The Isoquinoline-Sulfonamide Compound H-1337 Attenuates SU5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Proliferation Analyses
2.3. Western Blot Analyses
2.4. Design of Animal Experiments
2.5. Preparing Su5416/Hypoxia Model
2.6. Treatments with H-1337 for Su/Hx Rats
2.7. Histological Assessment of Pulmonary Vascular Remodeling
2.8. Immunofluorescence, Immunohistochemistry, Kinase Assays, and Measurement of Serum Concentrations of H-1337 and H-1337M1
2.9. Statistical Analyses
3. Results
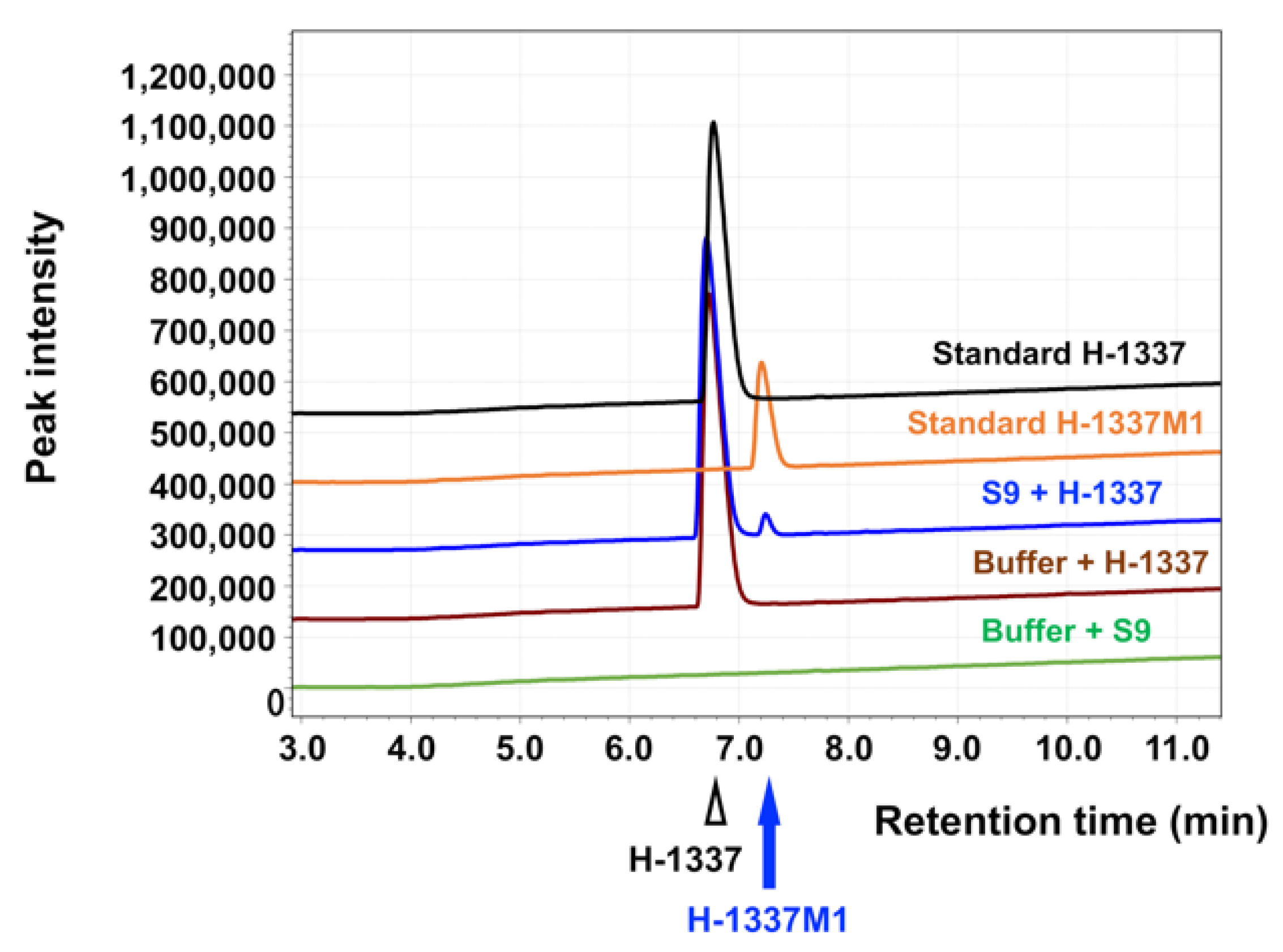
3.1. Characteristics of H-1337 and the Metabolite H-1337M1
3.2. H-1337 and H-1337M1 Suppressed the Phosphorylation of MLC in Human SMCs
3.3. H-1337 and H-1337M1 Suppressed the Phosphorylation of mTOR in Human SMCs
3.4. H-1337 and H-1337M1 Suppressed the Proliferation of hPASMCs
3.5. H-1337 Decreased Right Ventricular Pressure and Occlusive Vascular Lesions in Su/Hx Rats
3.6. H-1337 Suppressed the Phosphorylation of MLC and mTOR in the Pulmonary Vasculature
3.7. H-1337 Had a Suppressive Effect on RV Remodeling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Lau, E.M.; Montani, D.; Jaïs, X.; Sitbon, O.; Simonneau, G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014, 130, 2189–2208. [Google Scholar] [CrossRef]
- Tamura, Y.; Kumamaru, H.; Satoh, T.; Miyata, H.; Ogawa, A.; Tanabe, N.; Hatano, M.; Yao, A.; Abe, K.; Tsujino, I.; et al. Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ. J. 2017, 82, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef]
- Sakao, S.; Voelkel, N.F.; Tanabe, N.; Tatsumi, K. Determinants of an elevated pulmonary arterial pressure in patients with pulmonary arterial hypertension. Respir. Res. 2015, 16, 84. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2010, 43, 629–634. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K. Vascular remodeling in pulmonary arterial hypertension: Multiple cancer-like pathways and possible treatment modalities. Int. J. Cardiol. 2011, 147, 4–12. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Endothelial cells and pulmonary arterial hypertension: Apoptosis, proliferation, interaction and transdifferentiation. Respir. Res. 2009, 10, 95. [Google Scholar] [CrossRef]
- Loirand, G.; Guérin, P.; Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Sakao, S.; Taraseviciene-Stewart, L.; Lee, J.D.; Wood, K.; Cool, C.D.; Voelkel, N.F. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005, 19, 1178–1180. [Google Scholar] [CrossRef]
- Wu, F.; Yao, W.; Yang, J.; Zhang, M.; Xu, Y.; Hao, Y.; Yan, L.; Niu, Y.; Sun, T.; Yu, J.; et al. Protective effects of aloperin on monocroline-induced pulmonary hypertension via regulation of Rho A/Rho kinsase pathway in rats. Biomed. Pharmacother. 2017, 95, 1161–1168. [Google Scholar] [CrossRef]
- Wang, X.Y.; Mo, D.; Tian, W.; Liu, X.X.; Zhou, Y.G.; Sun, Y.; Feng, Y.D.; Xiao, X.; Hao, X.W.; Zhang, H.N.; et al. Inhibition of RhoA/ROCK signaling pathway ameliorates hypoxic pulmonary hypertension via HIF-1α-dependent functional TRPC channels. Toxicol. Appl. Pharmacol. 2019, 369, 60–72. [Google Scholar] [CrossRef]
- Dahal, B.K.; Kosanovic, D.; Pamarthi, P.K.; Sydykov, A.; Lai, Y.J.; Kast, R.; Schirok, H.; Stasch, J.P.; Ghofrani, H.A.; Weissmann, N.; et al. Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension. Eur. Respir. J. 2010, 36, 808–818. [Google Scholar] [CrossRef]
- Gary-Bobo, G.; Houssaini, A.; Amsellem, V.; Rideau, D.; Pacaud, P.; Perrin, A.; Brégeon, J.; Marcos, E.; Dubois-Randé, J.L.; Sitbon, O.; et al. Effects of HIV protease inhibitors on progression of monocrotaline- and hypoxia-induced pulmonary hypertension in rats. Circulation 2010, 122, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Do.e, Z.; Fukumoto, Y.; Takaki, A.; Tawara, S.; Ohashi, J.; Nakano, M.; Tada, T.; Saji, K.; Sugimura, K.; Fujita, H.; et al. Evidence for Rho-kinase activation in patients with pulmonary arterial hypertension. Circ. J. 2009, 73, 1731–1739. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Babicheva, A.; Makino, A.; Yuan, J.X. mTOR signaling in pulmonary vascular disease: Pathogenic role and therapeutic target. Int. J. Mol. Sci. 2021, 22, 2144. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.P.; Li, X.H.; Yang, Y.M.; Li, W.Q.; Zhang, W.; Hu, C.P.; Zhang, Z.; Li, Y.J. A critical role of the mTOR/eIF2α pathway in hypoxia-induced pulmonary hypertension. PLoS ONE 2015, 10, e0130806. [Google Scholar] [CrossRef]
- Houssaini, A.; Abid, S.; Mouraret, N.; Wan, F.; Rideau, D.; Saker, M.; Marcos, E.; Tissot, C.M.; Dubois-Randé, J.L.; Amsellem, V.; et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 48, 568–577. [Google Scholar] [CrossRef]
- Pena, A.; Kobir, A.; Goncharov, D.; Goda, A.; Kudryashova, T.V.; Ray, A.; Vanderpool, R.; Baust, J.; Chang, B.; Mora, A.L.; et al. Pharmacological inhibition of mTOR kinase reverses right ventricle remodeling and improves right ventricle structure and function in rats. Am. J. Respir. Cell Mol. Biol. 2017, 57, 615–625. [Google Scholar] [CrossRef]
- Hidaka, H.; Sumi, K.; Izuhara, T.; Kasai, A.; Tanimoto, H. A novel isoquinoline sulfonamide protein kinase inhibitor (H-1337) produces long-lasting reduction of IOP. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5712. [Google Scholar]
- Kasai, A.; Yoshida, Y.; Hasegawa, K.; Hidaka, H. Elucidation of molecular mechanism of H-1337, an anti-glaucoma agent. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5695. [Google Scholar]
- Montagnoli, T.L.; da Silva, J.S.; Sudo, S.Z.; Santos, A.D.; Gomide, G.F.; de Sá, M.P.L.; Zapata-Sudo, G. ROCK inhibition as potential target for treatment of pulmonary hypertension. Cells 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Sakao, S.; Takeuchi, T.; Suzuki, T.; Nishimura, R.; Yasuda, T.; Tanabe, N.; Tatsumi, K. Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L899–L915. [Google Scholar] [CrossRef]
- Toba, M.; Alzoubi, A.; O’Neill, K.D.; Gairhe, S.; Matsumoto, Y.; Oshima, K.; Abe, K.; Oka, M.; McMurtry, I.F. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H243–H250. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.H.; Piao, L.; Hong, Z.; Toth, P.T.; Marsboom, G.; Bache-Wiig, P.; Rehman, J.; Archer, S.L. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting Randle’s cycle. J. Mol. Med. 2012, 90, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Han, B.; Yu, T.; Zong, Z. Effect of apelin on the cardiac hemodynamics in hypertensive rats with heart failure. Int. J. Mol. Med. 2014, 34, 756–764. [Google Scholar] [CrossRef][Green Version]
- Hirano, K.; Derkach, D.N.; Hirano, M.; Nishimura, J.; Kanaide, H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol. Cell. Biochem. 2003, 248, 105–114. [Google Scholar] [CrossRef]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C., Jr.; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256–5267. [Google Scholar] [CrossRef]
- Wiza, C.; Nascimento, E.B.; Ouwens, D.M. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1453–E1460. [Google Scholar] [CrossRef]
- Vander Haar, E.; Lee, S.I.; Bandhakavi, S.; Griffin, T.J.; Kim, D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007, 9, 316–323. [Google Scholar] [CrossRef]
- Ramachandran, C.; Patil, R.V.; Combrink, K.; Sharif, N.A.; Srinivas, S.P. Rho-Rho kinase pathway in the actomyosin contraction and cell-matrix adhesion in immortalized human trabecular meshwork cells. Mol. Vis. 2011, 17, 1877–1890. [Google Scholar] [PubMed]
- Woodsome, T.P.; Polzin, A.; Kitazawa, K.; Eto, M.; Kitazawa, T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J. Cell Sci. 2006, 119 Pt 9, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.K.; Sung, M.L.; Yu, H.R.; Chang, H.I.; Kuo, H.C.; Tsai, T.C.; Yen, C.K.; Chen, C.N. Homocysteine induces smooth muscle cell proliferation through differential regulation of cyclins A and D1 expression. J. Cell. Physiol. 2011, 226, 1017–1026. [Google Scholar] [CrossRef]
- Pi, W.F.; Guo, X.J.; Su, L.P.; Xu, W.G. Troglitazone upregulates PTEN expression and induces the apoptosis of pulmonary artery smooth muscle cells under hypoxic conditions. Int. J. Mol. Med. 2013, 32, 1101–1109. [Google Scholar] [CrossRef]
- Morrell, N.W.; Adnot, S.; Archer, S.L.; Dupuis, J.; Lloyd Jones, P.; MacLean, M.R.; McMurtry, I.F.; Stenmark, K.R.; Thistlethwaite, P.A.; Weissmann, N.; et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. 1), S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Shimokawa, H.; Morikawa, K.; Uwatoku, T.; Oi, K.; Matsumoto, Y.; Hattori, T.; Nakashima, Y.; Kaibuchi, K.; Sueishi, K.; et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ. Res. 2004, 94, 385–393. [Google Scholar] [CrossRef]
- Abe, K.; Tawara, S.; Oi, K.; Hizume, T.; Uwatoku, T.; Fukumoto, Y.; Kaibuchi, K.; Shimokawa, H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J. Cardiovasc. Pharmacol. 2006, 48, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Paddenberg, R.; Stieger, P.; von Lilien, A.L.; Faulhammer, P.; Goldenberg, A.; Tillmanns, H.H.; Kummer, W.; Braun-Dullaeus, R.C. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Respir. Res. 2007, 8, 15. [Google Scholar] [CrossRef]
- Horita, H.; Furgeson, S.B.; Ostriker, A.; Olszewski, K.A.; Sullivan, T.; Villegas, L.R.; Levine, M.; Parr, J.E.; Cool, C.D.; Nemenoff, R.A.; et al. Selective inactivation of PTEN in smooth muscle cells synergizes with hypoxia to induce severe pulmonary hypertension. J. Am. Heart Assoc. 2013, 2, e000188. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Sakao, S.; Kato, F.; Naito, A.; Jujo, T.; Yasuda, T.; Tanabe, N.; Tatsumi, K. Pulmonary haemodynamics are correlated with intimal lesions in a rat model of severe PAH: Attenuation of pulmonary vascular remodelling with ambrisentan. Histol. Histopathol. 2016, 31, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Nielsen-Kudsk, J.E.; Vonk Noordegraaf, A.; de Man, F.S. Right ventricular fibrosis. Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Yamada, N.; Matsubara, H.; Mizoguchi, M.; Uchino, K.; Yao, A.; Kihara, Y.; Kawano, M.; Watanabe, H.; Takeda, Y.; et al. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ. J. 2013, 77, 2619–2625. [Google Scholar] [CrossRef]






| Kinase | H-1337 IC50 (µM) | H-1337M1 IC50 (µM) |
|---|---|---|
| ROCK1 | 0.24 | 0.02 |
| ROCK2 | 0.32 | 0.012 |
| Akt1 | 0.279 | 0.0042 |
| Akt2 | 1.662 | 0.054 |
| Akt3 | 0.112 | 0.0253 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, H.; Yoshida, Y.; Sanada, T.J.; Naito, A.; Maruyama, J.; Zhang, E.; Sumi, K.; Sakao, S.; Maruyama, K.; Hidaka, H.; et al. The Isoquinoline-Sulfonamide Compound H-1337 Attenuates SU5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Rats. Cells 2022, 11, 66. https://doi.org/10.3390/cells11010066
Shoji H, Yoshida Y, Sanada TJ, Naito A, Maruyama J, Zhang E, Sumi K, Sakao S, Maruyama K, Hidaka H, et al. The Isoquinoline-Sulfonamide Compound H-1337 Attenuates SU5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Rats. Cells. 2022; 11(1):66. https://doi.org/10.3390/cells11010066
Chicago/Turabian StyleShoji, Hiroki, Yoko Yoshida, Takayuki Jujo Sanada, Akira Naito, Junko Maruyama, Erquan Zhang, Kengo Sumi, Seiichiro Sakao, Kazuo Maruyama, Hiroyoshi Hidaka, and et al. 2022. "The Isoquinoline-Sulfonamide Compound H-1337 Attenuates SU5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Rats" Cells 11, no. 1: 66. https://doi.org/10.3390/cells11010066
APA StyleShoji, H., Yoshida, Y., Sanada, T. J., Naito, A., Maruyama, J., Zhang, E., Sumi, K., Sakao, S., Maruyama, K., Hidaka, H., & Tatsumi, K. (2022). The Isoquinoline-Sulfonamide Compound H-1337 Attenuates SU5416/Hypoxia-Induced Pulmonary Arterial Hypertension in Rats. Cells, 11(1), 66. https://doi.org/10.3390/cells11010066






