Metabolic Reprogramming of Innate Immune Cells as a Possible Source of New Therapeutic Approaches in Autoimmunity
Abstract
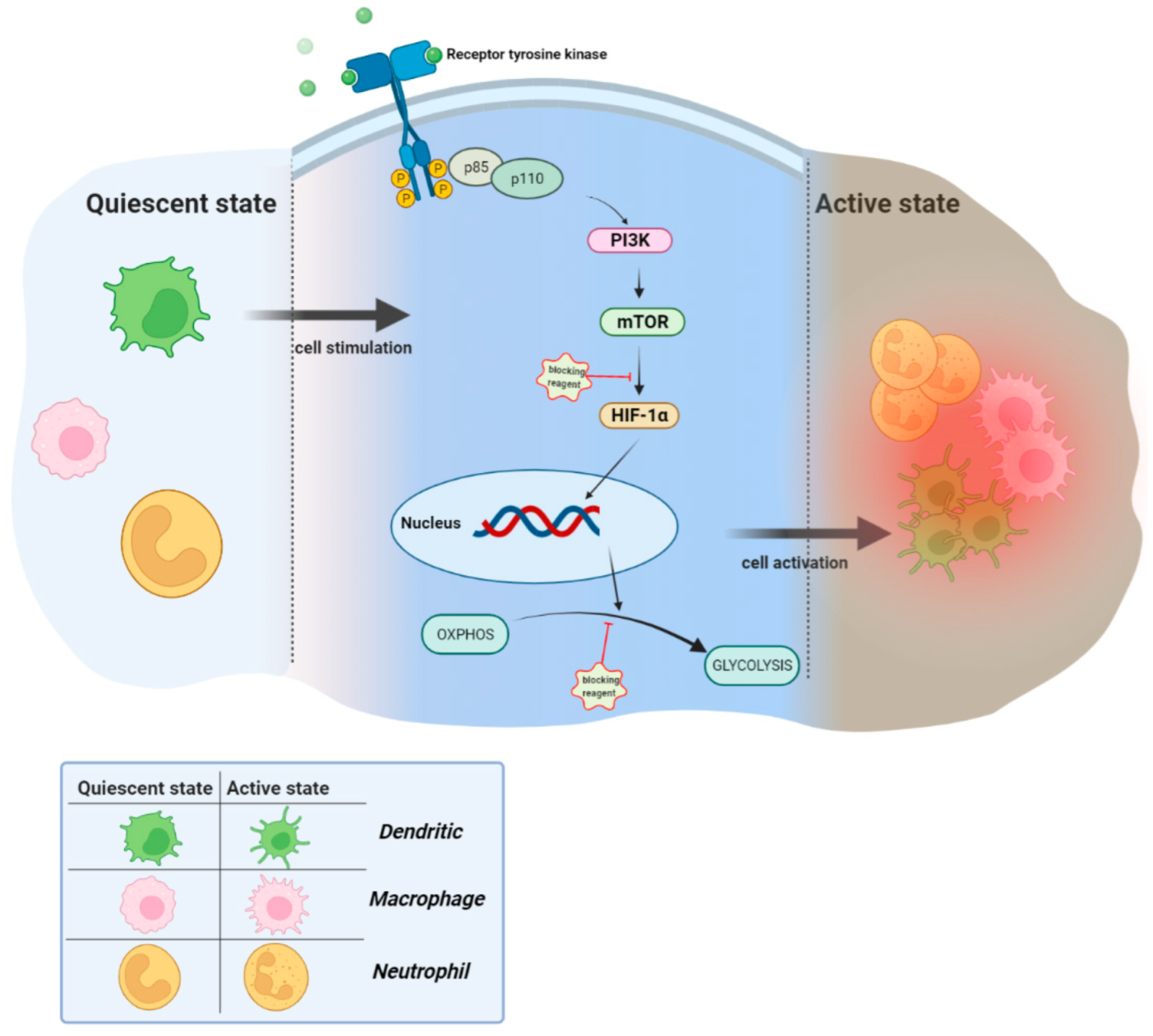
:1. Introduction
2. Metabolic Reprogramming of Macrophages, Dendritic Cells, and Neutrophils
2.1. Macrophages
2.2. Dendritic Cells
2.3. Neutrophils
3. The Possible Role of Mϕs, DCs, and Neutrophils in Autoimmune Diseases
3.1. Macrophages
3.2. Dendritic Cells
3.3. Neutrophils
4. Interfering with the Glycolytic Pathway: In Vitro and In Vivo Models
5. Conclusions
Funding
Conflicts of Interest
References
- Liaskou, E.; Wilson, D.V.; Oo, Y.H. Innate immune cells in liver inflammation. Mediat. Inflamm. 2012, 2012, 949157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanos, D.P.; Gao, B.; Gershwin, M.E. Liver immunology. Compr. Physiol. 2013, 3, 567. [Google Scholar] [PubMed] [Green Version]
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.-P. Glycolysis and oxidative phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions. Front. Immunol. 2020, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [Green Version]
- El Hassouni, B.; Granchi, C.; Vallés-Martí, A.; Supadmanaba, I.G.P.; Bononi, G.; Tuccinardi, T.; Funel, N.; Jimenez, C.R.; Peters, G.J.; Giovannetti, E. The dichotomous role of the glycolytic metabolism pathway in cancer metastasis: Interplay with the complex tumor microenvironment and novel therapeutic strategies. Semin. Cancer Biol. 2020, 60, 238–248. [Google Scholar] [CrossRef]
- Teng, X.; Cornaby, C.; Li, W.; Morel, L. Metabolic regulation of pathogenic autoimmunity: Therapeutic targeting. Curr. Opin. Immunol. 2019, 61, 10–16. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Chi, H. Immunometabolism at the intersection of metabolic signaling, cell fate, and systems immunology. Cell. Mol. Immunol. 2022, 19, 299–302. [Google Scholar] [CrossRef]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 295, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Mol. Innate Immun. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piranavan, P.; Bhamra, M.; Perl, A. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2020, 2, e200012. [Google Scholar] [PubMed] [Green Version]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef]
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519. [Google Scholar] [CrossRef]
- Halligan, D.N.; Murphy, S.J.E.; Taylor, C.T. The hypoxia-inducible factor (HIF) couples immunity with metabolism. Semin. Immunol. 2016, 28, 469–477. [Google Scholar] [CrossRef]
- Nowicki, S.; Gottlieb, E. Oncometabolites: Tailoring our genes. FEBS J. 2015, 282, 2796–2805. [Google Scholar] [CrossRef]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Starska, K.; Forma, E.; Jóźwiak, P.; Bryś, M.; Lewy-Trenda, I.; Brzezińska-Błaszczyk, E.; Krześlak, A. Gene and protein expression of glucose transporter 1 and glucose transporter 3 in human laryngeal cancer—the relationship with regulatory hypoxia-inducible factor-1α expression, tumor invasiveness, and patient prognosis. Tumor Biol. 2015, 36, 2309–2321. [Google Scholar] [CrossRef] [Green Version]
- Čuperlović-Culf, M. 2-Biology—Cancer Metabolic Phenotype. In NMR Metabolomics in Cancer Research; Čuperlović-Culf, M., Ed.; Woodhead Publishing Series in Biomedicine; Woodhead: Sawston, UK, 2013; pp. 15–138. [Google Scholar]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussien, R.; Brooks, G.A. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol. Genom. 2011, 43, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Mao, Y.; Zhao, Y.; Cao, Y.; Chen, X. Role of multifaceted regulators in cancer glucose metabolism and their clinical significance. Oncotarget 2016, 7, 31572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Laher, I. Systems Biology of Free Radicals and Antioxidants. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Bhattacharya, A.; Mirzaei, R.; Rawji, K.S.; Ahn, Y.; Rho, J.M.; Yong, V.W. Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. J. Clin. Investig. 2019, 129, 3277–3292. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Cai, W.-W.; Yu, Y.; Zong, S.-Y.; Wei, F. Metabolic reprogramming as a key regulator in the pathogenesis of rheumatoid arthritis. Inflamm. Res. 2020, 69, 1087–1101. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Yang, J.J.; Liu, X.; Liu, Z.-R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 2012, 45, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Li, Z.; Li, H.; Lu, Y.; Wu, H.; Li, Z. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell. Signal. 2015, 27, 1525–1532. [Google Scholar] [CrossRef]
- Yang, P.; Li, Z.; Fu, R.; Wu, H.; Li, Z. Pyruvate kinase M2 facilitates colon cancer cell migration via the modulation of STAT3 signalling. Cell. Signal. 2014, 26, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Soler Palacios, B.; Estrada-Capetillo, L.; Izquierdo, E.; Criado, G.; Nieto, C.; Municio, C.; González-Alvaro, I.; Sánchez-Mateos, P.; Pablos, J.L.; Corbí, A.L. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J. Pathol. 2015, 235, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Witmer, M.D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl. Acad. Sci. USA 1978, 75, 5132–5136. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, S.; Merlin, J.; Lee, M.K.S.; Murphy, A.J.; Guinamard, R.R. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis 2018, 271, 102–110. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: Relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tel, J.; Lambeck, A.J.A.; Cruz, L.J.; Tacken, P.J.; de Vries, I.J.M.; Figdor, C.G. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J. Immunol. 2010, 184, 4276–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, T.L.; Grajales-Reyes, G.E.; Wu, X.; Tussiwand, R.; Briseño, C.G.; Iwata, A.; Kretzer, N.M.; Durai, V.; Murphy, K.M. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 2016, 34, 93–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M. Origin of the lamina propria dendritic cell network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Varol, C.; Vallon-Eberhard, A.; Elinav, E.; Aychek, T.; Shapira, Y.; Luche, H.; Fehling, H.J.; Hardt, W.-D.; Shakhar, G.; Jung, S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 2009, 31, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.Y.J.; Ysebaert, D.; Berneman, Z.N.; Cools, N. Dendritic cells: Cellular mediators for immunological tolerance. Clin. Dev. Immunol. 2013, 2013, 972865. [Google Scholar] [CrossRef]
- Boer, M.C.; Joosten, S.A.; Ottenhoff, T.H.M. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front. Immunol. 2015, 6, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin-Cocon, L.; Aublin-Gex, A.; Sestito, S.E.; Shirey, K.A.; Patel, M.C.; André, P.; Blanco, J.C.; Vogel, S.N.; Peri, F.; Lotteau, V. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Perrin-Cocon, L.; Agaugue, S.; Diaz, O.; Vanbervliet, B.; Dollet, S.; Guironnet-Paquet, A.; Andre, P.; Lotteau, V. Th1 disabled function in response to TLR4 stimulation of monocyte-derived DC from patients chronically-infected by hepatitis C virus. PLoS ONE 2008, 3, e2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, K.-U. Oxygen sensing and cell metabolism in inflammation. Nat. Rev. Nephrol. 2017, 13, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Zezina, E.; Sercan-Alp, O.; Herrmann, M.; Biesemann, N. Glucose transporter 1 in rheumatoid arthritis and autoimmunity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1483. [Google Scholar] [CrossRef]
- O’Neill, L.A.J. Glycolytic reprogramming by TLRs in dendritic cells. Nat. Immunol. 2014, 15, 314–315. [Google Scholar] [CrossRef]
- Malinarich, F.; Duan, K.; Hamid, R.A.; Bijin, A.; Lin, W.X.; Poidinger, M.; Fairhurst, A.-M.; Connolly, J.E. High Mitochondrial Respiration and Glycolytic Capacity Represent a Metabolic Phenotype of Human Tolerogenic Dendritic Cells. J. Immunol. 2015, 194, 5174. [Google Scholar] [CrossRef] [Green Version]
- Ritprajak, P.; Kaewraemruaen, C.; Hirankarn, N. Current paradigms of tolerogenic dendritic cells and clinical implications for systemic lupus erythematosus. Cells 2019, 8, 1291. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Pierangeli, S.S.; Papalardo, E.; Ansari, G.A.S.; Khan, M.F. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: Correlation with disease activity. Arthritis Rheum. 2010, 62, 2064–2072. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Mir, A.R.; Islam, S.; Alam, K.; Ali, A. Immunochemical studies on HNE-modified HSA: Anti-HNE–HSA antibodies as a probe for HNE damaged albumin in SLE. Int. J. Biol. Macromol. 2016, 86, 145–154. [Google Scholar] [CrossRef]
- Wang, G.; Pierangeli, S.S.; Willis, R.; Gonzalez, E.B.; Petri, M.; Khan, M.F. Significance of lipid-derived reactive aldehyde-specific immune complexes in systemic lupus erythematosus. PLoS ONE 2016, 11, e0164739. [Google Scholar] [CrossRef] [PubMed]
- Leishangthem, B.D.; Sharma, A.; Bhatnagar, A. Role of altered mitochondria functions in the pathogenesis of systemic lupus erythematosus. Lupus 2016, 25, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic abnormalities and oxidative stress in lupus. Curr. Opin. Rheumatol. 2017, 29, 442. [Google Scholar] [CrossRef] [PubMed]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Means, T.K.; Latz, E.; Hayashi, F.; Murali, M.R.; Golenbock, D.T.; Luster, A.D. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Investig. 2005, 115, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.M.; Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004, 75, 995–1000. [Google Scholar] [CrossRef]
- Zhang, B.; Angelidou, A.; Alysandratos, K.-D.; Vasiadi, M.; Francis, K.; Asadi, S.; Theoharides, A.; Sideri, K.; Lykouras, L.; Kalogeromitros, D. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J. Neuroinflamm. 2010, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Athale, S.; Domic, B.; Murat, E.; Chandra, M.; Banchereau, R.; Baisch, J.; Phelps, K.; Clayton, S.; Gong, M. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016, 213, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.L.; Holt, G.E.; Lu, H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert Rev. Clin. Pharmacol. 2011, 4, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 2016, 12, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oaks, Z.; Winans, T.; Caza, T.; Fernandez, D.; Liu, Y.; Landas, S.K.; Banki, K.; Perl, A. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol. 2016, 68, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.; Bonilla, E.; Mirza, N.; Niland, B.; Perl, A. Rapamycin reduces disease activity and normalizes T cell activation–induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2983–2988. [Google Scholar] [CrossRef] [Green Version]
- Oaks, Z.; Winans, T.; Huang, N.; Banki, K.; Perl, A. Activation of the mechanistic target of rapamycin in SLE: Explosion of evidence in the last five years. Curr. Rheumatol. Rep. 2016, 18, 73. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, F.; Heit, A.; Dreher, S.; Eisenächer, K.; Mages, J.; Haas, T.; Krug, A.; Janssen, K.P.; Kirschning, C.J.; Wagner, H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 2008, 38, 2981–2992. [Google Scholar] [CrossRef]
- Cao, W.; Manicassamy, S.; Tang, H.; Kasturi, S.P.; Pirani, A.; Murthy, N.; Pulendran, B. Toll-like receptor–mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI (3) K-mTOR-p70S6K pathway. Nat. Immunol. 2008, 9, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Shimabukuro-Demoto, S.; Yoshida-Sugitani, R.; Furuyama-Tanaka, K.; Karyu, H.; Sugiura, Y.; Shimizu, Y.; Hosaka, T.; Goto, M.; Kato, N. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity 2014, 41, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; Facchinetti, V.; Voynova, E.; Hanabuchi, S.; Karnell, J.L.; Hanna, R.N.; Kolbeck, R.; Sanjuan, M.A.; Ettinger, R.; Liu, Y.-J. Galectin-9 inhibits TLR7-mediated autoimmunity in murine lupus models. J. Clin. Investig. 2018, 128, 1873–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Z.; Ke, Y.; Li, S.; Su, D.; Gan, C.; Hu, L.; Zhao, X.; Gao, B.; Song, Y.; Zhou, D.; et al. Pretreatment with Retro-2 protects cells from death caused by ricin toxin by retaining the capacity of protein synthesis. J. Appl. Toxicol. 2020, 40, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Dale, D.C.; Boxer, L.; Liles, W.C. The phagocytes: Neutrophils and monocytes. Blood J. Am. Soc. Hematol. 2008, 112, 935–945. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Rahman, F.Z.; Hayee, B.H.; Graham, S.J.; Marks, D.J.B.; Sewell, G.W.; Palmer, C.D.; Wilde, J.; Foxwell, B.M.J.; Gloger, I.S. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 2009, 206, 1883–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent pro-and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Furlan, R.; Cuomo, C.; Martino, G. Animal Models of Multiple Sclerosis. In Neural Cell Transplantation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 157–173. [Google Scholar]
- Jansen, A.; Homo-Delarche, F.; Hooijkaas, H.; Leenen, P.J.; Dardenne, M.; Drexhage, H.A. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 1994, 43, 667–675. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Herrada, A.A.; Escobedo, N.; Iruretagoyena, M.; Valenzuela, R.A.; Burgos, P.I.; Cuitino, L.; Llanos, C. Innate immune cells’ contribution to systemic lupus erythematosus. Front. Immunol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.W.; Quartier, P.; Botto, M.; Fossati-Jimack, L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann. Rheum. Dis. 2006, 65, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labonte, A.C.; Kegerreis, B.; Geraci, N.S.; Bachali, P.; Madamanchi, S.; Robl, R.; Catalina, M.D.; Lipsky, P.E.; Grammer, A.C. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS ONE 2018, 13, e0208132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Yang, Y.; Zhu, X.; Huang, L.; Xu, J. Macrophage polarization modulates development of systemic lupus erythematosus. Cell. Physiol. Biochem. 2015, 37, 1279–1288. [Google Scholar] [CrossRef]
- Mohammadi, S.; Saghaeian-Jazi, M.; Sedighi, S.; Memarian, A. Immunomodulation in systemic lupus erythematosus: Induction of M2 population in monocyte-derived macrophages by pioglitazone. Lupus 2017, 26, 1318–1327. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Onuora, S. Anti-TNF agents go head-to-head. Nat. Rev. Rheumatol. 2017, 13, 2. [Google Scholar] [CrossRef]
- Kinne, R.W.; Stuhlmüller, B.; Burmester, G.-R. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res. Ther. 2007, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Mulherin, D.; Fitzgerald, O.; Bresnihan, B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1996, 39, 115–124. [Google Scholar] [CrossRef]
- Smiljanovic, B.; Grützkau, A.; Sörensen, T.; Grün, J.R.; Vogl, T.; Bonin, M.; Schendel, P.; Stuhlmüller, B.; Claussnitzer, A.; Hermann, S. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, P.J.; Williams, A.S.; Goodfellow, R.M.; Williams, B.D. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis. Rheumatology 1999, 38, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambarus, C.A.; Noordenbos, T.; de Hair, M.J.H.; Tak, P.P.; Baeten, D.L.P. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chang, Y.; Wei, W. Endothelial dysfunction and inflammation: Immunity in rheumatoid arthritis. Mediat. Inflamm. 2016, 2016, 6813016. [Google Scholar] [CrossRef] [Green Version]
- Rohde, G.; Message, S.D.; Haas, J.J.; Kebadze, T.; Parker, H.; Laza-Stanca, V.; Khaitov, M.R.; Kon, O.M.; Stanciu, L.A.; Mallia, P. CXC chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin. Exp. Allergy 2014, 44, 930–939. [Google Scholar] [CrossRef] [Green Version]
- Tosiek, M.J.; Fiette, L.; El Daker, S.; Eberl, G.; Freitas, A.A. IL-15-dependent balance between Foxp3 and RORγt expression impacts inflammatory bowel disease. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Cholesterol and atherosclerosis. Biochim. Biophys. Acta 2000, 1529, 299–309. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Keping, Y.; Yunfeng, S.; Pengzhuo, X.; Liang, L.; Chenhong, X.; Jinghua, M. Sestrin1 inhibits oxidized low-density lipoprotein-induced activation of NLRP3 inflammasome in macrophages in a murine atherosclerosis model. Eur. J. Immunol. 2020, 50, 1154–1166. [Google Scholar] [CrossRef]
- Van Tits, L.J.H.; Stienstra, R.; Van Lent, P.L.; Netea, M.G.; Joosten, L.A.B.; Stalenhoef, A.F.H. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: A crucial role for Krüppel-like factor 2. Atherosclerosis 2011, 214, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, K.; Iwabuchi, K.; Shimada, K.; Kiyanagi, T.; Iwahara, C.; Nakayama, H.; Daida, H. Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis. 2011, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Lee, J.H.; Choi, S.H.; Kim, S.; Almazan, F.; Witztum, J.L.; Miller, Y.I. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4–and spleen tyrosine kinase–dependent activation of NADPH oxidase 2. Circ. Res. 2009, 104, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Touitou, I.; Koné-Paut, I. Autoinflammatory diseases. Best Pract. Res. Clin. Rheumatol. 2008, 22, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 291–301. [Google Scholar] [CrossRef]
- Kubota, K.; Ito, K.; Morooka, M.; Mitsumoto, T.; Kurihara, K.; Yamashita, H.; Takahashi, Y.; Mimori, A. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann. Nucl. Med. 2009, 23, 783–791. [Google Scholar] [CrossRef]
- Trebst, C.; Sørensen, T.L.; Kivisäkk, P.; Cathcart, M.K.; Hesselgesser, J.; Horuk, R.; Sellebjerg, F.; Lassmann, H.; Ransohoff, R.M. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am. J. Pathol. 2001, 159, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 318, 1–7. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Iqbal, A.J. Epigenetic control of macrophage polarisation and soluble mediator gene expression during inflammation. Mediat. Inflamm. 2016, 2016, 6591703. [Google Scholar] [CrossRef] [Green Version]
- Juhas, U.; Ryba-Stanisławowska, M.; Szargiej, P.; Myśliwska, J. Different pathways of macrophage activation and polarization. Adv. Hyg. Exp. Med./Postepy Hig. I Med. Dosw. 2015, 69, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and Crohn disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klüter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, U.D.T.; Tran, T.X.; Nguyen, T.V. Discordance in the diagnosis of diabetes: Comparison between HbA1c and fasting plasma glucose. PLoS ONE 2017, 12, e0182192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Jiménez, A.; Peón, A.N.; Terrazas, L.I. Alternatively activated macrophages in types 1 and 2 diabetes. Mediat. Inflamm. 2012, 2012, 815953. [Google Scholar] [CrossRef]
- Torres-Castro, I.; Arroyo-Camarena, Ú.D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016, 176, 81–89. [Google Scholar] [CrossRef]
- Wen, Y.; Gu, J.; Li, S.-L.; Reddy, M.A.; Natarajan, R.; Nadler, J.L. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 2006, 147, 2518–2525. [Google Scholar] [CrossRef]
- Hill, D.A.; Lim, H.-W.; Kim, Y.H.; Ho, W.Y.; Foong, Y.H.; Nelson, V.L.; Nguyen, H.C.B.; Chegireddy, K.; Kim, J.; Habertheuer, A. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E5096–E5105. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, S.; Wilson, A.; Akhter, N.; Shenouda, S.; Kochumon, S.; Behbehani, K.; Ahmad, R. Increased adipose tissue expression of Toll-like receptor (TLR)-7 in obese individuals: Significance in metabolic disease. J. Glycom. Lipidom. 2015, 5, 1. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The macrophage switch in obesity development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, J.; Goh, K.P.; Abbasi, A. Exercise and adipose tissue macrophages: New frontiers in obesity research? Front. Endocrinol. 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Kanter, J.E.; Kramer, F.; Barnhart, S.; Shen, X.; Vivekanandan-Giri, A.; Wall, V.Z.; Kowitz, J.; Devaraj, S.; O’Brien, K.D. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014, 7, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [Green Version]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chen, F.; Zhang, X.; Clinton, S.K.; Tang, X.; Sun, Z.; Chen, T. Suppression of oxidative stress and NFκB/MAPK signaling by lyophilized black raspberries for esophageal cancer prevention in rats. Nutrients 2017, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Marée, A.F.M.; Komba, M.; Finegood, D.T.; Edelstein-Keshet, L. A quantitative comparison of rates of phagocytosis and digestion of apoptotic cells by macrophages from normal (BALB/c) and diabetes-prone (NOD) mice. J. Appl. Physiol. 2008, 104, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.L.; Al-Hassi, H.O.; Rigby, R.J.; Bell, S.J.; Emmanuel, A.V.; Knight, S.C.; Kamm, M.A.; Stagg, A.J. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology 2005, 129, 50–65. [Google Scholar] [CrossRef]
- Chirdo, F.G.; Millington, O.R.; Beacock-Sharp, H.; Mowat, A.M. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 2005, 35, 1831–1840. [Google Scholar] [CrossRef]
- Bernardo, D.; Chaparro, M.; Gisbert, J.P. Human intestinal dendritic cells in inflammatory bowel diseases. Mol. Nutr. Food Res. 2018, 62, 1700931. [Google Scholar] [CrossRef]
- Middel, P.; Raddatz, D.; Gunawan, B.; Haller, F.; Radzun, H.-J. Increased number of mature dendritic cells in Crohn’s disease: Evidence for a chemokine mediated retention mechanism. Gut 2006, 55, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, H.; Kayama, H.; Nishimura, J.; Sekido, Y.; Osawa, H.; Barman, S.; Ogino, T.; Takahashi, H.; Haraguchi, N.; Hata, T. CD103+ dendritic cell function is altered in the colons of patients with ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 1524–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Abdel-Ghaffar, K.A.; Gamal, A.Y.; Baban, B.; Cutler, C.W. Blood dendritic cells:“canary in the coal mine” to predict chronic inflammatory disease? Front. Microbiol. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porsche, C.E.; Delproposto, J.B.; Patrick, E.; Zamarron, B.F.; Lumeng, C.N. Adipose tissue dendritic cell signals are required to maintain T cell homeostasis and obesity-induced expansion. Mol. Cell. Endocrinol. 2020, 505, 110740. [Google Scholar] [CrossRef]
- Ferris, S.T.; Carrero, J.A.; Mohan, J.F.; Calderon, B.; Murphy, K.M.; Unanue, E.R. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 2014, 41, 657–669. [Google Scholar] [CrossRef] [Green Version]
- De Laere, M.; Berneman, Z.N.; Cools, N. To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Sagar, D.; Lamontagne, A.; Foss, C.A.; Khan, Z.K.; Pomper, M.G.; Jain, P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood–brain barrier through paracellular transmigration and ERK activation. J. Neuroinflamm. 2012, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Rönnblom, L.; Eloranta, M.-L. The interferon signature in autoimmune diseases. Curr. Opin. Rheumatol. 2013, 25, 248–253. [Google Scholar] [CrossRef]
- Rönnblom, L.; Alm, G.V. A pivotal role for the natural interferon α–producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001, 194, F59–F64. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lövgren, T.; Eloranta, M.L.; Båve, U.; Alm, G.V.; Rönnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2004, 50, 1861–1872. [Google Scholar] [CrossRef]
- Barrat, F.J.; Meeker, T.; Gregorio, J.; Chan, J.H.; Uematsu, S.; Akira, S.; Chang, B.; Duramad, O.; Coffman, R.L. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005, 202, 1131–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, X.; Li, S.; Settlage, R.E.; Sun, S.; Ren, J.; Reihl, A.M.; Zhang, H.; Karyala, S.V.; Reilly, C.M.; Ahmed, S.A. Cutting edge: Plasmacytoid dendritic cells in late-stage lupus mice defective in producing IFN-α. J. Immunol. 2015, 195, 4578–4582. [Google Scholar] [CrossRef] [Green Version]
- Farkas, L.; Beiske, K.; Lund-Johansen, F.; Brandtzaeg, P.; Jahnsen, F.L. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001, 159, 237–243. [Google Scholar] [CrossRef]
- Rowland, S.L.; Riggs, J.M.; Gilfillan, S.; Bugatti, M.; Vermi, W.; Kolbeck, R.; Unanue, E.R.; Sanjuan, M.A.; Colonna, M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014, 211, 1977–1991. [Google Scholar] [CrossRef]
- You, S.; Chatenoud, L. Autoimmune diabetes: An overview of experimental models and novel therapeutics. Suppr. Regul. Immune Responses 2016, 1371, 117–142. [Google Scholar]
- Battaglia, M. Neutrophils and type 1 autoimmune diabetes. Curr. Opin. Hematol. 2014, 21, 8–15. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Y.; Xu, A.; Zhou, Z. Neutrophils in type 1 diabetes. J. Diabetes Investig. 2016, 7, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.L.; Chami, B.; Liu, Y.; Doyle, C.M.; El Kazzi, M.; Ahlenstiel, G.; Ahmad, G.; Pathma-Nathan, N.; Collins, G.; Toh, J. Neutrophil Extracellular Trap Density Increases With Increasing Histopathological Severity of Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 586–598. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, P.; Jackson, P.L.; Noerager, B.; Parker, S.; Dransfield, M.; Gaggar, A.; Blalock, J.E. N-α-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir. Res. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, R.I.; Ungurs, M.J.; Mumford, R.A.; Stockley, R.A. Aα-Val360: A marker of neutrophil elastase and COPD disease activity. Eur. Respir. J. 2013, 41, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Bilger, A.; Plowshay, J.; Ma, S. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget 2017, 8, 44266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, D.; Kagari, T.; Doi, H.; Shimozato, T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology 2006, 119, 195–202. [Google Scholar] [CrossRef]
- Odobasic, D.; Kitching, A.R.; Yang, Y.; O’Sullivan, K.M.; Muljadi, R.C.M.; Edgtton, K.L.; Tan, D.S.Y.; Summers, S.A.; Morand, E.F.; Holdsworth, S.R. Neutrophil myeloperoxidase regulates T-cell− driven tissue inflammation in mice by inhibiting dendritic cell function. Blood J. Am. Soc. Hematol. 2013, 121, 4195–4204. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [Green Version]
- Dzhagalov, I.; St. John, A.; He, Y.-W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 2007, 109, 1620–1626. [Google Scholar] [CrossRef] [Green Version]
- Barcellos-de-Souza, P.; Canetti, C.; Barja-Fidalgo, C.; Arruda, M.A. Leukotriene B4 inhibits neutrophil apoptosis via NADPH oxidase activity: Redox control of NF-κB pathway and mitochondrial stability. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Cross, A.; Barnes, T.; Bucknall, R.C.; Edwards, S.W.; Moots, R.J. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J. Leukoc. Biol. 2006, 80, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumble, J.M.; Huber, A.K.; Krishnamoorthy, G.; Srinivasan, A.; Giles, D.A.; Zhang, X.; Wang, L.; Segal, B.M. Neutrophil-related factors as biomarkers in EAE and MS. J. Exp. Med. 2015, 212, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minohara, M.; Matsuoka, T.; Li, W.; Osoegawa, M.; Ishizu, T.; Ohyagi, Y.; Kira, J.-i. Upregulation of myeloperoxidase in patients with opticospinal multiple sclerosis: Positive correlation with disease severity. J. Neuroimmunol. 2006, 178, 156–160. [Google Scholar] [CrossRef]
- Blagih, J.; Jones, R.G. Polarizing Macrophages through Reprogramming of Glucose Metabolism. Cell Metab. 2012, 15, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Haschemi, A.; Kosma, P.; Gille, L.; Evans, C.R.; Burant, C.F.; Starkl, P.; Knapp, B.; Haas, R.; Schmid, J.A.; Jandl, C. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012, 15, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Yu, Y.; Kang, R.; Zhu, S.; Yang, L.; Zeng, L.; Sun, X.; Yang, M.; Billiar, T.R.; Wang, H. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Dey, T.; Mishra, S.P.; Pandey, U. Pyruvate kinase M2 and cancer: The role of PKM2 in promoting tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Lan, H.; Yu, Z.; Wang, M.; Wang, S.; Chen, Y.; Rao, H.; Li, J.; Sheng, Z.; Shao, J. Blockage of glycolysis by targeting PFKFB3 alleviates sepsis-related acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2017, 491, 522–529. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, P.; Jiang, Z.; Bo, L.; Bian, J. Dexmedetomidine inhibits LPS-induced proinflammatory responses via suppressing HIF1α-dependent glycolysis in macrophages. Aging 2020, 12, 9534–9548. [Google Scholar] [CrossRef]
- Zhai, G.-Y.; Qie, S.-Y.; Guo, Q.-Y.; Qi, Y.; Zhou, Y.-J. sDR5-Fc inhibits macrophage M1 polarization by blocking the glycolysis. J. Geriatr. Cardiol. 2021, 18, 271–280. [Google Scholar] [CrossRef]
- Graves, J.D.; Kordich, J.J.; Huang, T.-H.; Piasecki, J.; Bush, T.L.; Sullivan, T.; Foltz, I.N.; Chang, W.; Douangpanya, H.; Dang, T. Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity. Cancer Cell 2014, 26, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.; Cudrici, C.; Zernetkina, V.; Niculescu, F.; Rus, H.; Drachenberg, C.; Rus, V. TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin. Immunol. 2009, 132, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hsu, H.-C.; Mountz, J.D. The Dynamic Duo–Inflammatory M1 macrophages and Th17 cells in Rheumatic Diseases. J. Orthop. Rheumatol. 2013, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basit, F.; Mathan, T.; Sancho, D.; de Vries, I.J.M. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front. Immunol. 2018, 9, 2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, P.G.; Satani, N.; Maxwell, D.; Lin, Y.-H.; Hammoudi, N.; Peng, Z.; Pisaneschi, F.; Link, T.M.; Lee, G.R.; Sun, D.; et al. SF2312 is a natural phosphonate inhibitor of enolase. Nat. Chem. Biol. 2016, 12, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Llanos, C.; Carreño, L.J.; Kalergis, A.M. Contribution of dendritic cell/T cell interactions to triggering and maintaining autoimmunity. Biol. Res. 2011, 44, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Vander Lugt, B.; Riddell, J.; Khan, A.A.; Hackney, J.A.; Lesch, J.; DeVoss, J.; Weirauch, M.T.; Singh, H.; Mellman, I. Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J. Cell Biol. 2017, 216, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Rutella, S.; Danese, S.; Leone, G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood 2006, 108, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Mansilla, M.J.; Sellès-Moreno, C.; Fàbregas-Puig, S.; Amoedo, J.; Navarro-Barriuso, J.; Teniente-Serra, A.; Grau-López, L.; Ramo-Tello, C.; Martínez-Cáceres, E.M. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2015, 21, 222–230. [Google Scholar] [CrossRef]
- Papenfuss, T.L.; Powell, N.D.; McClain, M.A.; Bedarf, A.; Singh, A.; Gienapp, I.E.; Shawler, T.; Whitacre, C.C. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 2011, 186, 3346–3355. [Google Scholar] [CrossRef]
- Dáňová, K.; Grohová, A.; Strnadová, P.; Funda, D.P.; Šumník, Z.; Lebl, J.; Cinek, O.; Průhová, Š.; Koloušková, S.; Obermannová, B. Tolerogenic dendritic cells from poorly compensated type 1 diabetes patients have decreased ability to induce stable antigen-specific T cell hyporesponsiveness and generation of suppressive regulatory T cells. J. Immunol. 2017, 198, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Barriuso, J.; Mansilla, M.J.; Quirant-Sánchez, B.; Ardiaca-Martínez, A.; Teniente-Serra, A.; Presas-Rodríguez, S.; ten Brinke, A.; Ramo-Tello, C.; Martínez-Cáceres, E.M. MAP7 and MUCL1 Are Biomarkers of Vitamin D3-Induced Tolerogenic Dendritic Cells in Multiple Sclerosis Patients. Front. Immunol. 2019, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L. Tolerogenic Dendritic Cells Induced by Vitamin D Receptor Ligands Enhance Regulatory T Cells Inhibiting Autoimmune Diabetes. Ann. N. Y. Acad. Sci. 2003, 987, 258–261. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.-M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Jauregui-Amezaga, A.; Cabezón, R.; Ramírez-Morros, A.; España, C.; Rimola, J.; Bru, C.; Pinó-Donnay, S.; Gallego, M.; Masamunt, M.C.; Ordás, I. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: A phase I study. J. Crohn’s Colitis 2015, 9, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borella, R.; De Biasi, S.; Paolini, A.; Boraldi, F.; Lo Tartaro, D.; Mattioli, M.; Fidanza, L.; Neroni, A.; Caro-Maldonado, A.; Meschiari, M.; et al. Metabolic reprograming shapes neutrophil functions in severe COVID-19. Eur. J. Immunol. 2022, 52, 484–502. [Google Scholar] [CrossRef]
- Willson, J.A.; Arienti, S.; Sadiku, P.; Reyes, L.; Coelho, P.; Morrison, T.; Rinaldi, G.; Dockrell, D.H.; Whyte, M.K.B.; Walmsley, S.R. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood 2022, 139, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sottnik, J.L.; Lori, J.C.; Rose, B.J.; Thamm, D.H. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin. Exp. Metastasis 2011, 28, 865–875. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [Green Version]
- Abboud, G.; Choi, S.-C.; Kanda, N.; Zeumer-Spataro, L.; Roopenian, D.C.; Morel, L. Inhibition of glycolysis reduces disease severity in an autoimmune model of rheumatoid arthritis. Front. Immunol. 2018, 9, 1973. [Google Scholar] [CrossRef]
- Raez, L.E.; Papadopoulos, K.; Ricart, A.D.; Chiorean, E.G.; DiPaola, R.S.; Stein, M.N.; Rocha Lima, C.M.; Schlesselman, J.J.; Tolba, K.; Langmuir, V.K.; et al. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-A.; Zhang, X.-D.; Guo, X.-Y.; Xian, S.-L.; Lu, Y.-F. 3-Bromopyruvate and sodium citrate target glycolysis, suppress survivin, and induce mitochondrial-mediated apoptosis in gastric cancer cells and inhibit gastric orthotopic transplantation tumor growth. Oncol. Rep. 2016, 35, 1287–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.-J.; Yang, H.-H.; Guan, X.-X.; Xiong, J.-B.; Sun, C.-C.; Zhang, C.-Y.; Luo, X.-Q.; Zhang, Y.-F.; Zhang, J.; Duan, J.-X.; et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell. Physiol. 2019, 234, 4641–4654. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qu, G.; Choi, S.-C.; Cornaby, C.; Titov, A.; Kanda, N.; Teng, X.; Wang, H.; Morel, L. Targeting T Cell Activation and Lupus Autoimmune Phenotypes by Inhibiting Glucose Transporters. Front. Immunol. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.A.; Sutphin, P.D.; Nguyen, P.; Turcotte, S.; Lai, E.W.; Banh, A.; Reynolds, G.E.; Chi, J.-T.; Wu, J.; Solow-Cordero, D.E. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 2011, 3, 94ra70. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cao, Y.; Zhang, W.; Bergmeier, S.; Qian, Y.; Akbar, H.; Colvin, R.; Ding, J.; Tong, L.; Wu, S. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.-W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: A single-arm, open-label, phase 1/2 trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Stathopoulou, C.; Nikoleri, D.; Bertsias, G. Immunometabolism: An overview and therapeutic prospects in autoimmune diseases. Immunotherapy 2019, 11, 813–829. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Lai, Z.-W.; Oaks, Z.; