.J.; Johnson, L.R. Polyamine regulation of ornithine decarboxylase and its antizyme in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G130–G138. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, H.Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 2012, 19, 31. [Google Scholar] [CrossRef] [Green Version]
- Lagishetty, C.V.; Naik, S.R. Polyamines: Potential anti-inflammatory agents and their possible mechanism of action. Indian J. Pharmacol. 2008, 40, 121–125. [Google Scholar]
- Lovaas, E.; Carlin, G. Spermine: An anti-oxidant and anti-inflammatory agent. Free Radic. Biol. Med. 1991, 11, 455–461. [Google Scholar] [CrossRef]
- Paul, S.; Kang, S.C. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm. Res. 2013, 62, 681–688. [Google Scholar] [CrossRef]
- Zhou, S.; Gu, J.; Liu, R.; Wei, S.; Wang, Q.; Shen, H.; Dai, Y.; Zhou, H.; Zhang, F.; Lu, L. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response through ATG5-Dependent Autophagy. Front. Immunol. 2018, 9, 948. [Google Scholar] [CrossRef]
- Belle, N.A.; Dalmolin, G.D.; Fonini, G.; Rubin, M.A.; Rocha, J.B. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004, 1008, 245–251. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 2003, 100, 2261–2265. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, S.; Kadoma, Y. Kinetic evaluation of polyamines as radical scavengers. Anticancer Res. 2005, 25, 965–969. [Google Scholar]
- Gaboriau, F.; Vaultier, M.; Moulinoux, J.P.; Delcros, J.G. Antioxidative properties of natural polyamines and dimethylsilane analogues. Redox. Rep. 2005, 10, 9–18. [Google Scholar] [CrossRef]
- Jeong, J.W.; Cha, H.J.; Han, M.H.; Hwang, S.J.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol. Ther. 2018, 26, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.L.; Oh, T.J.; Kim, I.G. Abnormal growth of polyamine-deficient Escherichia coli mutant is partially caused by oxidative stress-induced damage. Arch. Biochem. Biophys. 2003, 418, 125–132. [Google Scholar] [CrossRef]
- Nayvelt, I.; Hyvonen, M.T.; Alhonen, L.; Pandya, I.; Thomas, T.; Khomutov, A.R.; Vepsalainen, J.; Patel, R.; Keinanen, T.A.; Thomas, T.J. DNA condensation by chiral alpha-methylated polyamine analogues and protection of cellular DNA from oxidative damage. Biomacromolecules 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Sava, I.G.; Battaglia, V.; Rossi, C.A.; Salvi, M.; Toninello, A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic. Biol. Med. 2006, 41, 1272–1281. [Google Scholar] [CrossRef]
- von Deutsch, A.W.; Mitchell, C.D.; Williams, C.E.; Dutt, K.; Silvestrov, N.A.; Klement, B.J.; Abukhalaf, I.K.; von Deutsch, D.A. Polyamines protect against radiation-induced oxidative stress. Gravit. Space Biol. Bull. 2005, 18, 109–110. [Google Scholar]
- Arundel, C.M.; Nishioka, K.; Tofilon, P.J. Effects of alpha-difluoromethylornithine-induced polyamine depletion on the radiosensitivity of a human colon carcinoma cell line. Radiat. Res. 1988, 114, 634–640. [Google Scholar] [CrossRef]
- Chiu, S.; Oleinick, N.L. Radioprotection of cellular chromatin by the polyamines spermine and putrescine: Preferential action against formation of DNA-protein crosslinks. Radiat. Res. 1998, 149, 543–549. [Google Scholar] [CrossRef]
- Courdi, A.; Milano, G.; Bouclier, M.; Lalanne, C.M. Radiosensitization of human tumor cells by alpha-difluoromethylornithine. Int. J. Cancer 1986, 38, 103–107. [Google Scholar] [CrossRef]
- Douki, T.; Bretonniere, Y.; Cadet, J. Protection against radiation-induced degradation of DNA bases by polyamines. Radiat. Res. 2000, 153, 29–35. [Google Scholar] [CrossRef]
- Held, K.D.; Awad, S. Effects of polyamines and thiols on the radiation sensitivity of bacterial transforming DNA. Int. J. Radiat. Biol. 1991, 59, 699–710. [Google Scholar] [CrossRef]
- Newton, G.L.; Aguilera, J.A.; Ward, J.F.; Fahey, R.C. Polyamine-induced compaction and aggregation of DNA—A major factor in radioprotection of chromatin under physiological conditions. Radiat. Res. 1996, 145, 776–780. [Google Scholar] [CrossRef]
- Snyder, R.D.; Schroeder, K.K. Radiosensitivity of polyamine-depleted HeLa cells and modulation by the aminothiol WR-1065. Radiat. Res. 1994, 137, 67–75. [Google Scholar] [CrossRef]
- Sy, D.; Hugot, S.; Savoye, C.; Ruiz, S.; Charlier, M.; Spotheim-Maurizot, M. Radioprotection of DNA by spermine: A molecular modelling approach. Int. J. Radiat. Biol. 1999, 75, 953–961. [Google Scholar]
- Warters, R.L.; Newton, G.L.; Olive, P.L.; Fahey, R.C. Radioprotection of human cell nuclear DNA by polyamines: Radiosensitivity of chromatin is influenced by tightly bound spermine. Radiat. Res. 1999, 151, 354–362. [Google Scholar] [CrossRef]
- Pothipongsa, A.; Jantaro, S.; Incharoensakdi, A. Polyamines induced by osmotic stress protect Synechocystis sp. PCC 6803 cells and arginine decarboxylase transcripts against UV-B radiation. Appl. Biochem. Biotechnol. 2012, 168, 1476–1488. [Google Scholar] [CrossRef]
- Snyder, R.D.; Sunkara, P.S. Effect of polyamine depletion on DNA damage and repair following UV irradiation of HeLa cells. Photochem. Photobiol. 1990, 52, 525–532. [Google Scholar] [CrossRef]
- Williams, J.R.; Casero, R.A.; Dillehay, L.E. The effect of polyamine depletion on the cytotoxic response to PUVA, gamma rays and UVC in V79 cells in vitro. Biochem. Biophys. Res. Commun. 1994, 201, 1–7. [Google Scholar] [CrossRef]
- Chauhan, S.D.; Seggara, G.; Vo, P.A.; Macallister, R.J.; Hobbs, A.J.; Ahluwalia, A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003, 17, 773–775. [Google Scholar] [CrossRef]
- Di Mascio, P.; Teixeira, P.C.; Onuki, J.; Medeiros, M.H.; Dornemann, D.; Douki, T.; Cadet, J. DNA damage by 5-aminolevulinic and 4,5-dioxovaleric acids in the presence of ferritin. Arch. Biochem. Biophys. 2000, 373, 368–374. [Google Scholar] [CrossRef]
- Mackintosh, C.A.; Pegg, A.E. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts, and the sensitivity of fibroblasts to 1,3-bis-(2-chloroethyl)-N-nitrosourea. Biochem. J. 2000, 351 Pt 2, 439–447. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Rao, P.M.; Sarma, D.S. Studies on carcinogen chromatin--DNA interaction: Inhibition of N-methyl-N-nitrosourea-induced methylation of chromatin—DNA by spermine and distamycin A. Biochemistry 1978, 17, 4515–4518. [Google Scholar] [CrossRef]
- Gugliucci, A.; Menini, T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: A new role for old molecules? Life Sci. 2003, 72, 2603–2616. [Google Scholar] [CrossRef]
- Okumura, S.; Teratani, T.; Fujimoto, Y.; Zhao, X.; Tsuruyama, T.; Masano, Y.; Kasahara, N.; Iida, T.; Yagi, S.; Uemura, T.; et al. Oral administration of polyamines ameliorates liver ischemia/reperfusion injury and promotes liver regeneration in rats. Liver Transpl. 2016, 22, 1231–1244. [Google Scholar] [CrossRef] [Green Version]
- Sagor, G.H.; Berberich, T.; Takahashi, Y.; Niitsu, M.; Kusano, T. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res. 2013, 22, 595–605. [Google Scholar] [CrossRef]
- Sacitharan, P.K.; Lwin, S.; Gharios, G.B.; Edwards, J.R. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300. Exp. Mol. Med. 2018, 50, 123. [Google Scholar] [CrossRef] [Green Version]
- Chae, Y.B.; Kim, M.M. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int. J. Biol. Macromol. 2014, 63, 56–63. [Google Scholar] [CrossRef]
- Djajadikerta, A.; Keshri, S.; Pavel, M.; Prestil, R.; Ryan, L.; Rubinsztein, D.C. Autophagy Induction as a Therapeutic Strategy for Neurodegenerative Diseases. J. Mol. Biol. 2020, 432, 2799–2821. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [Green Version]
- Takabatake, Y.; Kimura, T.; Takahashi, A.; Isaka, Y. Autophagy and the kidney: Health and disease. Nephrol. Dial. Transplant. 2014, 29, 1639–1647. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M. Autophagy and aging: Keeping that old broom working. Trends Genet. 2008, 24, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Bai, X.Y.; Shi, S.; Cui, S.; Hong, Q.; Cai, G.; Chen, X. Age-related changes in the function of autophagy in rat kidneys. Age 2012, 34, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Del Roso, A.; Vittorini, S.; Cavallini, G.; Donati, A.; Gori, Z.; Masini, M.; Pollera, M.; Bergamini, E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp. Gerontol. 2003, 38, 519–527. [Google Scholar] [CrossRef]
- Lipinski, M.M.; Zheng, B.; Lu, T.; Yan, Z.; Py, B.F.; Ng, A.; Xavier, R.J.; Li, C.; Yankner, B.A.; Scherzer, C.R.; et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 107, 14164–14169. [Google Scholar] [CrossRef] [Green Version]
- Demirovic, D.; Nizard, C.; Rattan, S.I. Basal level of autophagy is increased in aging human skin fibroblasts in vitro, but not in old skin. PLoS ONE 2015, 10, e0126546. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Park, S.Y.; Moon, S.H.; Lee, J.D.; Kim, S. Autophagy in Human Skin Fibroblasts: Impact of Age. Int. J. Mol. Sci. 2018, 19, 2254. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Kitamura, H.; et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Melendez, A.; Talloczy, Z.; Seaman, M.; Eskelinen, E.L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef] [Green Version]
- Apfeld, J.; Kenyon, C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 1998, 95, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Hars, E.S.; Qi, H.; Ryazanov, A.G.; Jin, S.; Cai, L.; Hu, C.; Liu, L.F. Autophagy regulates ageing in C. elegans. Autophagy 2007, 3, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Toth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdelyi, P.; Takacs-Vellai, K.; Orosz, L.; Kovacs, A.L.; Csikos, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Tavernarakis, N.; Pasparaki, A.; Tasdemir, E.; Maiuri, M.C.; Kroemer, G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy 2008, 4, 870–873. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008, 4, e24. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.; Levine, B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy 2007, 3, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Finkel, S.E.; Tower, J. Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Exp. Gerontol. 2009, 44, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Ookuma, S.; Nishida, E. Lifespan extension by suppression of autophagy genes in Caenorhabditis elegans. Genes Cells 2009, 14, 717–726. [Google Scholar] [CrossRef]
- Kang, C.; You, Y.J.; Avery, L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007, 21, 2161–2171. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, M.; Rodriguez, E.; Birerdinc, A.; Baranova, A. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget 2015, 6, 1414–1421. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Powers, D.C.; Morley, J.E.; Flood, J.F. Age-related changes in LFA-1 expression, cell adhesion, and PHA-induced proliferation by lymphocytes from senescence-accelerated mouse (SAM)-P/8 and SAM-R/1 substrains. Cell Immunol. 1992, 141, 444–456. [Google Scholar] [CrossRef]
- Pallis, M.; Robins, A.; Powell, R. Quantitative analysis of lymphocyte CD11a using standardized flow cytometry. Scand. J. Immunol. 1993, 38, 559–564. [Google Scholar] [CrossRef]
- Okumura, M.; Fujii, Y.; Takeuchi, Y.; Inada, K.; Nakahara, K.; Matsuda, H. Age-related accumulation of LFA-1high cells in a CD8+CD45RAhigh T cell population. Eur. J. Immunol. 1993, 23, 1057–1063. [Google Scholar] [CrossRef]
- Chiricolo, M.; Morini, M.C.; Mancini, R.; Beltrandi, E.; Belletti, D.; Conte, R. Cell adhesion molecules CD11a and CD18 in blood monocytes in old age and the consequences for immunological dysfunction. Preliminary results. Gerontology 1995, 41, 227–234. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Flesher, D.L.; Sun, X.; Behrens, T.W.; Graham, R.R.; Criswell, L.A. Recent advances in the genetics of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2010, 6, 461–479. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Sridharan, A.; Prakash, S.; Agrawal, H. Dendritic cells and aging: Consequences for autoimmunity. Expert Rev. Clin. Immunol. 2012, 8, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef] [Green Version]
- La Ferla, K.; Reimann, C.; Jelkmann, W.; Hellwig-Burgel, T. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappaB. FASEB J. 2002, 16, 1811–1813. [Google Scholar] [CrossRef]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lum, A.F.; Green, C.E.; Lee, G.R.; Staunton, D.E.; Simon, S.I. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J. Biol. Chem. 2002, 277, 20660–20670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Deng, C.; Lu, Q.; Richardson, B. Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech. Ageing Dev. 2002, 123, 1257–1268. [Google Scholar] [CrossRef]
- Lu, Q.; Kaplan, M.; Ray, D.; Ray, D.; Zacharek, S.; Gutsch, D.; Richardson, B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum. 2002, 46, 1282–1291. [Google Scholar] [CrossRef]
- Kochmanski, J.; Marchlewicz, E.H.; Cavalcante, R.G.; Sartor, M.A.; Dolinoy, D.C. Age-related Epigenome-wide DNA Methylation and Hydroxymethylation in Longitudinal Mouse Blood. Epigenetics 2018, 13, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Guevara, E.E.; Lawler, R.R.; Staes, N.; White, C.M.; Sherwood, C.C.; Ely, J.J.; Hopkins, W.D.; Bradley, B.J. Age-associated epigenetic change in chimpanzees and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190616. [Google Scholar] [CrossRef]
