Distinct Effector Programs of Brain-Homing CD8+ T Cells in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Genotyping and Sampling
2.2. Flow Cytometry
2.3. Transmigration Assay
2.4. Statistics
3. Results
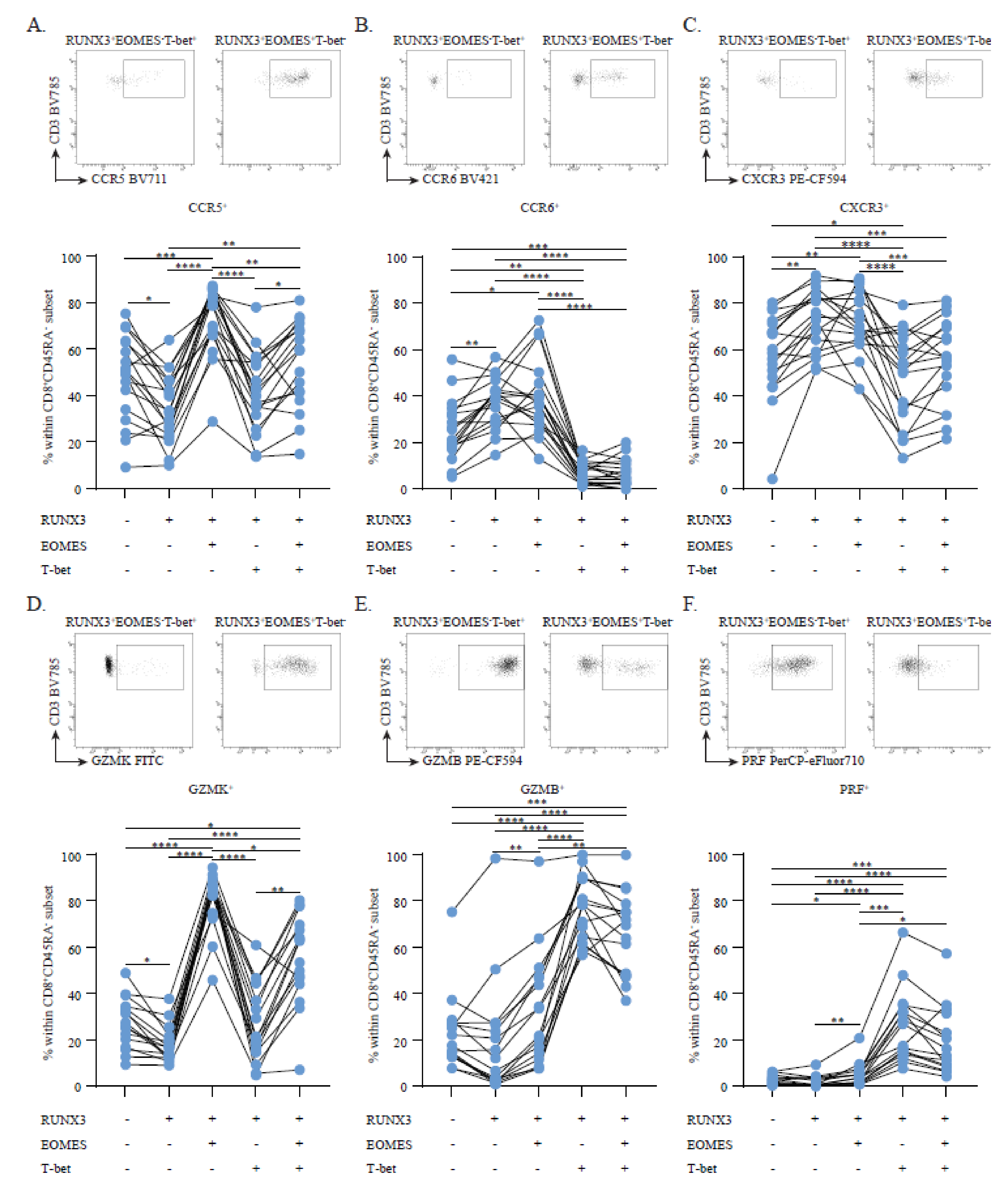
3.1. RUNX3 and Its Variant rs6672420 Disassociate with T-bet, but Not EOMES Expression in Blood CD8+ Memory T Cells
3.2. Circulating RUNX3+EOMES+T-bet− Memory CD8+ T Cells Show a Discriminative, Brain-Homing Phenotype
3.3. RUNX3+EOMES+T-bet−Memory CD8+ T Cells Are Enriched and Display a CD20dim CD69+ Brain Residency-Associated Phenotype in Early MS CSF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Nierop, G.P.; van Luijn, M.M.; Michels, S.S.; Melief, M.J.; Janssen, M.; Langerak, A.W.; Ouwendijk, W.J.D.; Hintzen, R.Q.; Verjans, G.M. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 2017, 134, 383–401. [Google Scholar] [CrossRef]
- Smolders, J.; Remmerswaal, E.B.M.; Schuurman, K.G.; Melief, J.; van Eden, C.G.; van Lier, R.A.W.; Huitinga, I.; Hamann, J. Characteristics of differentiated CD8+ and CD4+ T cells present in the human brain. Acta Neuropathol. 2013, 126, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Berge, I.J.M.T.; Van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fransen, N.L.; Hsiao, C.-C.; Van Der Poel, M.; Engelenburg, H.J.; Verdaasdonk, K.; Vincenten, M.C.J.; Remmerswaal, E.B.M.; Kuhlmann, T.; Mason, M.R.J.; Hamann, J.; et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 2020, 143, 1714–1730. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Fransen, N.L.; van den Bosch, A.M.; Brandwijk, K.I.; Huitinga, I.; Hamann, J.; Smolders, J. White matter lesions in multiple sclerosis are enriched for CD20dim CD8+ tissue-resident memory T cells. Eur. J. Immunol. 2021, 51, 483–486. [Google Scholar] [CrossRef]
- Kok, L.; Masopust, D.; Schumacher, T.N. The precursors of CD8+ tissue resident memory T cells: From lymphoid organs to infected tissues. Nat. Rev. Immunol. 2021, 22, 283–293. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Diao, H.; Getzler, A.J.; Rogal, W.; Frederick, M.A.; Milner, J.; Yu, B.; Crotty, S.; Goldrath, A.W.; Pipkin, M.E. The Transcription Factor Runx3 Establishes Chromatin Accessibility of cis-Regulatory Landscapes that Drive Memory Cytotoxic T Lymphocyte Formation. Immunity 2018, 48, 659–674.e6. [Google Scholar] [CrossRef] [Green Version]
- Milner, J.J.; Toma, C.; Yu, B.; Zhang, K.; Omilusik, K.; Phan, A.T.; Wang, D.; Getzler, A.J.; Nguyen, T.; Crotty, S.; et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 2017, 552, 253–257. [Google Scholar] [CrossRef]
- van der Gracht, E.; Behr, F.; Arens, R. Functional Heterogeneity and Therapeutic Targeting of Tissue-Resident Memory T Cells. Cells 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affandi, A.J.; Carvalheiro, T.; Ottria, A.; Broen, J.C.; Bossini-Castillo, L.; Tieland, R.G.; Van Bon, L.; Chouri, E.; Rossato, M.; Mertens, J.S.; et al. Low RUNX3 expression alters dendritic cell function in patients with systemic sclerosis and contributes to enhanced fibrosis. Ann. Rheum. Dis. 2019, 78, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Steinman, L. The discovery of natalizumab, a potent therapeutic for multiple sclerosis. J. Cell Biol. 2012, 199, 413–416. [Google Scholar] [CrossRef] [Green Version]
- van Langelaar, J.; van der Vuurst de Vries, R.M.; Janssen, M.; Wierenga-Wolf, A.F.; Spilt, I.M.; Siepman, T.A.; Dankers, W.; Verjans, G.M.G.M.; de Vries, H.E.; Lubberts, E.; et al. T helper 17.1 cells associate with multiple sclerosis disease activity: Perspectives for early intervention. Brain 2018, 141, 1334–1349. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Janssen, M.; Wierenga-Wolf, A.F.; Melief, M.J.; Siepman, T.A.; de Vries, H.E.; Unger, P.-P.A.; van Ham, S.M.; Hintzen, R.Q.; et al. Induction of brain-infiltrating T-bet-expressing B cells in multiple sclerosis. Ann. Neurol. 2019, 86, 264–278. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Trebst, C.; Tucky, B.; Wei, T.; Wu, L.; Baekkevold, E.S.; Lassmann, H.; Staugaitis, S.M.; et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 2003, 100, 8389–8394. [Google Scholar] [CrossRef] [Green Version]
- Smolders, J.; van Luijn, M.M.; Hsiao, C.-C.; Hamann, J. T-cell surveillance of the human brain in health and multiple sclerosis. Semin. Immunopathol. 2022, 1–13. [Google Scholar] [CrossRef]
- Clark, R.A. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef] [Green Version]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.-L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Hafler, D.A.; Lucchinetti, C.F. Multiple sclerosis—A quiet revolution. Nat. Rev. Neurol. 2015, 11, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Guilloty, F.; Pipkin, M.; Djuretic, I.M.; Levanon, D.; Lotem, J.; Lichtenheld, M.G.; Groner, Y.; Rao, A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009, 206, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, J.; Saji, E.; Tröscher, A.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Balashov, K.E.; Rottman, J.B.; Weiner, H.L.; Hancock, W.W. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1α and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6873–6878. [Google Scholar] [CrossRef] [Green Version]
- Annibali, V.; Ristori, G.; Angelini, D.F.; Serafini, B.; Mechelli, R.; Cannoni, S.; Romano, S.; Paolillo, A.; Abderrahim, H.; Diamantini, A.; et al. CD161highCD8+T cells bear pathogenetic potential in multiple sclerosis. Brain 2011, 134 Pt 2, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Parga-Vidal, L.; Behr, F.M.; Kragten, N.A.M.; Nota, B.; Wesselink, T.H.; Kavazović, I.; Covill, L.E.; Schuller, M.B.P.; Bryceson, Y.T.; Wensveen, F.M.; et al. Hobit identifies tissue-resident memory T cell precursors that are regulated by Eomes. Sci. Immunol. 2021, 6, eabg3533. [Google Scholar] [CrossRef]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.M.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Behr, F.M.; Parga-Vidal, L.; Kragten, N.A.M.; Van Dam, T.J.P.; Wesselink, T.H.; Sheridan, B.S.; Arens, R.; Van Lier, R.A.W.; Stark, R.; Van Gisbergen, K.P.J.M. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat. Immunol. 2020, 21, 1070–1081. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.A.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T Cell Help Guides Formation of CD103+ Lung-Resident Memory CD8+ T Cells during Influenza Viral Infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, L.K.; Wynne-Jones, E.; Freestone, D.; Pellicci, D.G.; Mielke, L.A.; Newman, D.M.; Braun, A.; Masson, F.; Kallies, A.; Belz, G.T.; et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 2015, 43, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes Induce Migration of Resting Memory CD8 T Cells into Mucosal Tissues. J. Immunol. 2017, 199, 2536–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Peripheral Blood | Healthy | MS (No Tx) b | NTZ-MS a,b |
|---|---|---|---|
| Individuals, n | 22 | 18 | 18 |
| Females, n (%) | 12 (55) | 9 (50) | 9 (50) |
| Age in years, median (range) | 38 (22–63) | 39 (24–48) | 35 (19–62) |
| Disease duration in months, median (range) c | NA | 2 (0–7) | 89 (32–256) |
| MP < 1 month prior to sampling, n (%) | NA | 2 (11) | NA |
| CNS | Paired early multiple sclerosis blood and CSF b | Late-stage postmortem multiple sclerosis brain tissue f | |
| Individuals, n | 7 | 4 | |
| Females, n (%) | 3 (43) | 3 (75) | |
| Age in years, median (range) | 41 (20–62) | 73 (66–77) | |
| Multiple sclerosis, n (%) | 7 (100) | 4 (100) | |
| Disease duration in months, median (range) c | 11 (9–315) | NA | |
| MP < 1 month prior to sampling, n (%) | 2 (29) | NA | |
| Other treatment prior to sampling, n (%) d | 3 (43) | NA | |
| Postmortem delay in hours, median (range) e | NA | 07:00 (06:50–07:04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koetzier, S.C.; van Langelaar, J.; Melief, M.-J.; Wierenga-Wolf, A.F.; Corsten, C.E.A.; Blok, K.M.; Hoeks, C.; Broux, B.; Wokke, B.; van Luijn, M.M.; et al. Distinct Effector Programs of Brain-Homing CD8+ T Cells in Multiple Sclerosis. Cells 2022, 11, 1634. https://doi.org/10.3390/cells11101634
Koetzier SC, van Langelaar J, Melief M-J, Wierenga-Wolf AF, Corsten CEA, Blok KM, Hoeks C, Broux B, Wokke B, van Luijn MM, et al. Distinct Effector Programs of Brain-Homing CD8+ T Cells in Multiple Sclerosis. Cells. 2022; 11(10):1634. https://doi.org/10.3390/cells11101634
Chicago/Turabian StyleKoetzier, Steven C., Jamie van Langelaar, Marie-José Melief, Annet F. Wierenga-Wolf, Cato E. A. Corsten, Katelijn M. Blok, Cindy Hoeks, Bieke Broux, Beatrijs Wokke, Marvin M. van Luijn, and et al. 2022. "Distinct Effector Programs of Brain-Homing CD8+ T Cells in Multiple Sclerosis" Cells 11, no. 10: 1634. https://doi.org/10.3390/cells11101634
APA StyleKoetzier, S. C., van Langelaar, J., Melief, M.-J., Wierenga-Wolf, A. F., Corsten, C. E. A., Blok, K. M., Hoeks, C., Broux, B., Wokke, B., van Luijn, M. M., & Smolders, J. (2022). Distinct Effector Programs of Brain-Homing CD8+ T Cells in Multiple Sclerosis. Cells, 11(10), 1634. https://doi.org/10.3390/cells11101634








