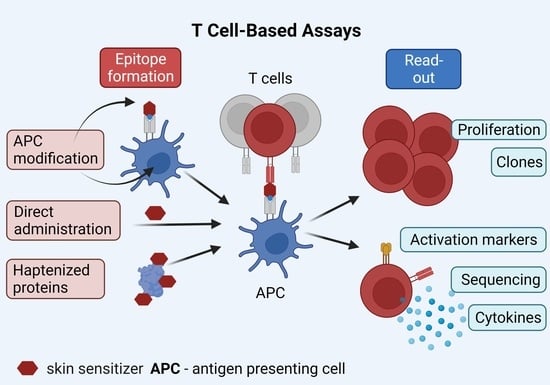
In Vitro Monitoring of Human T Cell Responses to Skin Sensitizing Chemicals—A Systematic Review
Abstract
:1. Introduction
1.1. Chemical-Induced T Cell Epitopes
1.2. Review Objectives
2. Methods
2.1. Search Strategy
2.1.1. PubMed
2.1.2. Scopus
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Collection
2.4. Scoring System for Antigen-Specific T Cell Activation
3. Results
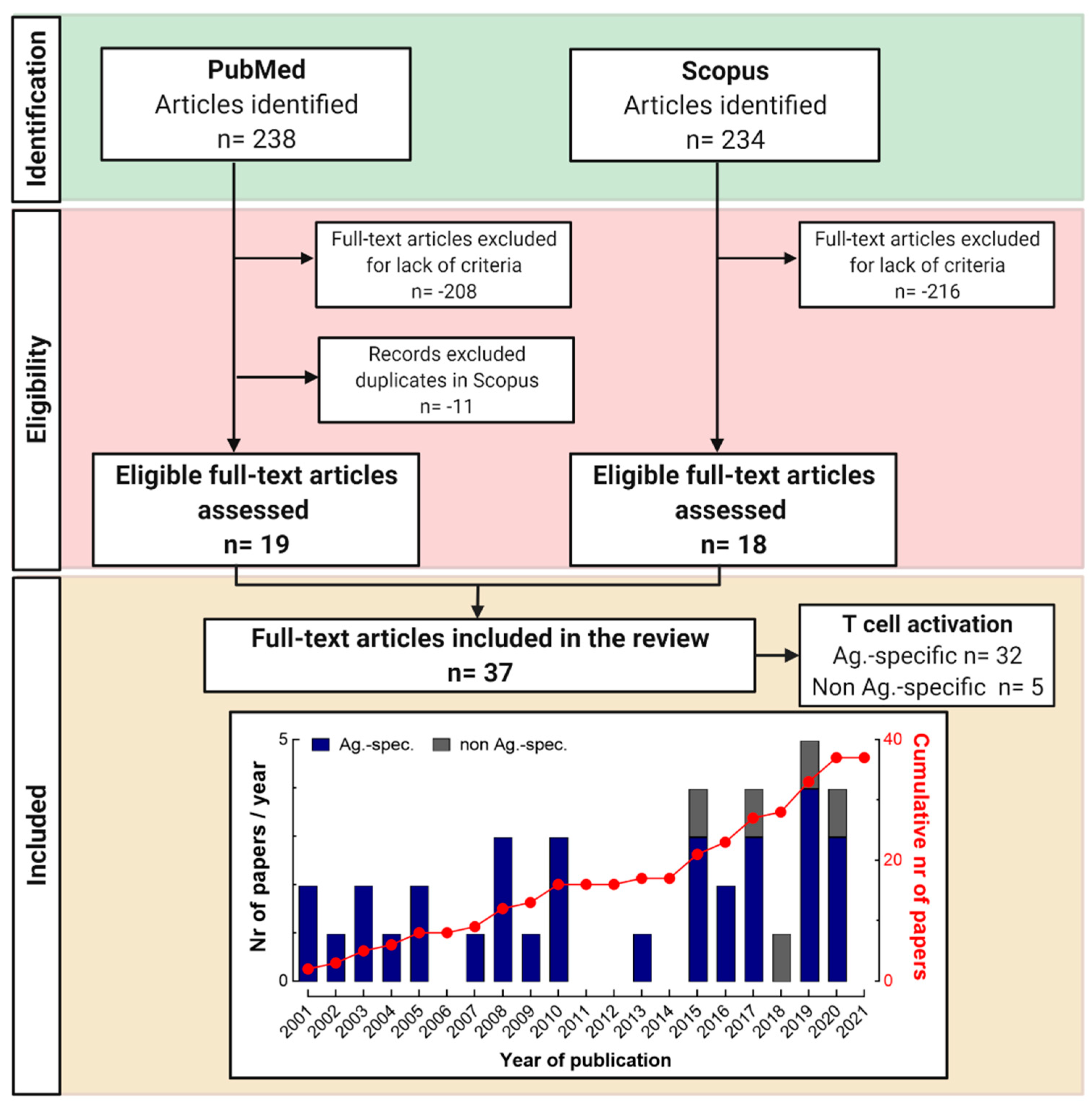
3.1. Selection of Articles Following PRISMA Guidelines
3.2. Monitoring Chemical-Specific T Cell Responses In Vitro
3.2.1. Investigated Chemical Allergens
3.2.2. Approaches for Chemical-Induced T Cell Epitope Formation
3.2.3. Blood as Major T Cell Source
3.2.4. Detection of Chemical-Specific T Cell Activation (Read-Outs)
3.2.5. Features of Chemical-Specific T Cell Responses in Patients
3.3. Monitoring Non-Antigen-Specific T Cell Activation
4. Discussion
4.1. APC Choice
4.2. T Cell Epitope Formation
4.3. T Cell Source
4.4. Read-Outs
4.5. Immune Monitoring of Allergic and Non-Allergic Individuals
4.6. Possible Uses of Assays Investigating Non-Antigen-Specific T Cell Activation
4.7. Limitations of Our Study
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Basketter, D.; Kimber, I. Contact Hypersensitivity. In Comprehensive Toxicology, 2nd ed.; McQueen, C., Ed.; Elsevier: Kidlington, UK, 2010; pp. 397–411. [Google Scholar]
- De Groot, A.C. Test Concentrations and Vehicles for 4900 Chemicals, 4th ed.; Acdegroot publishing: Wapserveen, The Netherlands, 2018. [Google Scholar]
- Kimber, I.; Basketter, D.A.; Dearman, R.J. Chemical allergens—what are the issues? Toxicology 2010, 268, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, A.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Derm. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Loman, L.; Uter, W.; Armario-Hita, J.C.; Ayala, F.; Balato, A.; Ballmer-Weber, B.K.; Bauer, A.; Bircher, A.J.; Buhl, T.; Czarnecka-Operacz, M.; et al. European Surveillance System on Contact Allergies (ESSCA): Characteristics of patients patch tested and diagnosed with irritant contact dermatitis. Contact Dermat. 2021, 85, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Bauer, A.; Belloni Fortina, A.; Bircher, A.J.; Brans, R.; Buhl, T.; Cooper, S.M.; Czarnecka-Operacz, M.; Dickel, H.; Dugonik, A.; et al. Patch test results with the European baseline series and additions thereof in the ESSCA network, 2015–2018. Contact Dermat. 2021, 84, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Werfel, T.; Lepoittevin, J.P.; White, I.R. Contact Allergy-Emerging Allergens and Public Health Impact. Int. J. Env. Res. Public Health 2020, 17, 2404. [Google Scholar] [CrossRef] [Green Version]
- Thierse, H.-J.; Luch, A. Consumer protection and risk assessment: Sensitising substances in consumer products. Allergo J. Int. 2019, 28, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimber, I.; Gerberick, G.F.; Van Loveren, H.; House, R.V. Chemical allergy: Molecular mechanisms and practical applications. Fundam Appl. Toxicol. 1992, 19, 479–483. [Google Scholar] [CrossRef]
- Kalboussi, H.; Kacem, I.; Aroui, H.; El Maalel, O.; Maoua, M.; Brahem, A.; El Guedri, S.; Chatti, S.; Ghariani, N.; Mrizak, N. Impact of Allergic Contact Dermatitis on the Quality of Life and Work Productivity. Derm. Res. Pract. 2019, 2019, 3797536. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.A.; Van Der Valk, P.G. Epicutaneous patch testing. Eur. J. Derm. 2002, 12, 506–513. [Google Scholar]
- McFadden, J.P.; Puangpet, P.; Pongpairoj, K.; Thaiwat, S.; Lee, S.X. Common Contact Allergens: A Practical Guide to Detecting Contact Dermatitis, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Christiansen, E.S.; Andersen, K.E.; Bindslev-Jensen, C.; Halken, S.; Kjaer, H.F.; Eller, E.; Host, A.; Mortz, C.G. Low patch test reactivity to nickel in unselected adolescents tested repeatedly with nickel in infancy. Pediatr. Allergy Immunol. 2016, 27, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.D.; Ahlstrom, M.G.; Johansen, J.D.; Dyring-Andersen, B.; Agerbeck, C.; Nielsen, M.M.; Poulsen, S.S.; Woetmann, A.; Odum, N.; Thomsen, A.R.; et al. Rapid allergen-induced interleukin-17 and interferon-gamma secretion by skin-resident memory CD8(+) T cells. Contact Dermat. 2017, 76, 218–227. [Google Scholar] [CrossRef]
- Tanno, L.K.; Darlenski, R.; Sanchez-Garcia, S.; Bonini, M.; Vereda, A.; Kolkhir, P.; Antolin-Amerigo, D.; Dimov, V.; Gallego-Corella, C.; Becerra, J.C.; et al. International survey on skin patch test procedures, attitudes and interpretation. World Allergy Organ. J. 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serup, J.; Hutton Carlsen, K. Patch test study of 90 patients with tattoo reactions: Negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermat. 2014, 71, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Kleverlaan, C.J.; Feilzer, A.J.; Rustemeyer, T. Sodium tetrachloropalladate (Na2[PdCl4]) as an improved test salt for palladium allergy patch testing. Contact Dermat. 2008, 58, 42–46. [Google Scholar] [CrossRef]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins; Part 1: Scientific Evidence. In OECD Series on Testing and Assessment; OECD: Paris, France, 2014. [Google Scholar] [CrossRef]
- Robins, H.S.; Campregher, P.V.; Srivastava, S.K.; Wacher, A.; Turtle, C.J.; Kahsai, O.; Riddell, S.R.; Warren, E.H.; Carlson, C.S. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 2009, 114, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Dudda, J.C.; Lembo, A.; Bachtanian, E.; Huehn, J.; Siewert, C.; Hamann, A.; Kremmer, E.; Forster, R.; Martin, S.F. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: Important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 2005, 35, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.R.; Martin, S.F. Pathomechanisms of Contact Sensitization. Curr. Allergy Asthma Rep. 2017, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Edele, F.; Molenaar, R.; Gutle, D.; Dudda, J.C.; Jakob, T.; Homey, B.; Mebius, R.; Hornef, M.; Martin, S.F. Cutting edge: Instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J. Immunol. 2008, 181, 3745–3749. [Google Scholar] [CrossRef] [Green Version]
- Hoper, T.; Mussotter, F.; Haase, A.; Luch, A.; Tralau, T. Application of proteomics in the elucidation of chemical-mediated allergic contact dermatitis. Toxicol. Res. (Camb.) 2017, 6, 595–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimber, I.; Dearman, R.J.; Basketter, D.A. Dendritic cells and the assessment in vitro of skin sensitizing potential. Cutan. Ocul. Toxicol. 2013, 32, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H.; Igyarto, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Hoper, T.; Siewert, K.; Dumit, V.I.; von Bergen, M.; Schubert, K.; Haase, A. The Contact Allergen NiSO4 Triggers a Distinct Molecular Response in Primary Human Dendritic Cells Compared to Bacterial LPS. Front. Immunol. 2021, 12, 644700. [Google Scholar] [CrossRef]
- Sasaki, Y.; Aiba, S. Dendritic cells and contact dermatitis. Clin. Rev. Allergy Immunol. 2007, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Toebak, M.J.; Gibbs, S.; Bruynzeel, D.P.; Scheper, R.J.; Rustemeyer, T. Dendritic cells: Biology of the skin. Contact Dermat. 2009, 60, 2–20. [Google Scholar] [CrossRef]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, E.M.; Shah, P.N.; Divito, S.J. Skin Resident Memory T Cells May Play Critical Role in Delayed-Type Drug Hypersensitivity Reactions. Front. Immunol. 2021, 12, 654190. [Google Scholar] [CrossRef]
- Murata, A.; Hayashi, S.I. CD4(+) Resident Memory T Cells Mediate Long-Term Local Skin Immune Memory of Contact Hypersensitivity in BALB/c Mice. Front. Immunol. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Gadsboll, A.O.; Jee, M.H.; Funch, A.B.; Alhede, M.; Mraz, V.; Weber, J.F.; Callender, L.A.; Carroll, E.C.; Bjarnsholt, T.; Woetmann, A.; et al. Pathogenic CD8(+) Epidermis-Resident Memory T Cells Displace Dendritic Epidermal T Cells in Allergic Dermatitis. J. Investig. Derm. 2020, 140, 806–815.e5. [Google Scholar] [CrossRef]
- Kish, D.D.; Li, X.; Fairchild, R.L. CD8 T cells producing IL-17 and IFN-gamma initiate the innate immune response required for responses to antigen skin challenge. J. Immunol. 2009, 182, 5949–5959. [Google Scholar] [CrossRef]
- Singleton, H.; Popple, A.; Gellatly, N.; Maxwell, G.; Williams, J.; Friedmann, P.S.; Kimber, I.; Dearman, R.J. Anti-hapten antibodies in response to skin sensitization. Contact Dermat. 2016, 74, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, S.; Thomson, P.; Meng, X.; Naisbitt, D. In-Vitro Approaches to Predict and Study T-Cell Mediated Hypersensitivity to Drugs. Front. Immunol. 2021, 12, 630530. [Google Scholar] [CrossRef] [PubMed]
- Gamerdinger, K.; Moulon, C.; Karp, D.R.; Van Bergen, J.; Koning, F.; Wild, D.; Pflugfelder, U.; Weltzien, H.U. A new type of metal recognition by human T cells: Contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J. Exp. Med. 2003, 197, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Soto, M.; Riedel, F.; Leddermann, M.; Bacher, P.; Scheffold, A.; Kuhl, H.; Timmermann, B.; Chudakov, D.M.; Molin, S.; Worm, M.; et al. TCRs with segment TRAV9-2 or a CDR3 histidine are overrepresented among nickel-specific CD4+ T cells. Allergy 2020, 75, 2574–2586. [Google Scholar] [CrossRef]
- Yin, L.; Crawford, F.; Marrack, P.; Kappler, J.W.; Dai, S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proc. Natl. Acad. Sci. USA 2012, 109, 18517–18522. [Google Scholar] [CrossRef] [Green Version]
- Thierse, H.J.; Moulon, C.; Allespach, Y.; Zimmermann, B.; Doetze, A.; Kuppig, S.; Wild, D.; Herberg, F.; Weltzien, H.U. Metal-protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation. J. Immunol. 2004, 172, 1926–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thierse, H.J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef]
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Env. Res. Public Health 2021, 18, 10867. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; von Bonin, A.; Fessler, C.; Pflugfelder, U.; Weltzien, H.U. Structural complexity of antigenic determinants for class I MHC-restricted, hapten-specific T cells. Two qualitatively differing types of H-2Kb-restricted TNP epitopes. J. Immunol. 1993, 151, 678–687. [Google Scholar]
- Puig, M.; Ananthula, S.; Venna, R.; Kumar Polumuri, S.; Mattson, E.; Walker, L.M.; Cardone, M.; Takahashi, M.; Su, S.; Boyd, L.F.; et al. Alterations in the HLA-B*57:01 Immunopeptidome by Flucloxacillin and Immunogenicity of Drug-Haptenated Peptides. Front. Immunol. 2021, 11, 629399. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Immune pathomechanism and classification of drug hypersensitivity. Allergy 2019, 74, 1457–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichler, W.J.; Beeler, A.; Keller, M.; Lerch, M.; Posadas, S.; Schmid, D.; Spanou, Z.; Zawodniak, A.; Gerber, B. Pharmacological interaction of drugs with immune receptors: The p-i concept. Allergol. Int. 2006, 55, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef] [Green Version]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef]
- Watkins, S.; Pichler, W.J. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVbeta20-1, altering pHLA recognition. PLoS ONE 2013, 8, e76211. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, S.; Wegrecki, M.; Cheng, T.Y.; Bourgeois, E.A.; Cotton, R.N.; Mayfield, J.A.; Monnot, G.C.; Le Nours, J.; Van Rhijn, I.; Rossjohn, J.; et al. Human T cell response to CD1a and contact dermatitis allergens in botanical extracts and commercial skin care products. Sci. Immunol. 2020, 5, eaax5430. [Google Scholar] [CrossRef]
- Rudolph, M.G.; Stanfield, R.L.; Wilson, I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006, 24, 419–466. [Google Scholar] [CrossRef]
- Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 1998, 19, 395–404. [Google Scholar] [CrossRef]
- Lee, C.H.; Salio, M.; Napolitani, G.; Ogg, G.; Simmons, A.; Koohy, H. Predicting Cross-Reactivity and Antigen Specificity of T Cell Receptors. Front. Immunol. 2020, 11, 565096. [Google Scholar] [CrossRef]
- Ortmann, B.; Martin, S.; von Bonin, A.; Schiltz, E.; Hoschutzky, H.; Weltzien, H.U. Synthetic peptides anchor T cell-specific TNP epitopes to MHC antigens. J. Immunol. 1992, 148, 1445–1450. [Google Scholar]
- Dietz, L.; Esser, P.R.; Schmucker, S.S.; Goette, I.; Richter, A.; Schnolzer, M.; Martin, S.F.; Thierse, H.J. Tracking human contact allergens: From mass spectrometric identification of peptide-bound reactive small chemicals to chemical-specific naive human T-cell priming. Toxicol. Sci. 2010, 117, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Shearer, G.M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur. J. Immunol. 1974, 4, 527–533. [Google Scholar] [CrossRef]
- Richter, A.; Schmucker, S.S.; Esser, P.R.; Traska, V.; Weber, V.; Dietz, L.; Thierse, H.J.; Pennino, D.; Cavani, A.; Martin, S.F. Human T cell priming assay (hTCPA) for the identification of contact allergens based on naive T cells and DC--IFN-gamma and TNF-alpha readout. Toxicol. Vitr. 2013, 27, 1180–1185. [Google Scholar] [CrossRef]
- Meng, X.; Yerly, D.; Naisbitt, D.J. Mechanisms leading to T-cell activation in drug hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, U.; Eichmann, K.; Krammer, P.H. Frequencies and regulation of trinitrophenyl-specific cytotoxic T precursor cells: Immunization results in release from suppression. J. Immunol. 1983, 130, 7–14. [Google Scholar]
- Iglesias, A.; Hansen-Hagge, T.; Von Bonin, A.; Weltzien, H.U. Increased frequency of 2,4,6-trinitrophenyl (TNP)-specific, H-2b-restricted cytotoxic T lymphocyte precursors in transgenic mice expressing a T cell receptor beta chain gene from an H-2b-restricted, TNP-specific cytolytic T cell clone. Eur. J. Immunol. 1992, 22, 335–341. [Google Scholar] [CrossRef]
- Martin, S.; Delattre, V.; Leicht, C.; Weltzien, H.U.; Simon, J.C. A high frequency of allergen-specific CD8+ Tc1 cells is associated with the murine immune response to the contact sensitizer trinitrophenyl. Exp. Derm. 2003, 12, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kempkes, B.; Palmer, E.; Martin, S.; von Bonin, A.; Eichmann, K.; Ortmann, B.; Weltzien, H.U. Predominant T cell receptor gene elements in TNP-specific cytotoxic T cells. J. Immunol. 1991, 147, 2467–2473. [Google Scholar] [PubMed]
- Levine, B.B.; Ovary, Z. Studies on the mechanism of the formation of the penicillin antigen. III. The N-(D-alpha-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J. Exp. Med. 1961, 114, 875–904. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, F.S.; Saide, K.; Kim, S.H.; Monshi, M.; Tailor, A.; Wood, S.; Meng, X.; Jenkins, R.; Faulkner, L.; Daly, A.K.; et al. Promiscuous T-cell responses to drugs and drug-haptens. J. Allergy Clin. Immunol. 2015, 136, 474–476.e8. [Google Scholar] [CrossRef] [Green Version]
- Monshi, M.M.; Faulkner, L.; Gibson, A.; Jenkins, R.E.; Farrell, J.; Earnshaw, C.J.; Alfirevic, A.; Cederbrant, K.; Daly, A.K.; French, N.; et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology 2013, 57, 727–739. [Google Scholar] [CrossRef]
- Wuillemin, N.; Adam, J.; Fontana, S.; Krahenbuhl, S.; Pichler, W.J.; Yerly, D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. J. Immunol. 2013, 190, 4956–4964. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Abe, R.; Pan, R.Y.; Wang, C.W.; Hung, S.I.; Tsai, Y.G.; Chung, W.H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Hertzman, R.J.; Palubinsky, A.M.; Giles, J.B.; Karnes, J.H.; Gibson, A.; Phillips, E.J. Immunopharmacogenomics: Mechanisms of HLA-Associated Drug Reactions. Clin. Pharm. 2021, 110, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deshpande, P.; Hertzman, R.J.; Palubinsky, A.M.; Gibson, A.; Phillips, E.J. Genomic Risk Factors Driving Immune-Mediated Delayed Drug Hypersensitivity Reactions. Front. Genet. 2021, 12, 641905. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Yip, V.; Mayorga, C.; Blanca, M.; Barbaud, A.; Nakonechna, A.; Cernadas, J.; Gotua, M.; Brockow, K.; Caubet, J.C.; et al. Genetic variants associated with T cell-mediated cutaneous adverse drug reactions: A PRISMA-compliant systematic review-An EAACI position paper. Allergy 2020, 75, 1069–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illing, P.T.; Vivian, J.P.; Purcell, A.W.; Rossjohn, J.; McCluskey, J. Human leukocyte antigen-associated drug hypersensitivity. Curr. Opin. Immunol. 2013, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Illing, P.T.; Purcell, A.W.; McCluskey, J. The role of HLA genes in pharmacogenomics: Unravelling HLA associated adverse drug reactions. Immunogenetics 2017, 69, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Hertzman, R.J.; Deshpande, P.; Gibson, A.; Phillips, E.J. Role of pharmacogenomics in T-cell hypersensitivity drug reactions. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 327–334. [Google Scholar] [CrossRef]
- Chessman, D.; Kostenko, L.; Lethborg, T.; Purcell, A.W.; Williamson, N.A.; Chen, Z.; Kjer-Nielsen, L.; Mifsud, N.A.; Tait, B.D.; Holdsworth, R.; et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 2008, 28, 822–832. [Google Scholar] [CrossRef] [Green Version]
- de Jong, A.; Pena-Cruz, V.; Cheng, T.Y.; Clark, R.A.; Van Rhijn, I.; Moody, D.B. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat. Immunol. 2010, 11, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, A.; Ogg, G. CD1a function in human skin disease. Mol. Immunol. 2021, 130, 14–19. [Google Scholar] [CrossRef]
- Betts, R.J.; Perkovic, A.; Mahapatra, S.; Del Bufalo, A.; Camara, K.; Howell, A.R.; Martinozzi Teissier, S.; De Libero, G.; Mori, L. Contact sensitizers trigger human CD1-autoreactive T-cell responses. Eur. J. Immunol. 2017, 47, 1171–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C.; Jenkins, R.E.; Aleksic, M.; Pirmohamed, M.; Naisbitt, D.J.; Park, B.K. Characterization of p-phenylenediamine-albumin binding sites and T-cell responses to hapten-modified protein. J. Investig. Derm. 2010, 130, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieben, S.; Kawakubo, Y.; Al Masaoudi, T.; Merk, H.F.; Blomeke, B. Delayed-type hypersensitivity reaction to paraphenylenediamine is mediated by 2 different pathways of antigen recognition by specific alphabeta human T-cell clones. J. Allergy Clin. Immunol. 2002, 109, 1005–1011. [Google Scholar] [CrossRef]
- Chipinda, I.; Hettick, J.M.; Siegel, P.D. Haptenation: Chemical reactivity and protein binding. J. Allergy (Cairo) 2011, 2011, 839682. [Google Scholar] [CrossRef] [Green Version]
- Johansen, J.D.; Malher, V.; Lepoittevin, J.P.; Frosch, P.J. Contact Dermatitis, 6th ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sykulev, Y.; Cohen, R.J.; Eisen, H.N. The law of mass action governs antigen-stimulated cytolytic activity of CD8+ cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 11990–11992. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Brameshuber, M.; Zeng, X.; Xie, J.; Li, Q.J.; Chien, Y.H.; Valitutti, S.; Davis, M.M. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 2013, 39, 846–857. [Google Scholar] [CrossRef] [Green Version]
- Ndreu, L.; Erber, L.N.; Tornqvist, M.; Tretyakova, N.Y.; Karlsson, I. Characterizing Adduct Formation of Electrophilic Skin Allergens with Human Serum Albumin and Hemoglobin. Chem. Res. Toxicol. 2020, 33, 2623–2636. [Google Scholar] [CrossRef]
- Parkinson, E.; Aleksic, M.; Arthur, R.; Regufe Da Mota, S.; Cubberley, R.; Skipp, P.J. Proteomic analysis of haptenation by skin sensitisers: Diphencyprone and ethyl acrylate. Toxicol. Vitr. 2020, 62, 104697. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Frombach, J.; Sonnenburg, A.; Krapohl, B.D.; Zuberbier, T.; Peiser, M.; Stahlmann, R.; Schreiner, M. Lymphocyte surface markers and cytokines are suitable for detection and potency assessment of skin-sensitizing chemicals in an in vitro model of allergic contact dermatitis: The LCSA-ly. Arch. Toxicol. 2018, 92, 1495–1505. [Google Scholar] [CrossRef]
- Hou, F.; Xing, C.; Li, B.; Cheng, J.; Chen, W. Performance of a novel in vitro assay for skin sensitization based on activation of T lymphocytes. ALTEX 2020, 37, 451–468. [Google Scholar] [CrossRef]
- Balo-Banga, J.M.; Schweitzer, K.; Lakatos, S.; Sipka, S. A novel rapid (20-min) IL-6 release assay using blood mononuclear cells of patients with various clinical forms of drug induced skin injuries. World Allergy Organ. J. 2015, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Mai, W.; Liu, X.; Su, G.; Zhou, W.; Wen, Z.; Lu, D. Elevation of Circulating Th17/Th22 Cells Exposed to Low-Level Formaldehyde and Its Relevance to Formaldehyde-Induced Occupational Allergic Contact Dermatitis. J. Occup. Env. Med. 2017, 59, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Clouet, E.; Bechara, R.; Raffalli, C.; Damiens, M.H.; Groux, H.; Pallardy, M.; Ferret, P.J.; Kerdine-Romer, S. The THP-1 cell toolbox: A new concept integrating the key events of skin sensitization. Arch. Toxicol. 2019, 93, 941–951. [Google Scholar] [CrossRef]
- Coulter, E.M.; Jenkinson, C.; Farrell, J.; Lavergne, S.N.; Pease, C.; White, A.; Aleksic, M.; Basketter, D.; Williams, D.P.; King, C.; et al. Measurement of CD4+ and CD8+ T-lymphocyte cytokine secretion and gene expression changes in p-phenylenediamine allergic patients and tolerant individuals. J. Investig. Derm. 2010, 130, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.; Kim, S.H.; Faulkner, L.; Evely, J.; Pirmohamed, M.; Park, K.B.; Naisbitt, D.J. In Vitro Priming of Naive T-cells with p-Phenylenediamine and Bandrowski’s Base. Chem. Res. Toxicol. 2015, 28, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Moed, H.; von Blomberg, M.; Bruynzeel, D.P.; Scheper, R.; Gibbs, S.; Rustemeyer, T. Improved detection of allergen-specific T-cell responses in allergic contact dermatitis through the addition of ‘cytokine cocktails’. Exp. Derm. 2005, 14, 634–640. [Google Scholar] [CrossRef]
- Bordignon, V.; Palamara, F.; Altomonte, G.; Sperduti, I.; Pietravalle, M.; Cavallotti, C.; Cordiali-Fei, P.; Fuggetta, M.P.; Cristaudo, A.; Ensoli, F. A laboratory test based on determination of cytokine profiles: A promising assay to identify exposition to contact allergens and predict the clinical outcome in occupational allergic contact dermatitis. BMC Immunol. 2015, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Coulter, E.M.; Farrell, J.; Mathews, K.L.; Maggs, J.L.; Pease, C.K.; Lockley, D.J.; Basketter, D.A.; Park, B.K.; Naisbitt, D.J. Activation of human dendritic cells by p-phenylenediamine. J. Pharm. Exp. 2007, 320, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C.; Jenkins, R.E.; Maggs, J.L.; Kitteringham, N.R.; Aleksic, M.; Park, B.K.; Naisbitt, D.J. A mechanistic investigation into the irreversible protein binding and antigenicity of p-phenylenediamine. Chem. Res. Toxicol. 2009, 22, 1172–1180. [Google Scholar] [CrossRef]
- Kneilling, M.; Caroli, U.; Grimmel, C.; Fischer, J.; Eichner, M.; Wieder, T.; Maier, F.C.; Rocken, M.; Biedermann, T. Para-phenylenediamine-specific lymphocyte activation test: A sensitive in vitro assay to detect para-phenylenediamine sensitization in patients with severe allergic reactions. Exp. Derm. 2010, 19, 435–441. [Google Scholar] [CrossRef]
- Oakes, T.; Popple, A.L.; Williams, J.; Best, K.; Heather, J.M.; Ismail, M.; Maxwell, G.; Gellatly, N.; Dearman, R.J.; Kimber, I.; et al. The T Cell Response to the Contact Sensitizer Paraphenylenediamine Is Characterized by a Polyclonal Diverse Repertoire of Antigen-Specific Receptors. Front. Immunol. 2017, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Skazik, C.; Grannemann, S.; Wilbers, L.; Merk, H.F.; Coenraads, P.J.; Breuer, S.; Blomeke, B. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermat. 2008, 59, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Wicks, K.; Stretton, C.; Popple, A.; Beresford, L.; Williams, J.; Maxwell, G.; Gosling, J.P.; Kimber, I.; Dearman, R.J. T lymphocyte phenotype of contact-allergic patients: Experience with nickel and p-phenylenediamine. Contact Dermat. 2019, 81, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Newell, L.; Polak, M.E.; Perera, J.; Owen, C.; Boyd, P.; Pickard, C.; Howarth, P.H.; Healy, E.; Holloway, J.W.; Friedmann, P.S.; et al. Sensitization via healthy skin programs Th2 responses in individuals with atopic dermatitis. J. Investig. Derm. 2013, 133, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutte, R.J.; Zhang, X.; An, N.; Ostrov, D.A.; Vukmanovic, S. Molecular docking predictions of fragrance binding to human leukocyte antigen molecules. Contact Dermat. 2019, 81, 174–183. [Google Scholar] [CrossRef]
- Sieben, S.; Hertl, M.; Al Masaoudi, T.; Merk, H.F.; Blomeke, B. Characterization of T cell responses to fragrances. Toxicol. Appl. Pharm. 2001, 172, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cortial, A.; Nosbaum, A.; Rozieres, A.; Baeck, M.; de Montjoye, L.; Grande, S.; Briancon, S.; Nicolas, J.F.; Vocanson, M. Encapsulation of hydrophobic allergens into nanoparticles improves the in vitro immunological diagnosis of allergic contact dermatitis. Nanomedicine 2015, 11, 1029–1033. [Google Scholar] [CrossRef]
- Masjedi, K.; Ahlborg, N.; Gruvberger, B.; Bruze, M.; Karlberg, A.T. Methylisothiazolinones elicit increased production of both T helper (Th)1- and Th2-like cytokines by peripheral blood mononuclear cells from contact allergic individuals. Br. J. Derm. 2003, 149, 1172–1182. [Google Scholar] [CrossRef]
- Kim, D.; Kobayashi, T.; Voisin, B.; Jo, J.H.; Sakamoto, K.; Jin, S.P.; Kelly, M.; Pasieka, H.B.; Naff, J.L.; Meyerle, J.H.; et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: A case report. Nat. Med. 2020, 26, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Iyer, V.J.; Cherian, K.M. A rapid in vitro method of identifying contact allergens and irritants. Toxicol. Mech. Methods 2003, 13, 103–109. [Google Scholar] [CrossRef]
- Gildea, L.A.; Ryan, C.A.; Hulette, B.C.; Dearman, R.J.; Kimber, D.; Gerberick, G.F. Transcript profiling of T lymphocytes and dendritic cells in a co-culture system using anti-CD3 and allergen activation. J. Toxicol. Cutan. Ocul. 2004, 23, 277–292. [Google Scholar] [CrossRef]
- Sachs, B.; Erdmann, S.; al Masaoudi, T.; Merk, H.F. Molecular features determining lymphocyte reactivity in allergic contact dermatitis to chloramphenicol and azdiamphenicol. Allergy 2001, 56, 69–72. [Google Scholar] [CrossRef]
- Vilchez-Sanchez, F.; Dominguez-Ortega, J.; Munoz, M.G.; Loli-Ausejo, D.; Heredia-Revuelto, R.; Roman, A.F.; Quirce, S. Two case reports of delayed-allergic reactions to clindamycin confirmed with a positive lymphocyte transformation test. Eur. Ann. Allergy Clin. 2020, 52, 91–93. [Google Scholar] [CrossRef]
- Girardi, M.; Duncan, K.O.; Tigelaar, R.E.; Imaeda, S.; Watsky, K.L.; McNiff, J.M. Cross-comparison of patch test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am. J. Derm. 2005, 27, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.S.; Haddadeen, C.; Lai, C.; Healy, E. In vitro human T cell responses to diphencyprone. Contact Dermat. 2017, 76, 251–253. [Google Scholar] [CrossRef]
- Hansel, K.; Murgia, N.; Russano, A.; Crescenzi, F.; Tramontana, M.; Bianchi, L.; Neve, D.; Muzi, G.; Stingeni, L. Airborne allergic contact dermatitis caused by Machaerium scleroxylon: Confirmation by in vivo and in vitro tests. Contact Dermat. 2019, 81, 296–298. [Google Scholar] [CrossRef]
- Popple, A.; Williams, J.; Maxwell, G.; Gellatly, N.; Dearman, R.J.; Kimber, I. T lymphocyte dynamics in methylisothiazolinone-allergic patients. Contact Dermat. 2016, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wahlkvist, H.; Masjedi, K.; Gruvberger, B.; Zuber, B.; Karlberg, A.T.; Bruze, M.; Ahlborg, N. The lipophilic hapten parthenolide induces interferon-gamma and interleukin-13 production by peripheral blood-derived CD8+ T cells from contact allergic subjects in vitro. Br. J. Derm. 2008, 158, 70–77. [Google Scholar] [CrossRef]
- Camouse, M.M.; Swick, A.R.; Ryan, C.A.; Hulette, B.; Gerberick, F.; Tinkle, S.S.; Nedorost, S.T.; Cooper, K.D.; Stevens, S.R.; Baron, E.D. Determination of in vivo dose response and allergen-specific T cells in subjects contact-sensitized to squaric acid dibutyl ester. Dermatitis 2008, 19, 95–99. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yang, X.; Chen, Y.; Pang, Y.; Qi, G.; Chen, L.; Zhuang, Z. Effect of trichloroacetaldehyde on the activation of CD4(+)T cells in occupational medicamentosa-like dermatitis: An in vivo and in vitro study. Toxicology 2019, 423, 95–104. [Google Scholar] [CrossRef]
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.W.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166. [Google Scholar] [CrossRef]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicol. Vitr. 2006, 20, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Lindstedt, M.; Albrekt, A.S.; Borrebaeck, C.A. A genomic biomarker signature can predict skin sensitizers using a cell-based in vitro alternative to animal tests. BMC Genom. 2011, 12, 399. [Google Scholar] [CrossRef] [Green Version]
- Kapsenberg, M.L.; Res, P.; Bos, J.D.; Schootemijer, A.; Teunissen, M.B.; Van Schooten, W. Nickel-specific T lymphocyte clones derived from allergic nickel-contact dermatitis lesions in man: Heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur. J. Immunol. 1987, 17, 861–865. [Google Scholar] [CrossRef]
- Moulon, C.; Choleva, Y.; Thierse, H.J.; Wild, D.; Weltzien, H.U. T cell receptor transfection shows non-HLA-restricted recognition of nickel by CD8+ human T cells to be mediated by alphabeta T cell receptors. J. Investig. Derm. 2003, 121, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Bacher, P.; Schink, C.; Teutschbein, J.; Kniemeyer, O.; Assenmacher, M.; Brakhage, A.A.; Scheffold, A. Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. J. Immunol. 2013, 190, 3967–3976. [Google Scholar] [CrossRef]
- Su, L.F.; Kidd, B.A.; Han, A.; Kotzin, J.J.; Davis, M.M. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity 2013, 38, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Vocanson, M.; Cluzel-Tailhardat, M.; Poyet, G.; Valeyrie, M.; Chavagnac, C.; Levarlet, B.; Courtellemont, P.; Rozieres, A.; Hennino, A.; Nicolas, J.F. Depletion of human peripheral blood lymphocytes in CD25+ cells allows for the sensitive in vitro screening of contact allergens. J. Investig. Derm. 2008, 128, 2119–2122. [Google Scholar] [CrossRef] [Green Version]
- Yerly, D.; Pompeu, Y.A.; Schutte, R.J.; Eriksson, K.K.; Strhyn, A.; Bracey, A.W.; Buus, S.; Ostrov, D.A. Structural Elements Recognized by Abacavir-Induced T Cells. Int. J. Mol. Sci. 2017, 18, 1464. [Google Scholar] [CrossRef] [Green Version]
- Kalish, R.S.; Johnson, K.L. Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J. Immunol. 1990, 145, 3706–3713. [Google Scholar]
- Villani, A.P.; Rozieres, A.; Bensaid, B.; Eriksson, K.K.; Mosnier, A.; Albert, F.; Mutez, V.; Brassard, O.; Baysal, T.; Tardieu, M.; et al. Massive clonal expansion of polycytotoxic skin and blood CD8(+) T cells in patients with toxic epidermal necrolysis. Sci. Adv. 2021, 7, eabe0013. [Google Scholar] [CrossRef]
- Steinert, E.M.; Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Manlove, L.S.; Igyarto, B.Z.; Southern, P.J.; Masopust, D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 2015, 161, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Chong, B.; Mirchandani, N.; Brinster, N.K.; Yamanaka, K.; Dowgiert, R.K.; Kupper, T.S. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006, 176, 4431–4439. [Google Scholar] [CrossRef] [Green Version]
- Geiger, R.; Duhen, T.; Lanzavecchia, A.; Sallusto, F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J. Exp. Med. 2009, 206, 1525–1534. [Google Scholar] [CrossRef] [Green Version]
- de Graaf, N.P.J.; Bontkes, H.J.; Roffel, S.; Kleverlaan, C.J.; Rustemeyer, T.; Gibbs, S.; Feilzer, A.J. Non-heat inactivated autologous serum increases accuracy of in vitro CFSE lymphocyte proliferation test (LPT) for nickel. Clin. Exp. Allergy 2020, 50, 722–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Baehr, V.; Mayer, W.; Liebenthal, C.; von Baehr, R.; Bieger, W.; Volk, H.D. Improving the in vitro antigen specific T cell proliferation assay: The use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J. Immunol. Methods 2001, 251, 63–71. [Google Scholar] [CrossRef]
- Gibson, A.; Faulkner, L.; Lichtenfels, M.; Ogese, M.; Al-Attar, Z.; Alfirevic, A.; Esser, P.R.; Martin, S.F.; Pirmohamed, M.; Park, B.K.; et al. The Effect of Inhibitory Signals on the Priming of Drug Hapten-Specific T Cells That Express Distinct Vbeta Receptors. J. Immunol. 2017, 199, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Mei, D.; Guerra, E.; Corinti, S.; Giani, M.; Pirrotta, L.; Puddu, P.; Girolomoni, G. Patients with allergic contact dermatitis to nickel and nonallergic individuals display different nickel-specific T cell responses. Evidence for the presence of effector CD8+ and regulatory CD4+ T cells. J. Investig. Derm. 1998, 111, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, L.; Flossdorf, M.; Mir, J.; Cho, Y.L.; Plambeck, M.; Treise, I.; Toska, A.; Heinzel, S.; Schiemann, M.; Busch, D.H.; et al. Differential expansion of T central memory precursor and effector subsets is regulated by division speed. Nat. Commun. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Kim, T.S.; Braciale, T.J. The cell cycle time of CD8+ T cells responding in vivo is controlled by the type of antigenic stimulus. PLoS ONE 2010, 5, e15423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, N.; Shemer, A.; Correa da Rosa, J.; Rozenblit, M.; Fuentes-Duculan, J.; Gittler, J.K.; Finney, R.; Czarnowicki, T.; Zheng, X.; Xu, H.; et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J. Allergy Clin. Immunol. 2014, 134, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Elias, G.; Ogunjimi, B.; Van Tendeloo, V. Activation-induced surface proteins in the identification of antigen-responsive CD4 T cells. Immunol. Lett. 2020, 219, 1–7. [Google Scholar] [CrossRef]
- Wolfl, M.; Kuball, J.; Ho, W.Y.; Nguyen, H.; Manley, T.J.; Bleakley, M.; Greenberg, P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007, 110, 201–210. [Google Scholar] [CrossRef]
- Ronel, T.; Harries, M.; Wicks, K.; Oakes, T.; Singleton, H.; Dearman, R.; Maxwell, G.; Chain, B. The clonal structure and dynamics of the human T cell response to an organic chemical hapten. Elife 2021, 10, 1–23. [Google Scholar] [CrossRef]
- Ko, T.M.; Chung, W.H.; Wei, C.Y.; Shih, H.Y.; Chen, J.K.; Lin, C.H.; Chen, Y.T.; Hung, S.I. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2011, 128, 1266–1276.e11. [Google Scholar] [CrossRef]
- Emerson, R.O.; DeWitt, W.S.; Vignali, M.; Gravley, J.; Hu, J.K.; Osborne, E.J.; Desmarais, C.; Klinger, M.; Carlson, C.S.; Hansen, J.A.; et al. Immunosequencing identifies signatures of cytomegalovirus exposure history and HLA-mediated effects on the T cell repertoire. Nat. Genet. 2017, 49, 659–665. [Google Scholar] [CrossRef]
- Snyder, T.M.; Gittelman, R.M.; Klinger, M.; May, D.H.; Osborne, E.J.; Taniguchi, R.; Zahid, H.J.; Kaplan, I.M.; Dines, J.N.; Noakes, M.T.; et al. Magnitude and Dynamics of the T-Cell Response to SARS-CoV-2 Infection at Both Individual and Population Levels. MedRxiv 2020. [Google Scholar] [CrossRef]
- Pan, R.Y.; Chu, M.T.; Wang, C.W.; Lee, Y.S.; Lemonnier, F.; Michels, A.W.; Schutte, R.; Ostrov, D.A.; Chen, C.B.; Phillips, E.J.; et al. Identification of drug-specific public TCR driving severe cutaneous adverse reactions. Nat. Commun. 2019, 10, 3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.F.; Esser, P.R.; Schmucker, S.; Dietz, L.; Naisbitt, D.J.; Park, B.K.; Vocanson, M.; Nicolas, J.F.; Keller, M.; Pichler, W.J.; et al. T-cell recognition of chemicals, protein allergens and drugs: Towards the development of in vitro assays. Cell. Mol. Life Sci. 2010, 67, 4171–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation. In OECD Guidelines for the Testing of Chemicals, Section; OECD: Paris, France, 2021. [Google Scholar] [CrossRef]

| N° | Chemical | Main Use | Score * | References |
|---|---|---|---|---|
| 1 | Bandrowski’s Base (BB) | ** | +++ | Coulter, 2010 [93]; Gibson, 2015 [94]; Moed, 2005 [95]; Sieben, 2002 [80] |
| 2 | p-Phenylenediamine (PPD) | hair dye and dye | +++ | Bordignon, 2015 [96]; Coulter, 2007 [97]; Coulter, 2010 [93]; Gibson, 2015 [94]; Jenkinson, 2009 [98]; Jenkinson, 2010 [79]; Kneilling, 2009 [99]; Moed, 2005 [95]; Oakes, 2017 [100]; Sieben 2002 [80]; Skazik, 2008 [101]; Wicks, 2019 [102] |
| 3 | 2,4-Dinitrochlorobenzene (DNCB) | model chemical | ++ | Betts, 2017 [78]; Newell, 2013 [103] |
| 4 | Balsam of Peru | fragrance | ++ | Nicolai, 2020 [51] |
| 5 | Benzyl benzoate | fragrance | ++ | Nicolai, 2020 [51] |
| 6 | Benzyl cinnamate | fragrance | ++ | Nicolai, 2020 [51]; Schutte, 2019 [104] |
| 7 | Coenzyme Q2 | fragrance | ++ | Nicolai, 2020 [51] |
| 8 | Eugenol | fragrance | ++ | Sieben, 2001 [105] |
| 9 | Farnesol | fragrance | ++ | Nicolai, 2020 [51] |
| 10 | Fragrance mix | fragrance | ++ | Cortial, 2015 [106]; Moed, 2005 [95] |
| 11 | Methylchloroisothiazolinone (MCI) | preservative | ++ | Moed, 2005 [95] |
| Methylchloroisothiazolinone/Methylisothiazolinone (MCI/MI) | preservative | ++ | Masjedi, 2003 [107] | |
| 12 | Sulfamethoxazole/Trimethoprim (SMX/TMP) | drugs | ++ | Kim, 2020 [108] |
| 13 | 1-Fluoro-2,4-dinitrobenzene (DNFB) | model chemical | + | Banerjee, 2003 [109] |
| 14 | 2,4-Dinitrobenzenesulfoniacid (DNBS) | model chemical | + | Gildea, 2004 [110] |
| 15 | Azidamphenicol | drug | + | Sachs, 2001 [111] |
| 16 | Benzyl salicylate | fragrance | + | Schutte, 2019 [104] |
| 17 | Chloramphenicol | drug | + | Sachs, 2001 [111] |
| 18 | Clindamycin | drug | + | Vilchez-Sánchez, 2020 [112] |
| 19 | Diltiazem | drug | + | Girardi, 2005 [113] |
| 20 | Diphenylcyclopropenone (DPCP) | drug | + | Friedmann, 2017 [114] |
| 21 | Geraniol | fragrance | + | Sieben, 2001 [105] |
| 22 | Hydroxycitronellal | fragrance | + | Sieben, 2001 [105] |
| 24 | Isoeugenol | fragrance | + | Banerjee, 2003 [109]; Sieben, 2001 [105] |
| 23 | Machaerium scleroxylon | plant | + | Hansel, 2019 [115] |
| 24 | Methylisothiazolinone (MI) | preservative | + | Popple, 2016 [116] |
| 25 | Metronidazole | drug | + | Girardi, 2005 [113] |
| 26 | Oak moss | fragrance | + | Sieben, 2001 [105] |
| 27 | Parthenolide | *** | + | Wahlkvist, 2008 [117] |
| 28 | Squaric acid dibutylester (SADBE) | drug $ | + | Camouse, 2008 [118] |
| 29 | Trichloroethylene (TCE) | pollutant | + | Li, 2019 [119] |
| 31 | Urushiol | *** | + | Kim, 2016 [120] |
| APC | Epitope Formation | Chemicals | References |
|---|---|---|---|
| PBMC | Direct administration in culture | Azidamphenicol, BB, Benzyl cinnamate, Benzyl salicylate, Chloramphenicol, Clindamycin, Diltiazem, DNCB, DNFB, Eugenol, Fragrance mix, Geraniol, Hydroxycitronellal, Isoeugenol, Metronidazole, Machaerium scleroxylon, MCI/MI, MI, Oak mos, Parthenolide, PPD, SMX/TMP, TCE | Banerjee, 2003 [109]; Bordignon, 2015 [96]; Cortial, 2015 [106]; Coulter, 2010 [93]; Friedmann, 2017 [114]; Girardi, 2005 [113]; Hansel, 2019 [115]; Jenkinson, 2009 [98]; Kim, 2020 [108]; Knelling, 2010 [99]; Li, 2019 [119]; Masjedi, 2003 [107]; Moed, 2005 [95]; Newell, 2013 [103]; Popple, 2016 [116]; Sachs, 2001 [111]; Schutte, 2019 [104]; Sieben, 2001 [105]; Sieben, 2002 [80]; Skazik, 2008 [101]; Vilchez-Sánchez, 2020 [112]; Wahlkvist, 2008 [117]; Wicks, 2019 [102] |
| Modification (e.g., pulsed APC) | BB, PPD | Sieben, 2002 [80]; Wicks, 2019 [102] | |
| Protein conjugation (e.g., to HSA) | MI, PPD | Oakes, 2017 [100]; Popple, 2016 [116]; Wicks, 2019 [102] | |
| Dendritic cells | Direct administration in culture | BB, PPD | Coulter, 2010 [93]; Gibson, 2015 [94] |
| Modification (e.g., pulsed APC) | BB, DNBS, Fragrance mix, MCI, PPD, SADBE | Camouse, 2008 [118]; Coulter, 2007 [97]; Gildea, 2004 [110]; Moed, 2005 [95] | |
| EBV-transformed B cells | Direct administration in culture | Eugenol, Geraniol, Hydroxycitronellal, Isoeugenol, Oak moss, PPD | Jenkinson, 2010 [79]; Gibson, 2015 [94]; Sieben, 2001 [105] |
| Protein conjugation (e.g., to HSA) | PPD | Jenkinson, 2010 [79] | |
| Cell lines (CD1a-expressing) | Direct administration in culture | Balsam of Peru, Benzyl benzoate, Benzyl cinnamate, Coenzyme Q2, Farnesol | Nicolai, 2020 [51] |
| Modification (e.g., pulsed APC) | DNCB, Urushiol | Betts, 2017 [78]; Kim, 2016 [120]; Nicolai, 2020 [51] |
| Read-outs | Method/Assay | Chemicals | References |
|---|---|---|---|
| Proliferation | Thymidine | Azidamphenicol, ** BB, Chloramphenicol, Clindamycin, Diltiazem, DNBS, DPCP, Eugenol, ** Fragrance mix, ** Geraniol, ** Hydroxycitronellal, ** Isoeugenol, ** MCI, ** MCI/MI, ** MI, Metronidazole, ** Oak moss, ** PPD, SADBE | Camouse, 2008 [118]; ** Cortial, 2015 [106]; Coulter, 2007 [97]; ** Coulter, 2010 [93]; Friedmann, 2017 [114]; ** Gibson, 2015 [94]; Gildea, 2004 [110]; Girardi, 2005 [113]; Jenkinson, 2009 [98]; ** Jenkinson, 2010 [79]; Kneilling, 2010 [99]; ** Masjedi, 2003 [107]; ** Moed, 2005 [95]; Oakes, 2017 [100]; Popple, 2016 [116]; Sachs, 2001 [111]; ** Sieben, 2001 [105]; ** Sieben, 2002 [80]; Skazik, 2008 [101]; Vilchez-Sánchez, 2020 [112]; ** Wicks, 2016 [102] |
| CFSE | Machaerium scleroxylon, SMX/TMP | Kim, 2020 [108]; Hansel, 2019 [115] | |
| Other | Benzyl cinnamate, ** Benzyl salicylate, ** DNFB, DPCP, ** Isoeugenol, ** TCE | ** Banerjee, 2003 [109]; Friedmann, 2017 [114]; ** Li, 2019 [119]; ** Schutte, 2019 [104] | |
| Cytokine production | ELISA | Balsam of Peru, ** BB, Benzyl benzoate, Benzyl cinnamate, Coenzyme Q2, DNCB, ** DNFB, Eugenol, Farnesol, ** Fragrance mix, ** Geraniol, ** Hydroxycitronellal, ** Isoeugenol, ** MCI, ** MI, ** Oak moss, ** PPD, ** TCE | Banerjee, 2003 [109]; Betts, 2017 [78]; ** Cortial, 2015 [106]; ** Coulter, 2010 [93]; ** Jenkinson, 2010 [79]; ** Li, 2019 [119]; ** Masjedi, 2003 [107]; ** Moed, 2005 [95]; Nicolai, 2020 [51]; ** Sieben, 2001 [105]; ** Sieben, 2002 [80] |
| ELISpot | ** Benzyl salicylate DNCB, PPD, Parthenolide | Bordignon, 2015 [96]; Gibson, 2015 [94]; Newell, 2013 [103]; ** Schutte, 2019 [104]; Wahlkvist, 2008 [117] | |
| Other | DNCB, Urushiol | Betts, 2017 [78]; Newell, 2013 [103]; Kim 2016 [120] | |
| Gene expression | RT-PCR | BB, PPD, Urushiol | Coulter, 2010 [93]; Kim, 2016 [120] |
| Microarray/RNA seq | DNBS, SMX/TMP | Gildea, 2004 [110]; Kim, 2020 [108] | |
| T cell phenotype (e.g., activation markers, cytotoxicity) | BB, DNCB, Eugenol, Geraniol, Hydroxycitronellal, Isoeugenol, Machaerium scleroxylon, Oak moss, PPD, SMX/TMP, TCE | Hansel, 2019 [115]; Kim, 2020 [108]; Li, 2019 [119]; Sieben, 2001 [105]; Sieben, 2002 [80]; Wicks, 2019 [102] | |
| T cell clone Proliferation | w/o HLA blocking | PPD | Gibson, 2015 [94]; Jenkinson, 2010 [79]; Skazik, 2008 [101] |
| with HLA blocking | BB, PPD | Sieben, 2002 [80] | |
| T cell receptor repertoire | NGS | PPD | Oakes, 2017 [100] |
| other | PPD | Skazik, 2008 [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Soto, M.; Curato, C.; Riedel, F.; Thierse, H.-J.; Luch, A.; Siewert, K. In Vitro Monitoring of Human T Cell Responses to Skin Sensitizing Chemicals—A Systematic Review. Cells 2022, 11, 83. https://doi.org/10.3390/cells11010083
Aparicio-Soto M, Curato C, Riedel F, Thierse H-J, Luch A, Siewert K. In Vitro Monitoring of Human T Cell Responses to Skin Sensitizing Chemicals—A Systematic Review. Cells. 2022; 11(1):83. https://doi.org/10.3390/cells11010083
Chicago/Turabian StyleAparicio-Soto, Marina, Caterina Curato, Franziska Riedel, Hermann-Josef Thierse, Andreas Luch, and Katherina Siewert. 2022. "In Vitro Monitoring of Human T Cell Responses to Skin Sensitizing Chemicals—A Systematic Review" Cells 11, no. 1: 83. https://doi.org/10.3390/cells11010083
APA StyleAparicio-Soto, M., Curato, C., Riedel, F., Thierse, H.-J., Luch, A., & Siewert, K. (2022). In Vitro Monitoring of Human T Cell Responses to Skin Sensitizing Chemicals—A Systematic Review. Cells, 11(1), 83. https://doi.org/10.3390/cells11010083