- Avrahami, D.; Li, C.; Zhang, J.; Schug, J.; Avrahami, R.; Rao, S.; Stadler, M.B.; Burger, L.; Schubeler, D.; Glaser, B.; et al. Aging-Dependent Demethylation of Regulatory Elements Correlates with Chromatin State and Improved beta Cell Function. Cell Metab. 2015, 22, 619–632. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Leblond, F.; Mamarbachi, M.; Geoffroy, S.; Thorin, E. Age-Dependent Demethylation of Sod2 Promoter in the Mouse Femoral Artery. Oxid. Med. Cell. Longev. 2016, 2016, 8627384. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M.; Hayakawa, K.; Arai, D.; Shiota, K. Age- and sex-dependent DNA hypomethylation controlled by growth hormone in mouse liver. Mech. Ageing Dev. 2013, 134, 331–337. [Google Scholar] [CrossRef]
- Khalil, H.; Tazi, M.; Caution, K.; Ahmed, A.; Kanneganti, A.; Assani, K.; Kopp, B.; Marsh, C.; Dakhlallah, D.; Amer, A.O. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics 2016, 11, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Thalheim, T.; Herberg, M.; Galle, J. Linking DNA Damage and Age-Related Promoter DNA Hyper-Methylation in the Intestine. Genes 2018, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Cruickshanks, H.A.; McBryan, T.; Nelson, D.M.; Vanderkraats, N.D.; Shah, P.P.; van Tuyn, J.; Singh Rai, T.; Brock, C.; Donahue, G.; Dunican, D.S.; et al. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 2013, 15, 1495–1506. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guo, Z.; Guo, Y.; Li, M.; Yan, H.; Cheng, J.; Wang, C.; Hong, G. Common DNA methylation alterations of Alzheimer’s disease and aging in peripheral whole blood. Oncotarget 2016, 7, 19089–19098. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Ramos, R.B.; Fabris, V.; Lecke, S.B.; Maturana, M.A.; Spritzer, P.M. Association between global leukocyte DNA methylation and cardiovascular risk in postmenopausal women. BMC Med. Genet. 2016, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Joyce, B.T.; Gao, T.; Zheng, Y.; Liu, L.; Zhang, W.; Dai, Q.; Shrubsole, M.J.; Hibler, E.A.; Cristofanilli, M.; Zhang, H.; et al. Prospective changes in global DNA methylation and cancer incidence and mortality. Br. J. Cancer 2016, 115, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Kresovich, J.K.; Joyce, B.T.; Gao, T.; Zheng, Y.; Zhang, Z.; Achenbach, C.J.; Murphy, R.L.; Just, A.C.; Shen, J.; Yang, H.; et al. Promoter methylation of PGC1A and PGC1B predicts cancer incidence in a veteran cohort. Epigenomics 2018, 10, 733–743. [Google Scholar] [CrossRef]
- Maeda, M.; Nakajima, T.; Oda, I.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Asada, K.; Yokoi, C.; Ando, T.; Yoshida, T.; et al. High impact of methylation accumulation on metachronous gastric cancer: 5-year follow-up of a multicentre prospective cohort study. Gut 2017, 66, 1721–1723. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Iwanishi, M.; Minami, T.; Chishina, H.; Arizumi, T.; Takita, M.; Kitai, S.; Yada, N.; Ida, H.; Hagiwara, S.; et al. Hepatic DNA Methylation Is Affected by Hepatocellular Carcinoma Risk in Patients with and without Hepatitis Virus. Dig. Dis. 2015, 33, 745–750. [Google Scholar] [CrossRef]
- Vaz, M.; Hwang, S.Y.; Kagiampakis, I.; Phallen, J.; Patil, A.; O’Hagan, H.M.; Murphy, L.; Zahnow, C.A.; Gabrielson, E.; Velculescu, V.E.; et al. Chronic Cigarette Smoke-Induced Epigenomic Changes Precede Sensitization of Bronchial Epithelial Cells to Single-Step Transformation by KRAS Mutations. Cancer Cell 2017, 32, 360–376.e6. [Google Scholar] [CrossRef] [Green Version]
- Ianov, L.; Riva, A.; Kumar, A.; Foster, T.C. DNA Methylation of Synaptic Genes in the Prefrontal Cortex Is Associated with Aging and Age-Related Cognitive Impairment. Front. Aging Neurosci. 2017, 9, 249. [Google Scholar] [CrossRef]
- Jin, L.; Jiang, Z.; Xia, Y.; Lou, P.; Chen, L.; Wang, H.; Bai, L.; Xie, Y.; Liu, Y.; Li, W.; et al. Genome-wide DNA methylation changes in skeletal muscle between young and middle-aged pigs. BMC Genom. 2014, 15, 653. [Google Scholar] [CrossRef] [Green Version]
- Kananen, L.; Marttila, S.; Nevalainen, T.; Jylhava, J.; Mononen, N.; Kahonen, M.; Raitakari, O.T.; Lehtimaki, T.; Hurme, M. Aging-associated DNA methylation changes in middle-aged individuals: The Young Finns study. BMC Genom. 2016, 17, 103. [Google Scholar] [CrossRef] [Green Version]
- Spiers, H.; Hannon, E.; Wells, S.; Williams, B.; Fernandes, C.; Mill, J. Age-associated changes in DNA methylation across multiple tissues in an inbred mouse model. Mech. Ageing Dev. 2016, 154, 20–23. [Google Scholar] [CrossRef]
- De, F.C.L.A.J.; van der Plaat, D.A.; de Jong, K.; van Diemen, C.C.; Postma, D.S.; Nedeljkovic, I.; van Duijn, C.M.; Amin, N.; la Bastide-van Gemert, S.; de Vries, M.; et al. Long-term Air Pollution Exposure, Genome-wide DNA Methylation and Lung Function in the LifeLines Cohort Study. Environ. Health Perspect. 2018, 126, 027004. [Google Scholar]
- Nwanaji-Enwerem, J.C.; Colicino, E.; Dai, L.; Cayir, A.; Sanchez-Guerra, M.; Laue, H.E.; Nguyen, V.T.; Di, Q.; Just, A.C.; Hou, L.; et al. Impacts of the Mitochondrial Genome on the Relationship of Long-Term Ambient Fine Particle Exposure with Blood DNA Methylation Age. Environ. Sci. Technol. 2017, 51, 8185–8195. [Google Scholar] [CrossRef]
- Panni, T.; Mehta, A.J.; Schwartz, J.D.; Baccarelli, A.A.; Just, A.C.; Wolf, K.; Wahl, S.; Cyrys, J.; Kunze, S.; Strauch, K.; et al. Genome-Wide Analysis of DNA Methylation and Fine Particulate Matter Air Pollution in Three Study Populations: KORA F3, KORA F4, and the Normative Aging Study. Environ. Health Perspect. 2016, 124, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, R.; Shi, M.; Cai, J.; Shi, J.; Yang, C.; Li, H.; Lin, Z.; Meng, X.; Liu, C.; et al. Possible Mediation by Methylation in Acute Inflammation Following Personal Exposure to Fine Particulate Air Pollution. Am. J. Epidemiol. 2018, 187, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Ward-Caviness, C.K.; Nwanaji-Enwerem, J.C.; Wolf, K.; Wahl, S.; Colicino, E.; Trevisi, L.; Kloog, I.; Just, A.C.; Vokonas, P.; Cyrys, J.; et al. Long-term exposure to air pollution is associated with biological aging. Oncotarget 2016, 7, 74510–74525. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Fink, B.; Thurmann, L.; Eszlinger, M.; Herberth, G.; Lehmann, I. Tobacco smoking differently influences cell types of the innate and adaptive immune system-indications from CpG site methylation. Clin. Epigenetics 2015, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, Y.; Saum, K.U.; Schottker, B.; Breitling, L.P.; Brenner, H. Tobacco smoking and smoking-related DNA methylation are associated with the development of frailty among older adults. Epigenetics 2017, 12, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Ligthart, S.; Steenaard, R.V.; Peters, M.J.; van Meurs, J.B.; Sijbrands, E.J.; Uitterlinden, A.G.; Bonder, M.J.; consortium, B.; Hofman, A.; Franco, O.H.; et al. Tobacco smoking is associated with DNA methylation of diabetes susceptibility genes. Diabetologia 2016, 59, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Steenaard, R.V.; Ligthart, S.; Stolk, L.; Peters, M.J.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; Franco, O.H.; Dehghan, A. Tobacco smoking is associated with methylation of genes related to coronary artery disease. Clin. Epigenetics 2015, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schottker, B.; Florath, I.; Stock, C.; Butterbach, K.; Holleczek, B.; Mons, U.; Brenner, H. Smoking-Associated DNA Methylation Biomarkers and Their Predictive Value for All-Cause and Cardiovascular Mortality. Environ. Health Perspect. 2016, 124, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Marioni, R.E.; Hedman, A.K.; Pfeiffer, L.; Tsai, P.C.; Reynolds, L.M.; Just, A.C.; Duan, Q.; Boer, C.G.; Tanaka, T.; et al. A DNA methylation biomarker of alcohol consumption. Mol. Psychiatry 2018, 23, 422–433. [Google Scholar] [CrossRef]
- Dugue, P.A.; Wilson, R.; Lehne, B.; Jayasekara, H.; Wang, X.; Jung, C.H.; Joo, J.E.; Makalic, E.; Schmidt, D.F.; Baglietto, L.; et al. Alcohol consumption is associated with widespread changes in blood DNA methylation: Analysis of cross-sectional and longitudinal data. Addict. Biol. 2021, 26, e12855. [Google Scholar] [CrossRef]
- Friedel, E.; Walter, H.; Veer, I.M.; Zimmermann, U.S.; Heinz, A.; Frieling, H.; Zindler, T. Impact of Long-Term Alcohol Consumption and Relapse on Genome-Wide DNA Methylation Changes in Alcohol-Dependent Subjects: A Longitudinal Study. Alcohol. Clin. Exp. Res. 2020, 44, 1356–1365. [Google Scholar] [CrossRef]
- Lu, M.; Xueying, Q.; Hexiang, P.; Wenjing, G.; Hagg, S.; Weihua, C.; Chunxiao, L.; Canqing, Y.; Jun, L.; Zengchang, P.; et al. Genome-wide associations between alcohol consumption and blood DNA methylation: Evidence from twin study. Epigenomics 2021, 13, 939–951. [Google Scholar] [CrossRef]
- O’Hagan, H.M.; Wang, W.; Sen, S.; Destefano Shields, C.; Lee, S.S.; Zhang, Y.W.; Clements, E.G.; Cai, Y.; Van Neste, L.; Easwaran, H.; et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 2011, 20, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Elgizouli, M.; Schottker, B.; Holleczek, B.; Nieters, A.; Brenner, H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clin. Epigenetics 2016, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, Y.; Breitling, L.P.; Brenner, H. Relationship of tobacco smoking and smoking-related DNA methylation with epigenetic age acceleration. Oncotarget 2016, 7, 46878–46889. [Google Scholar] [CrossRef] [Green Version]
- Barres, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Denham, J.; O’Brien, B.J.; Harvey, J.T.; Charchar, F.J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 2015, 7, 717–731. [Google Scholar] [CrossRef]
- Maejima, H.; Kanemura, N.; Kokubun, T.; Murata, K.; Takayanagi, K. Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci. Lett. 2018, 665, 67–73. [Google Scholar] [CrossRef]
- Ronn, T.; Volkov, P.; Davegardh, C.; Dayeh, T.; Hall, E.; Olsson, A.H.; Nilsson, E.; Tornberg, A.; Dekker Nitert, M.; Eriksson, K.F.; et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013, 9, e1003572. [Google Scholar] [CrossRef]
- Barres, R.; Kirchner, H.; Rasmussen, M.; Yan, J.; Kantor, F.R.; Krook, A.; Naslund, E.; Zierath, J.R. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013, 3, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Marioni, R.E.; Colicino, E.; Peters, M.J.; Ward-Caviness, C.K.; Tsai, P.C.; Roetker, N.S.; Just, A.C.; Demerath, E.W.; Guan, W.; et al. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging Albany NY 2016, 8, 1844–1865. [Google Scholar] [CrossRef] [Green Version]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging Albany NY 2011, 3, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Shain, S.A.; Schultz, J.J.; Lancaster, C.M. Aging in the AXC/SSh rat: Diminished prostate L-ornithine decarboxylase (ODC) activity reflects diminished prostate ODC protein and transcript content. Endocrinology 1986, 119, 1830–1838. [Google Scholar] [CrossRef]
- Casillas, M.A., Jr.; Lopatina, N.; Andrews, L.G.; Tollefsbol, T.O. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol. Cell. Biochem. 2003, 252, 33–43. [Google Scholar] [CrossRef]
- Lopatina, N.; Haskell, J.F.; Andrews, L.G.; Poole, J.C.; Saldanha, S.; Tollefsbol, T. Differential maintenance and de novo methylating activity by three DNA methyltransferases in aging and immortalized fibroblasts. J. Cell. Biochem. 2002, 84, 324–334. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Hemstedt, T.J.; Bading, H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat. Neurosci. 2012, 15, 1111–1113. [Google Scholar] [CrossRef]
- Romanenko, E.B.; Demidenko, Z.N.; Vanyushin, B.F. RNA-polymerase, DNA-polymerase, DNA-methyltransferase and sphingomyelinase activities in liver nuclei of rats of different Age. Biochemistry 1998, 63, 159–163. [Google Scholar]
- Frostesjo, L.; Holm, I.; Grahn, B.; Page, A.W.; Bestor, T.H.; Heby, O. Interference with DNA methyltransferase activity and genome methylation during F9 teratocarcinoma stem cell differentiation induced by polyamine depletion. J. Biol. Chem. 1997, 272, 4359–4366. [Google Scholar] [CrossRef] [Green Version]
- Poomipark, N.; Flatley, J.E.; Hill, M.H.; Mangnall, B.; Azar, E.; Grabowski, P.; Powers, H.J. Methyl Donor Status Influences DNMT Expression and Global DNA Methylation in Cervical Cancer Cells. Asian Pac. J. Cancer Prev. 2016, 17, 3213–3222. [Google Scholar]
- Tsuji, T.; Usui, S.; Aida, T.; Tachikawa, T.; Hu, G.F.; Sasaki, A.; Matsumura, T.; Todd, R.; Wong, D.T. Induction of epithelial differentiation and DNA demethylation in hamster malignant oral keratinocyte by ornithine decarboxylase antizyme. Oncogene 2001, 20, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, D.; Shima, K.; Matsuo, K.; Nishioka, T.; Chen, C.Y.; Hu, G.F.; Sasaki, A.; Tsuji, T. Ornithine decarboxylase antizyme induces hypomethylation of genome DNA and histone H3 lysine 9 dimethylation (H3K9me2) in human oral cancer cell line. PLoS ONE 2010, 5, e12554. [Google Scholar] [CrossRef] [Green Version]
- Pegg, A.E.; Wang, X.; Schwartz, C.E.; McCloskey, D.E. Spermine synthase activity affects the content of decarboxylated S-adenosylmethionine. Biochem. J. 2011, 433, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Shantz, L.M.; Holm, I.; Janne, O.A.; Pegg, A.E. Regulation of S-adenosylmethionine decarboxylase activity by alterations in the intracellular polyamine content. Biochem. J. 1992, 288 Pt 2, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Papazafiri, P.; Osborne, H.B. Effect of alpha-difluoromethylornithine on DNA methylation in murine erythroleukaemic cells. Relationship to stimulation of induced differentiation. Biochem. J. 1987, 242, 479–483. [Google Scholar] [CrossRef]
- Bell, J.T.; Tsai, P.C.; Yang, T.P.; Pidsley, R.; Nisbet, J.; Glass, D.; Mangino, M.; Zhai, G.; Zhang, F.; Valdes, A.; et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012, 8, e1002629. [Google Scholar] [CrossRef] [Green Version]
- Florath, I.; Butterbach, K.; Muller, H.; Bewerunge-Hudler, M.; Brenner, H. Cross-sectional and longitudinal changes in DNA methylation with age: An epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Hum. Mol. Genet. 2014, 23, 1186–1201. [Google Scholar] [CrossRef]
- McClay, J.L.; Aberg, K.A.; Clark, S.L.; Nerella, S.; Kumar, G.; Xie, L.Y.; Hudson, A.D.; Harada, A.; Hultman, C.M.; Magnusson, P.K.; et al. A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum. Mol. Genet. 2014, 23, 1175–1185. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.F.; Tejedor, J.R.; Bayon, G.F.; Fernandez, A.F.; Fraga, M.F. Distinct chromatin signatures of DNA hypomethylation in aging and cancer. Aging Cell 2018, 17, e12744. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, X.; Ning, C.; Zhu, Q.; Yao, Y.; Zhao, Y.; Luan, F. Methylation of the genes ROD1, NLRC5, and HKR1 is associated with aging in Hainan centenarians. BMC Med. Genom. 2018, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Ray, D.; Gutsch, D.; Richardson, B. Effect of DNA methylation and chromatin structure on ITGAL expression. Blood 2002, 99, 4503–4508. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, C.A.; Thompson, P.A. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am. J. Clin. Nutr. 2015, 102, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Wada, M.; Funada-Wada, U.; Mano, H.; Udaka, S. Effects of Dietary Polyamines on the Promotion of Mammary Tumor in Rats. J. Health Sci. 2002, 48, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Soda, K. Polyamine Metabolism and Gene Methylation in Conjunction with One-Carbon Metabolism. Int. J. Mol. Sci. 2018, 19, 3106. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Dong, B.; Yang, M.; Dong, B.; Wei, Y. Frailty and Cognitive Impairment in Predicting Mortality among Oldest-Old People. Front. Aging Neurosci. 2018, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Langa, K.M.; Larson, E.B.; Karlawish, J.H.; Cutler, D.M.; Kabeto, M.U.; Kim, S.Y.; Rosen, A.B. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008, 4, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Perna, L.; Wahl, H.W.; Mons, U.; Saum, K.U.; Holleczek, B.; Brenner, H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing 2015, 44, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Kwon, D.Y.; Jung, J.M.; Han, C.; Jo, I.; Jo, S.A. Mini-Mental Status Examination as predictors of mortality in the elderly. Acta Psychiatr. Scand. 2013, 127, 298–304. [Google Scholar] [CrossRef]
- Sachs, G.A.; Carter, R.; Holtz, L.R.; Smith, F.; Stump, T.E.; Tu, W.; Callahan, C.M. Cognitive impairment: An independent predictor of excess mortality: A cohort study. Ann. Intern. Med. 2011, 155, 300–308. [Google Scholar] [CrossRef]
- Santabarbara, J.; Gracia-Garcia, P.; Pirez, G.; Lopez-Anton, R.; De La Camara, C.; Ventura, T.; Perez-Sastre, M.; Lobo, E.; Saz, P.; Marcos, G.; et al. Mortality in Mild Cognitive Impairment Diagnosed with DSM-5 Criteria and with Petersen’s Criteria: A 17-Year Follow-Up in a Community Study. Am. J. Geriatr. Psychiatry 2016, 24, 977–986. [Google Scholar] [CrossRef]
- Vassilaki, M.; Cha, R.H.; Aakre, J.A.; Therneau, T.M.; Geda, Y.E.; Mielke, M.M.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Mortality in mild cognitive impairment varies by subtype, sex, and lifestyle factors: The Mayo Clinic Study of Aging. J. Alzheimers Dis. 2015, 45, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Scheunemann, L.; Eisenberg, T.; Mertel, S.; Bhukel, A.; Koemans, T.S.; Kramer, J.M.; Liu, K.S.; Schroeder, S.; Stunnenberg, H.G.; et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 2013, 16, 1453–1460. [Google Scholar] [CrossRef]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Bartels, A.L. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr. Pharm. Des. 2011, 17, 2771–2777. [Google Scholar] [CrossRef]
- Chung, Y.C.; Ko, H.W.; Bok, E.; Park, E.S.; Huh, S.H.; Nam, J.H.; Jin, B.K. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010, 43, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Prineas, J.W.; Parratt, J.D. Oligodendrocytes and the early multiple sclerosis lesion. Ann. Neurol. 2012, 72, 18–31. [Google Scholar] [CrossRef]
- Tourdias, T.; Dousset, V. Neuroinflammatory imaging biomarkers: Relevance to multiple sclerosis and its therapy. Neurotherapeutics 2013, 10, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Garbuzova-Davis, S.; Sanberg, P.R. Blood-CNS Barrier Impairment in ALS patients versus an animal model. Front. Cell. Neurosci. 2014, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.C.; Hernandez-Ontiveros, D.G.; Louis, M.K.; Willing, A.E.; Borlongan, C.V.; Sanberg, P.R.; Voltarelli, J.C.; Garbuzova-Davis, S. Neurovascular aspects of amyotrophic lateral sclerosis. Int. Rev. Neurobiol. 2012, 102, 91–106. [Google Scholar]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, 2364. [Google Scholar] [CrossRef] [Green Version]
- Akanuma, S.I.; Shimada, H.; Kubo, Y.; Hosoya, K.I. Involvement of Carrier-Mediated Transport at the Blood-Cerebrospinal Fluid Barrier in Spermine Clearance from Rat Brain. Biol. Pharm. Bull. 2017, 40, 1599–1603. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Trolin, C.; Nygren, I.; Aquilonius, S.M.; Askmark, H. Increased red blood cell polyamines in ALS and Parkinson’s disease. Exp. Neurol. 2002, 177, 515–520. [Google Scholar] [CrossRef]
- Graham, S.F.; Chevallier, O.P.; Elliott, C.T.; Holscher, C.; Johnston, J.; McGuinness, B.; Kehoe, P.G.; Passmore, A.P.; Green, B.D. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 2015, 10, e0119452. [Google Scholar] [CrossRef]
- Holmes, C.; Butchart, J. Systemic inflammation and Alzheimer’s disease. Biochem. Soc. Trans. 2011, 39, 898–901. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 2010, 33, 1539–1556. [Google Scholar] [CrossRef]
- Tao, Q.; Ang, T.F.A.; DeCarli, C.; Auerbach, S.H.; Devine, S.; Stein, T.D.; Zhang, X.; Massaro, J.; Au, R.; Qiu, W.Q. Association of Chronic Low-grade Inflammation With Risk of Alzheimer Disease in ApoE4 Carriers. JAMA Netw. Open 2018, 1, e183597. [Google Scholar] [CrossRef] [Green Version]
- Morrison, L.D.; Bergeron, C.; Kish, S.J. Brain S-adenosylmethionine decarboxylase activity is increased in Alzheimer’s disease. Neurosci. Lett. 1993, 154, 141–144. [Google Scholar] [CrossRef]
- Karouzakis, E.; Gay, R.E.; Michel, B.A.; Gay, S.; Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis. Rheum. 2009, 60, 3613–3622. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.J.; Goodman, S.J.; Kobor, M.S. DNA methylation and healthy human aging. Aging Cell 2015, 14, 924–932. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Wu, Y.; Bockmuhl, Y.; Spengler, D. The Janus face of DNA methylation in aging. Aging Albany NY 2010, 2, 107–110. [Google Scholar]
- Vanyushin, B.F.; Nemirovsky, L.E.; Klimenko, V.V.; Vasiliev, V.K.; Belozersky, A.N. The 5-methylcytosine in DNA of rats. Tissue and age specificity and the changes induced by hydrocortisone and other agents. Gerontologia 1973, 19, 138–152. [Google Scholar] [CrossRef]
- Wilson, V.L.; Smith, R.A.; Ma, S.; Cutler, R.G. Genomic 5-methyldeoxycytidine decreases with age. J. Biol. Chem. 1987, 262, 9948–9951. [Google Scholar] [CrossRef]
- Day, J.J.; Sweatt, J.D. DNA methylation and memory formation. Nat. Neurosci. 2010, 13, 1319–1323. [Google Scholar] [CrossRef]
- Day, J.J.; Childs, D.; Guzman-Karlsson, M.C.; Kibe, M.; Moulden, J.; Song, E.; Tahir, A.; Sweatt, J.D. DNA methylation regulates associative reward learning. Nat. Neurosci. 2013, 16, 1445–1452. [Google Scholar] [CrossRef]
- Rosenbaum, J.F.; Fava, M.; Falk, W.E.; Pollack, M.H.; Cohen, L.S.; Cohen, B.M.; Zubenko, G.S. The antidepressant potential of oral S-adenosyl-l-methionine. Acta Psychiatr. Scand. 1990, 81, 432–436. [Google Scholar] [CrossRef]
- Chan, A.; Shea, T.B. Effects of dietary supplementation with N-acetyl cysteine, acetyl-L-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromol. Med. 2007, 9, 264–269. [Google Scholar] [CrossRef]
- Tchantchou, F.; Graves, M.; Ortiz, D.; Rogers, E.; Shea, T.B. Dietary supplementation with 3-deaza adenosine, N-acetyl cysteine, and S-adenosyl methionine provide neuroprotection against multiple consequences of vitamin deficiency and oxidative challenge: Relevance to age-related neurodegeneration. Neuromol. Med. 2004, 6, 93–103. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soda, K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. https://doi.org/10.3390/cells11010164
Soda K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells. 2022; 11(1):164. https://doi.org/10.3390/cells11010164
Chicago/Turabian StyleSoda, Kuniyasu. 2022. "Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation" Cells 11, no. 1: 164. https://doi.org/10.3390/cells11010164
APA StyleSoda, K. (2022). Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells, 11(1), 164. https://doi.org/10.3390/cells11010164





