Influences of Long-Term Exercise and High-Fat Diet on Age-Related Telomere Shortening in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Design and Treatment
2.3. Euthanasia and Sample Preparation
2.4. Analysis of Relative Telomere Length (RTL) in PBMCs and Solid Organs
- Telomere For: 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′;
- Telomere Rev: 3′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-5′;
- GAPDH For: 5′-CACCTAGACAAGGATGCAGAG-3′;
- GAPDH Rev: 3′-GCATGACTGGAGGAATCACA-5′.
2.5. The mRNA Expression Analyses in Blood Cells and Solid Organs
- 5.
- Β-actin: 5′-CTTCCTTCCTGGGTATGGAATCCTG-3′;
- 6.
- Tert: 5′-ATCGAGCAGAGCATCTCCATGAATG-3′;
- 7.
- Terf-1: 5′-AAAACAGACATGGCTTTGGGAAGAA-3′;
- 8.
- Terf-2: 5′-GAGAAAATTTAGACTGTTCCTTTGA-3′.
2.6. Statistical Analyses
3. Results
3.1. Characterization of the Animal Model
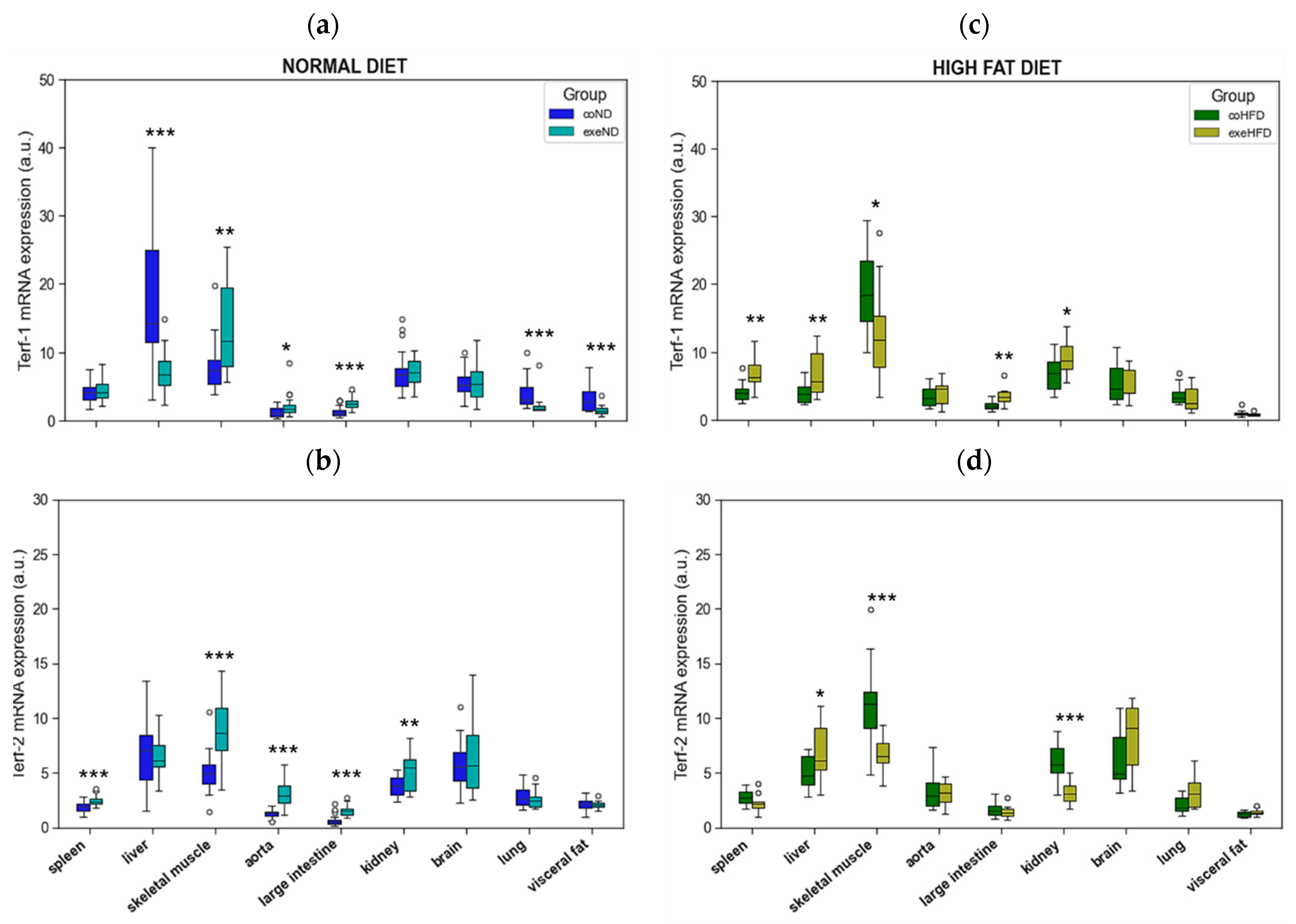
3.2. Influence of HFD on RTL and the Expression of Telomere-Regulating Genes in Different Tissues
3.3. Influence of Exercise on RTL and the Expression of Telomere-Regulating Genes in Different Tissues
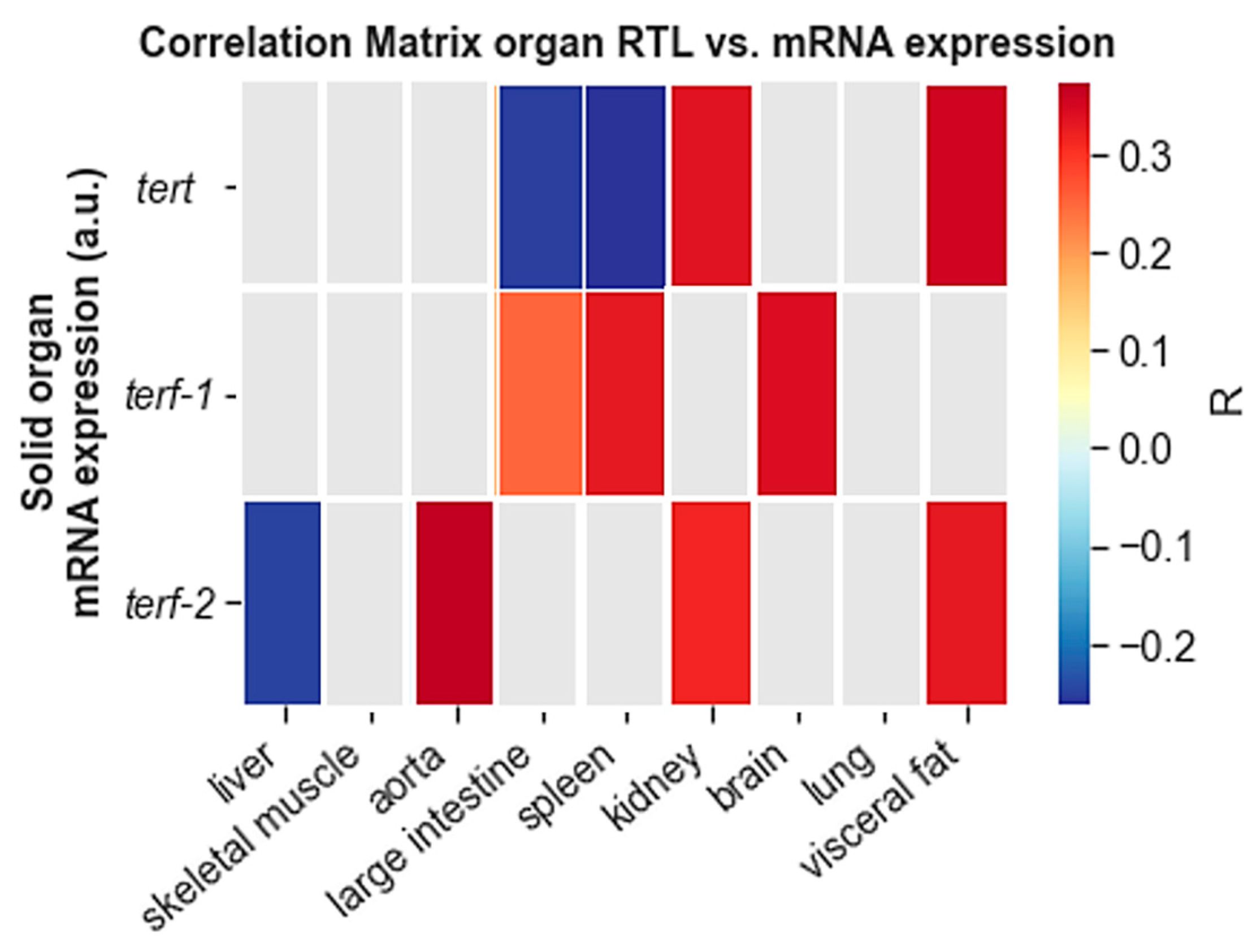
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetos, A.; Gardner, J.P.; Zureik, M.; Labat, C.; Xiaobin, L.; Adamopoulos, C.; Temmar, M.; Bean, K.E.; Thomas, F.; Aviv, A. Short Telomeres Are Associated With Increased Carotid Atherosclerosis in Hypertensive Subjects. Hypertension 2004, 43, 182–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, L.S.; de Boer, R.A.; Samani, N.J.; van Veldhuisen, D.J.; van der Harst, P. Telomere biology in heart failure. Eur. J. Heart Fail. 2008, 10, 1049–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaneh-Far, R.; Cawthon, R.M.; Na, B.; Browner, W.S.; Schiller, N.B.; Whooley, M.A. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: Data from the Heart and Soul Study. Arter. Thromb Vasc. Biol. 2008, 28, 1379–1384. [Google Scholar] [CrossRef]
- Willeit, P.; Willeit, J.; Brandstätter, A.; Ehrlenbach, S.; Mayr, A.; Gasperi, A.; Weger, S.; Oberhollenzer, F.; Reindl, M.; Kronenberg, F.; et al. Cellular Aging Reflected by Leukocyte Telomere Length Predicts Advanced Atherosclerosis and Cardiovascular Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1649–1656. [Google Scholar] [CrossRef] [Green Version]
- Testa, R.; Olivieri, F.; Sirolla, C.; Spazzafumo, L.; Rippo, M.R.; Marra, M.; Bonfigli, A.R.; Ceriello, A.; Antonicelli, R.; Franceschi, C.; et al. Leukocyte telomere length is associated with complications of Type 2 diabetes mellitus. Diabet. Med. 2011, 28, 1388–1394. [Google Scholar] [CrossRef]
- Ehrlenbach, S.; Willeit, P.; Kiechl, S.; Willeit, J.; Reindl, M.; Schanda, K.; Kronenberg, F.; Brandstatter, A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: Introduction of a well-controlled high-throughput assay. Int. J. Epidemiol. 2009, 38, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Honig, L.S.; Kang, M.S.; Schupf, N.; Lee, J.H.; Mayeux, R. Association of Shorter Leukocyte Telomere Repeat Length With Dementia and Mortality. Arch. Neurol. 2012, 69, 1332–1339. [Google Scholar] [CrossRef] [Green Version]
- Pusceddu, I.; Kleber, M.; Delgado, G.; Herrmann, W.; Marz, W.; Herrmann, M. Telomere length and mortality in the Ludwigshafen Risk and Cardiovascular Health study. PLoS ONE 2018, 13, e0198373. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hägg, S. Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res. Rev. 2018, 48, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas-Simoes, T.M.; Ros, E.; Sala-Vila, A. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 2016, 65, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Astuti, Y.; Wardhana, A.; Watkins, J.; Wulaningsih, W.; Network, P.R. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Env. Res. 2017, 158, 480–489. [Google Scholar] [CrossRef]
- Semeraro, M.D.; Smith, C.; Kaiser, M.; Levinger, I.; Duque, G.; Gruber, H.-J.; Herrmann, M. Physical activity, a modulator of aging through effects on telomere biology. Aging 2020, 12, 13803–13823. [Google Scholar] [CrossRef]
- Werner, C.; Hanhoun, M.; Widmann, T.; Kazakov, A.; Semenov, A.; Pöss, J.; Bauersachs, J.; Thum, T.; Pfreundschuh, M.; Müller, P.; et al. Effects of Physical Exercise on Myocardial Telomere-Regulating Proteins, Survival Pathways, and Apoptosis. J. Am. Coll. Cardiol. 2008, 52, 470. [Google Scholar] [CrossRef] [Green Version]
- Werner, C.M.; Furster, T.; Widmann, T.; Poss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Buchner, N.; Meyer, T.; Kindermann, W.; et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2018, 40, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, A.T.; Zimmerman, J.B.; Witkowski, S.; Hearn, J.W.; Hatfield, B.D.; Roth, S.M. Relationship between physical activity level, telomere length, and telomerase activity. Med. Sci. Sports Exerc. 2008, 40, 1764. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, A.T.; Lima, L.C.J.; Wang, J.; Hanson, E.D.; Guth, L.M.; Spangenburg, E.E.; Roth, S.M. Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. J. Appl. Physiol. 2012, 113, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, A.T.; Witkowski, S.; Marshall, M.R.; Wang, J.; Lima, L.C.; Guth, L.M.; Spangenburg, E.E.; Roth, S.M. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 911–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Takatsu, M.; Hattori, T.; Murase, T.; Ohura, S.; Takeshita, Y.; Watanabe, S.; Murohara, T.; Nagata, K. Premature cardiac senescence in DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Nagoya J. Med. Sci. 2014, 76, 35–49. [Google Scholar] [PubMed]
- Burchfield, J.G.; Kebede, M.A.; Meoli, C.C.; Stöckli, J.; Whitworth, P.T.; Wright, A.L.; Hoffman, N.J.; Minard, A.Y.; Ma, X.; Krycer, J.R.; et al. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 2018, 293, 5731–5745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Rev. Esp. Cardiol. 2016, 69, 939. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, M.; Pusceddu, I.; Marz, W.; Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Lab. Med. 2018, 56, 1210–1222. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Paul, L. Diet, nutrition and telomere length. J. Nutr. Biochem. 2011, 22, 895–901. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, A.T.; Ludlow, L.W.; Roth, S.M. Do Telomeres Adapt to Physiological Stress? Exploring the Effect of Exercise on Telomere Length and Telomere-Related Proteins. BioMed Res. Int. 2013, 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Bandaria, J.N.; Qin, P.; Berk, V.; Chu, S.; Yildiz, A. Shelterin Protects Chromosome Ends by Compacting Telomeric Chromatin. Cell 2016, 164, 735–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.G.; Donato, A.J.; Walker, A.E. Telomere uncapping and vascular aging. Am. J. Physiol Heart Circ. Physiol. 2018, 315, H1–H5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaput, J.-P.; Tremblay, A. Obesity and physical inactivity: The relevance of reconsidering the notion of sedentariness. Obes Facts 2009, 2, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Review, W.P. Most Obese Countries 2022. Available online: https://worldpopulationreview.com/country-rankings/most-obese-countries (accessed on 14 April 2022).
- Division of Nutrition, Physical Activity and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Available online: https://www.cdc.gov/physicalactivity/data/inactivity-prevalence-maps/index.html (accessed on 29 March 2022).
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Shi, L.; Prescott, J.; Chiuve, S.E.; Hu, F.B.; De Vivo, I.; Stampfer, M.J.; Franks, P.W.; Manson, J.E.; Rexrode, K.M. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS ONE 2012, 7, e38374. [Google Scholar] [CrossRef]
- Buxton, J.L.; Walters, R.G.; Visvikis-Siest, S.; Meyre, D.; Froguel, P.; Blakemore, A.I. Childhood obesity is associated with shorter leukocyte telomere length. J. Clin. Endocrinol. Metab. 2011, 96, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- MacEneaney, O.J.; Kushner, E.J.; Westby, C.M.; Cech, J.N.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity 2010, 18, 1677–1682. [Google Scholar] [CrossRef]
- Kim, S.; Parks, C.G.; DeRoo, L.A.; Chen, H.; Taylor, J.A.; Cawthon, R.M.; Sandler, D.P. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomark. Prev. 2009, 18, 816–820. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Gao, Y.T.; Cai, Q.; Qu, S.; Cai, H.; Li, H.L.; Wu, J.; Ji, B.T.; Yang, G.; Chow, W.H.; et al. Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity 2013, 21, 2582–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordfjall, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Sabater, M.; Ricart, W.; Fernandez-Real, J.M. Telomere length of subcutaneous adipose tissue cells is shorter in obese and formerly obese subjects. Int. J. Obes. 2010, 34, 1345–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.C. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. 2012, 36, 1176–1179. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.A.; Prior, S.L.; Barry, J.D.; Caplin, S.; Baxter, J.N.; Stephens, J.W. Changes in markers of oxidative stress and DNA damage in human visceral adipose tissue from subjects with obesity and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 106, 627–633. [Google Scholar] [CrossRef]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F.T.; Mazzola, J.; Epifanio, M.; Stein, R.T.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Mangge, H.; Herrmann, M.; Almer, G.; Zelzer, S.; Moeller, R.; Horejsi, R.; Renner, W. Telomere shortening associates with elevated insulin and nuchal fat accumulation. Sci. Rep. 2020, 10, 6863. [Google Scholar] [CrossRef] [Green Version]
- Paffenbarger Jr, R.S.; Hyde, R.; Wing, A.L.; Hsieh, C.-c. Physical activity, all-cause mortality, and longevity of college alumni. New Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef]
- Fraser, G.E.; Shavlik, D.J. Ten years of life: Is it a matter of choice? Arch. Intern. Med. 2001, 161, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Franco, O.H.; de Laet, C.; Peeters, A.; Jonker, J.; Mackenbach, J.; Nusselder, W. Effects of physical activity on life expectancy with cardiovascular disease. Arch. Intern. Med. 2005, 165, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Melhus, H.; Gedeborg, R.; Sundström, J.; Ahlbom, A.; Zethelius, B.; Berglund, L.G.; Wolk, A.; Michaëlsson, K. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ 2009, 338, b688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Barrow, D.R.; Abbate, L.M.; Paquette, M.R.; Driban, J.B.; Vincent, H.K.; Newman, C.; Messier, S.P.; Ambrose, K.R.; Shultz, S.P. Exercise prescription for weight management in obese adults at risk for osteoarthritis: Synthesis from a systematic review. BMC Musculoskelet Disord 2019, 20, 610. [Google Scholar] [CrossRef] [Green Version]
- Cherkas, L.F.; Hunkin, J.L.; Kato, B.S.; Richards, J.B.; Gardner, J.P.; Surdulescu, G.L.; Kimura, M.; Lu, X.; Spector, T.D.; Aviv, A. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008, 168, 154–158. [Google Scholar] [CrossRef]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; De Vivo, I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Soares-Miranda, L.; Imamura, F.; Siscovick, D.; Jenny, N.S.; Fitzpatrick, A.L.; Mozaffarian, D. Physical activity, physical fitness and leukocyte telomere length: The cardiovascular health study. Med. Sci. Sports Exerc. 2015, 47, 2525. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, A.T.; Gratidao, L.; Ludlow, L.W.; Spangenburg, E.E.; Roth, S.M. Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle. Exp. Physiol. 2017, 102, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Rodrigues, S.; Rodríguez, A.; Gonçalves, I.O.; Moreira, A.; Maciel, E.; Santos, S.; Domingues, M.R.; Frühbeck, G.; Ascensão, A.; Magalhães, J. Impact of physical exercise on visceral adipose tissue fatty acid profile and inflammation in response to a high-fat diet regimen. Int. J. Biochem Cell Biol. 2017, 87, 114–124. [Google Scholar] [CrossRef]
- Li, F.H.; Sun, L.; Zhu, M.; Li, T.; Gao, H.E.; Wu, D.S.; Zhu, L.; Duan, R.; Liu, T.C. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Exp. Gerontol. 2018, 113, 150–162. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S.; Bunner, W.P.; Chang, Y.; Shi, R. Exercise induced Right Ventricular Fibrosis is Associated with Myocardial Damage and Inflammation. Korean Circ. J. 2018, 48, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Luan, J.; Hu, F.; Zhao, Y.; Lv, B.; Wang, W.; Xia, M.; Zhao, X.; Lao, K. Aerobic exercise-stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney. Exp. Med. 2018, 16, 3511–3517. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic. Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Peña, E.; León-Mengíbar, J.; Powell, T.R.; Caixàs, A.; Cardoner, N.; Rosa, A. Telomere length in patients with obesity submitted to bariatric surgery: A systematic review. Eur. Eat. Disord Rev. 2021, 29, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.D.; Almer, G.; Renner, W.; Gruber, H.J.; Herrmann, M. Telomere length in leucocytes and solid tissues of young and aged rats. Aging 2022, 14, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Tarry, J.L.; Ozanne, S.E.; Hales, C.N. Ageing and telomeres: A study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003, 31, 1576–1583. [Google Scholar] [CrossRef]
- Lai, T.-P.; Zhang, N.; Noh, J.; Mender, I.; Tedone, E.; Huang, E.; Wright, W.E.; Danuser, G.; Shay, J.W. A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat. Commun. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
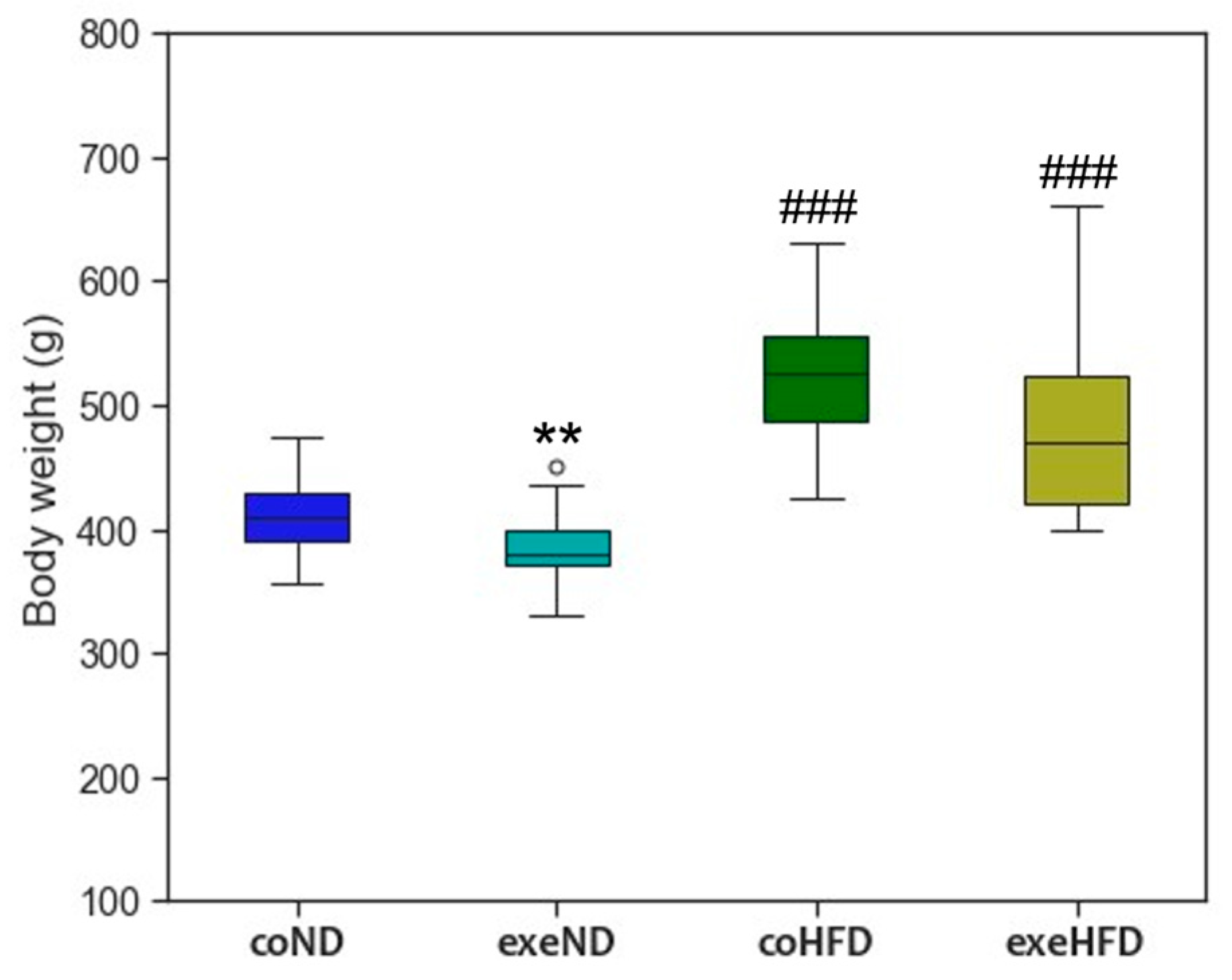
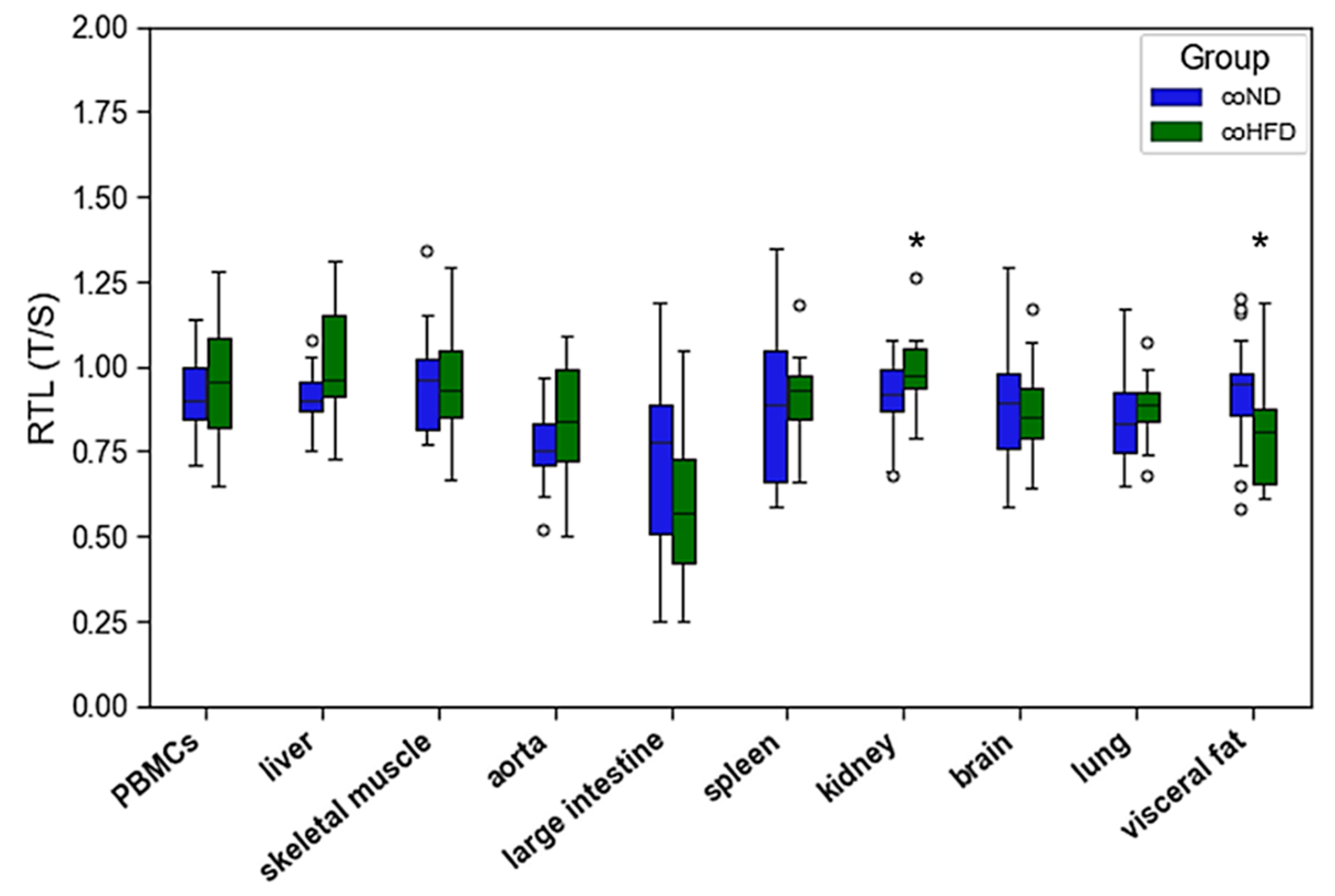

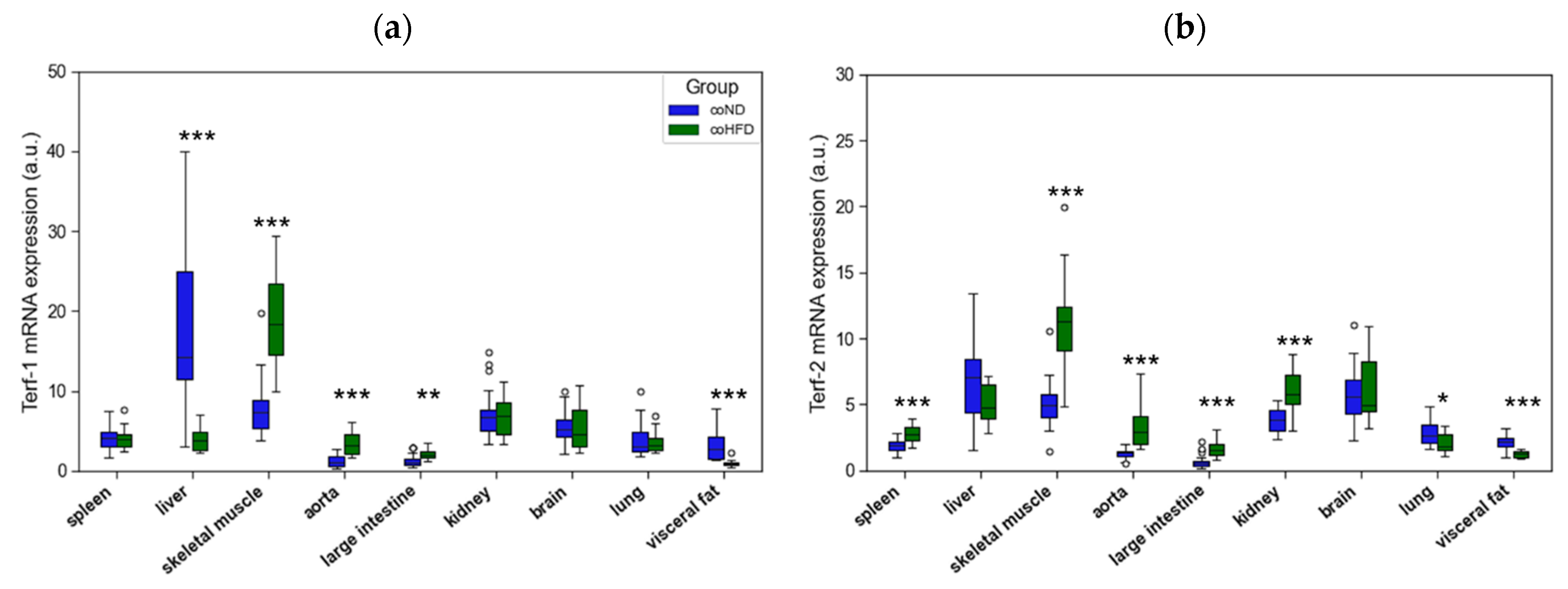

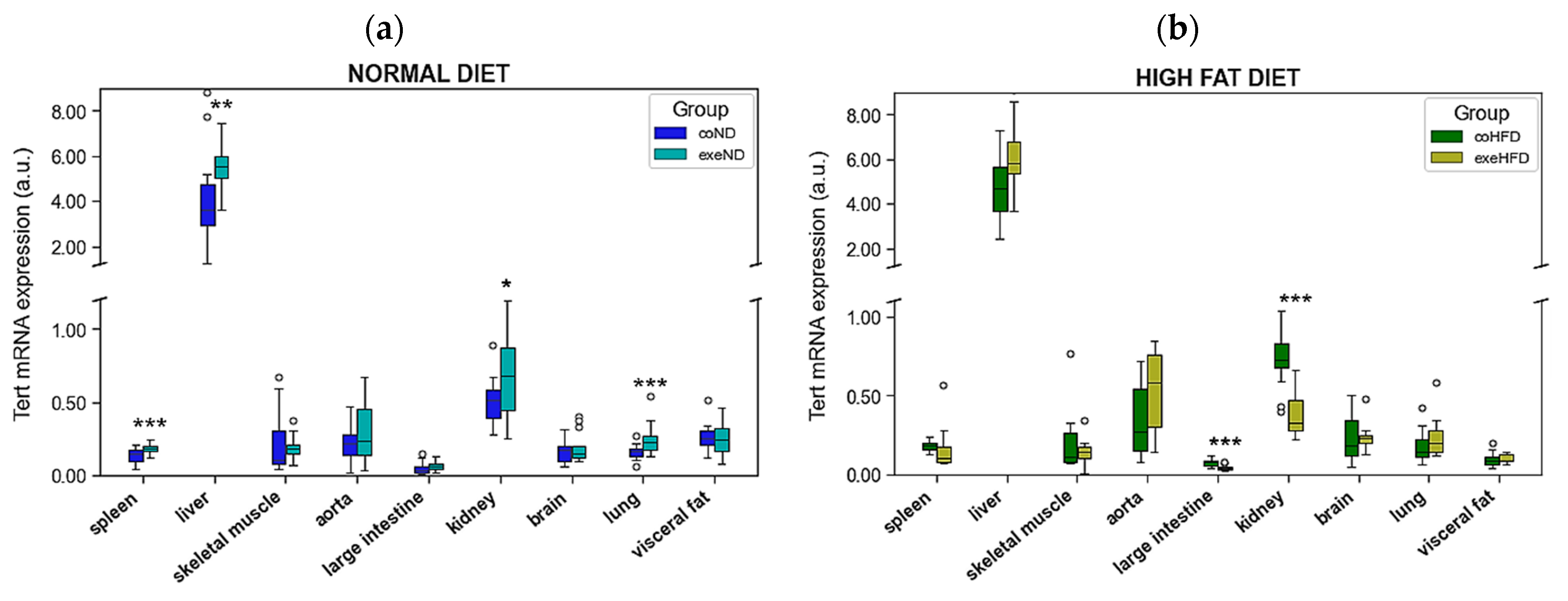
| Organs | Measurement | ND | HFD | ||
|---|---|---|---|---|---|
| Sedentary n = 22 | Exercising n = 22 | Sedentary n = 16 | Exercising n = 12 | ||
| heart | average weight | 1.31 ± 0.21 | 1.24 ± 0.11 | 1.40 ± 0.14 | 1.46 ± 0.19 ### |
| normalized weight | 0.28 ± 0.04 | 0.27 ± 0.03 | 0.30 ± 0.03 | 0.31 ± 0.03 ### | |
| spleen | average weight | 0.98 ± 0.16 | 0.97 ± 0.15 | 1.20 ± 0.16 ### | 1.18 ± 0.23 ## |
| normalized weight | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.24 ± 0.07 | 0.25 ± 0.04 ### | |
| liver | average weight | 12.53 ± 1.72 | 12.50 ± 1.80 | 14.03 ± 2.36 # | 15.16 ± 4.40 # |
| normalized weight | 2.67 ± 0.31 | 2.56 ± 0.68 | 2.98 ± 0.52 # | 3.28 ± 0.89 # | |
| visceral fat | average weight | 13.20 ± 5.26 | 10.46 ± 4.48 | 40.13 ± 12.81 ### | 39.46 ± 23.20 ### |
| normalized weight | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.018 ### | 0.07 ± 0.03 ### | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeraro, M.D.; Almer, G.; Renner, W.; Gruber, H.-J.; Herrmann, M. Influences of Long-Term Exercise and High-Fat Diet on Age-Related Telomere Shortening in Rats. Cells 2022, 11, 1605. https://doi.org/10.3390/cells11101605
Semeraro MD, Almer G, Renner W, Gruber H-J, Herrmann M. Influences of Long-Term Exercise and High-Fat Diet on Age-Related Telomere Shortening in Rats. Cells. 2022; 11(10):1605. https://doi.org/10.3390/cells11101605
Chicago/Turabian StyleSemeraro, Maria Donatella, Gunter Almer, Wilfried Renner, Hans-Jürgen Gruber, and Markus Herrmann. 2022. "Influences of Long-Term Exercise and High-Fat Diet on Age-Related Telomere Shortening in Rats" Cells 11, no. 10: 1605. https://doi.org/10.3390/cells11101605
APA StyleSemeraro, M. D., Almer, G., Renner, W., Gruber, H. -J., & Herrmann, M. (2022). Influences of Long-Term Exercise and High-Fat Diet on Age-Related Telomere Shortening in Rats. Cells, 11(10), 1605. https://doi.org/10.3390/cells11101605







