The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neural Stem Cell Cultures
2.2. Chronic Cell Aging Model
2.3. Evaluation of Cell Death and Viability
2.4. Proliferation Index
2.5. Transfection Assays
2.6. Mitochondrial ROS Detection
2.7. ATP Measurement
2.8. Total Protein Extraction
2.9. Immunoblotting
2.10. Immnunoprecipitation Assay
2.11. Total RNA Extraction and Quantitative RT-PCR (qRT-PCR)
2.12. Senescence Associated β-Galactosidase Activity
2.13. Animal Model of Depressive-Like Behavior and Physical Exercise (PE) Protocol
2.14. Free-Floating Immunohistochemistry
2.15. Statistical Analysis
3. Results
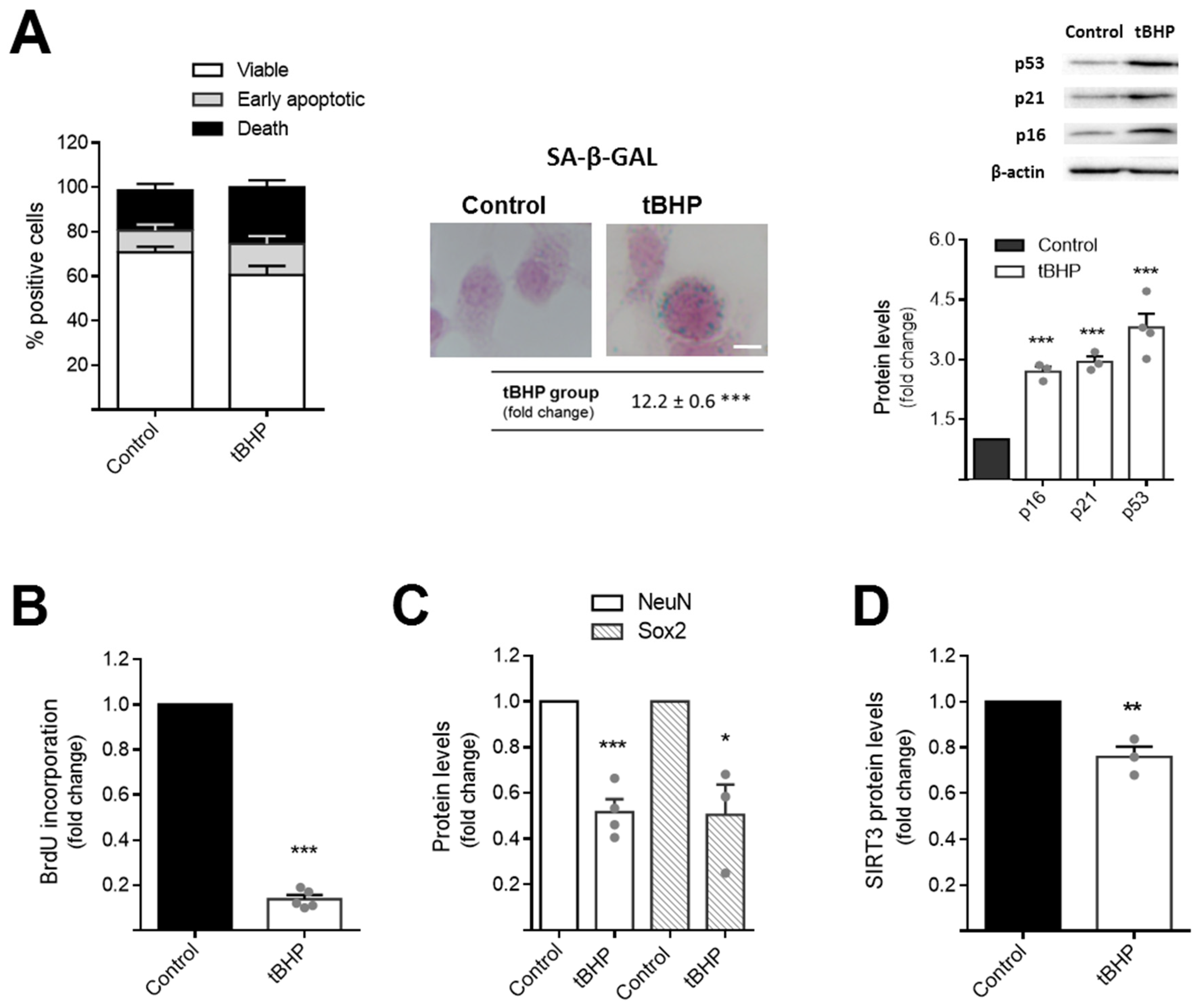
3.1. Tert-Butyl Hydroperoxide Induces Aging Features in NSCs and Reduces Mitochondrial Antioxidant Defence System
3.2. SIRT3 Rescues Mitochondrial Oxidative Stress and Differentiation Potential of Aged NSCs
3.3. SIRT3 Further Activates the Long Chain Acyl-CoA Dehydrogenase (LCAD) in Aged NSCs
3.4. SIRT3 Requires LCAD and Oxidative Control to Decelerate NSC Aging
3.5. Reduced Neurogenesis in Depressive-Like Mice Is Associated with LCAD Downregulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.-L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef]
- Lin, R.; Iacovitti, L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015, 1628 Pt B, 327–342. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.-L.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional Implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Mathews, K.J.; Allen, K.M.; Boerrigter, D.; Ball, H.; Weickert, C.S.; Double, K.L. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell 2017, 16, 1195–1199. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Berger, T.; Lee, H.; Young, A.H.; Aarsland, D.; Thuret, S. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol. Med. 2020, 26, 803–818. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Simões do Couto, F.; Lunet, N.; Ginó, S.; Chester, C.; Freitas, V.; Maruta, C.; Figueira, M.L.; De Mendonça, A. Depression with melancholic features is associated with higher long-term risk for dementia. J. Affect. Disord. 2016, 15, 202–229. [Google Scholar] [CrossRef]
- Kuring, J.K.; Mathias, J.L.; Ward, L. Risk of Dementia in persons who have previously experienced clinically-significant Depression, Anxiety, or PTSD: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 274, 247–261. [Google Scholar] [CrossRef]
- Siopi, E.; Denizet, M.; Gabellec, M.M.; de Chaumont, F.; Olivo-Marin, J.C.; Guilloux, J.P.; Lledo, P.M.; Lazarini, F. Anxiety- and Depression-Like States Lead to Pronounced Olfactory Deficits and Impaired Adult Neurogenesis in Mice. J. Neurosci. 2016, 36, 518–531. [Google Scholar] [CrossRef]
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat. Rev. Neurosci. 2019, 20, 34–48. [Google Scholar] [CrossRef]
- Xavier, J.M.; Rodrigues, C.M.P.; Solá, S. Mitochondria: Major Regulators of Neural Development. Neuroscientist 2016, 22, 346–358. [Google Scholar] [CrossRef]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef]
- Lisowski, P.; Kannan, P.; Mlody, B.; Prigione, A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018, 19, e45432. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Costa, M.; Ribeiro, M.F.; Siquenique, S.; Sá Santos, S.; Martins, J.; Coelho, A.V.; Silva, M.F.B.; Rodrigues, C.M.P.; Solá, S. Reprograming of lipid metabolism as a new driving force behind tauroursodeoxycholic acid-induced neural stem cell proliferation. Front. Cell Dev. Biol. 2020, 8, 335. [Google Scholar] [CrossRef]
- Knobloch, M.; Jessberger, S. Metabolism and neurogenesis. Curr. Opin. Neurobiol. 2017, 42, 45–52. [Google Scholar] [CrossRef]
- Stoll, E.A.; Cheung, W.; Mikheev, A.M.; Sweet, I.R.; Bielas, J.H.; Zhang, J.; Rostomily, R.C.; Horner, P.J. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J. Biol. Chem. 2011, 286, 38592–38601. [Google Scholar] [CrossRef]
- Zuccoli, G.S.; Saia-Cereda, V.M.; Nascimento, J.M.; Martins-De-Souza, D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front. Neurosci. 2017, 11, 493. [Google Scholar] [CrossRef]
- Schwer, B.; North, B.J.; Frye, R.A.; Ott, M.; Verdin, E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 2002, 158, 647–657. [Google Scholar] [CrossRef]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef]
- Giralt, A.; Villarroya, F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem. J. 2012, 444, 1–10. [Google Scholar] [CrossRef]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef]
- Lombard, D.B.; Alt, F.W.; Cheng, H.L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef]
- Ozden, O.; Park, S.H.; Wagner, B.A.; Song, H.Y.; Zhu, Y.; Vassilopoulos, A.; Jung, B.; Buettner, G.R.; Gius, D. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic. Biol. Med. 2014, 76, 163–172. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef]
- Le, W.; Abbas, A.S.; Sprecher, H.; Vockley, J.; Schulz, H. Long-chain acyl-CoA dehydrogenase is a key enzyme in the mitochondrial β-oxidation of unsaturated fatty acids. Biochim. Biophys. Acta 2000, 1485, 121–128. [Google Scholar] [CrossRef]
- Spiliotopoulos, D.; Goffredo, D.; Conti, L.; Di Febo, F.; Biella, G.; Toselli, M.; Cattaneo, E. An optimized experimental strategy for efficient conversion of embryonic stem (ES)-derived mouse neural stem (NS) cells into a nearly homogeneous mature neuronal population. Neurobiol. Dis. 2009, 34, 320–331. [Google Scholar] [CrossRef]
- Conti, L.; Pollard, S.M.; Gorba, T.; Reitano, E.; Toselli, M.; Biella, G.; Sun, Y.; Sanzone, S.; Ying, Q.L.; Cattaneo, E.; et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005, 3, e283. [Google Scholar] [CrossRef]
- Pollard, S.M.; Benchoua, A.; Lowell, S. Neural stem cells, neurons, and glia. Methods Enzymol. 2006, 418, 151–169. [Google Scholar] [CrossRef]
- Glaser, T.; Pollard, S.M.; Smith, A.; Brüstle, O. Tripotential differentiation of adherently expandable neural stem (NS) cells. PLoS ONE 2007, 2, e298. [Google Scholar] [CrossRef]
- Rabin, D.M.; Rabin, R.L.; Blenkinsop, T.A.; Temple, S.; Stern, J.H. Chronic oxidative stress upregulates drusen-related protein expression in adult human RPE stem cell-derived RPE cells: A novel culture model for dry AMD. Aging 2013, 5, 51–66. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods Mol. Biol. 2007, 371, 21–31. [Google Scholar] [CrossRef]
- Patki, G.; Li, L.; Allam, F.; Solanki, N.; Dao, A.T.; Alkadhi, K.; Salim, S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol. Behav. 2014, 130, 47–53. [Google Scholar] [CrossRef]
- Patki, G.; Solanki, N.; Atrooz, F.; Ansari, A.; Allam, F.; Jannise, B.; Maturi, J.; Salim, S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol. Behav. 2014, 130, 135–144. [Google Scholar] [CrossRef]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Lehmann, M.; Martinowich, K.; Manji, H.K.; Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 2010, 15, 1152–1163. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Y.; Pi, C.; Wang, H.; Sun, H.; Yu, X.; Shi, Y.; He, X. Sirt3 Attenuates Oxidative Stress Damage and Rescues Cellular Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Superoxide Dismutase 2. Front. Cell Dev. Biol. 2020, 8, 599376. [Google Scholar] [CrossRef]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef]
- Zhang, T.; Ke, W.; Zhou, X.; Qian, Y.; Feng, S.; Wang, R.; Cui, G.; Tao, R.; Guo, W.; Duan, Y.; et al. Human Neural Stem Cells Reinforce Hippocampal Synaptic Network and Rescue Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Stem Cell Rep. 2019, 13, 1022–1037. [Google Scholar] [CrossRef]
- Berdugo-Vega, G.; Arias-Gil, G.; López-Fernández, A.; Artegiani, B.; Wasielewska, J.M.; Lee, C.C.; Lippert, M.T.; Kempermann, G.; Takagaki, K.; Calegari, F. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 2020, 11, 135. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wong, N.-K.; Xiao, J.; So, K.-F. Oxidative Stress in Stem Cell Aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef]
- Sabaretnam, T.; Kritharides, L.; O’Reilly, J.N.; Le Couteur, D.G. The effect of aging on the response of isolated hepatocytes to hydrogen peroxide and tert-butyl hydroperoxide. Toxicol. Vitr. 2010, 24, 123–128. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Ji, Q.; Wu, Z.; Zhang, W.; Cai, Y.; Wang, Z.; Hu, J.; Liu, Z.; Wang, Q.; Bi, S.; et al. SIRT3 consolidates heterochromatin and counteracts senescence. Nucleic Acids Res. 2021, 49, 4203–4219. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Cameron, H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015, 40, 113–128. [Google Scholar] [CrossRef]
- Dioli, C.; Patrício, P.; Pinto, L.G.; Marie, C.; Morais, M.; Vyas, S.; Bessa, J.M.; Pinto, L.; Sotiropoulos, I. Adult neurogenic process in the subventricular zone-olfactory bulb system is regulated by Tau protein under prolonged stress. Cell Prolif. 2021, 54, e13027. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Defteralı, Ç.; Moreno-Estellés, M.; Crespo, C.; Díaz-Guerra, E.; Díaz-Moreno, M.; Vergaño-Vera, E.; Nieto-Estévez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H.; et al. Neural stem cells in the adult olfactory bulb core generate mature neurons in vivo. Stem Cells 2021, 39, 1253–1269. [Google Scholar] [CrossRef]
- Wang, F.; Jin, J.; Wang, J.; He, R.; Li, K.; Hu, X.; Li, Y.; Zhu, Y. Association between olfactory function and inhibition of emotional competing distractors in major depressive disorder. Sci. Rep. 2020, 10, 6322. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.R.; Kokovay, E. Rejuvenating subventricular zone neurogenesis in the aging brain. Curr. Opin. Pharmacol. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
- Xavier, J.M.; Morgado, A.L.; Solá, S.; Rodrigues, C.M.P. Mitochondrial translocation of p53 modulates neuronal fate by preventing differentiation-induced mitochondrial stress. Antioxid. Redox Signal. 2014, 21, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Ribeiro, F.F.; Xapelli, S.; Genebra, T.; Ribeiro, M.F.; Sebastião, A.M.; Rodrigues, C.M.P.; Solá, S. Tauroursodeoxycholic Acid Enhances Mitochondrial Biogenesis, Neural Stem Cell Pool, and Early Neurogenesis in Adult Rats. Mol. Neurobiol. 2018, 55, 3725–3738. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.M.; Morgado, A.L.; Rodrigues, C.M.P.; Solá, S. Tauroursodeoxycholic acid increases neural stem cell pool and neuronal conversion by regulating mitochondria-cell cycle retrograde signaling. Cell Cycle 2014, 13, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.; Santos, A.A.; Afonso, M.B.; Rodrigues, P.M.; Sá Santos, S.; Castro, R.E.; Rodrigues, C.M.P.; Solá, S. Diet-dependent gut microbiota impacts on adult neurogenesis through mitochondrial stress modulation. Brain Commun. 2020, 2, fcaa165. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.P.; Solá, S.; Nan, Z.; Castro, R.E.; Ribeiro, P.S.; Low, W.C.; Steer, C.J. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]




| Gene | Sequence (5′-3′) |
|---|---|
| Hprt | 5′ -GGTGAAAAGGACCTCTCGAAGTG- 3′ (fwd) |
| 5′ -ATAGTCAAGGGCATATCCAACAACA- 3′ (rev) | |
| Mki67 (for ki67) | 5′ -CCTTTGCTGTCCCCGAAGA- 3′ (fwd) |
| 5′ -GGCTTCTCATCTGTTGCTTCCT- 3′ (rev) | |
| Ppargc1a (for PGC-1α) | 5′ -GGACATGTGCAGCCAAGACTCT- 3′ (fwd) |
| 5′ -CACTTCAATCCACCCAGAAAGCT- 3′ (rev) | |
| Map2 | 5′ -GTTCAGGCCCACTCTCCTTC- 3′ (fwd) |
| 5′ -CTTGCTGCTGTGGTTTTCCG- 3′ (rev) | |
| Tfam | 5′ -CACCCAGATGCAAAACTTTCAG- 3′ (fwd) |
| 5′ -CTGCTCTTTATACTTGCTCACAG- 3′ (rev) | |
| PLIN2 | 5′ -TGCTGTGTGGTGATCTGGAC- 3′ (fwd) |
| 5′ -CGGAGGACACAAGGTCGTAG- 3′ (rev) | |
| VLCAD | 5′ -CAGCGACTTTATGCCAGGGA- 3′ (fwd) |
| 5′ -TGGCAGGGTCATTCACTTCC- 3′ (rev) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.S.; Moreira, J.B.; Costa, M.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S.; Solá, S. The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells 2022, 11, 90. https://doi.org/10.3390/cells11010090
Santos SS, Moreira JB, Costa M, Rodrigues RS, Sebastião AM, Xapelli S, Solá S. The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells. 2022; 11(1):90. https://doi.org/10.3390/cells11010090
Chicago/Turabian StyleSantos, Sónia Sá, João B. Moreira, Márcia Costa, Rui S. Rodrigues, Ana M. Sebastião, Sara Xapelli, and Susana Solá. 2022. "The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression" Cells 11, no. 1: 90. https://doi.org/10.3390/cells11010090






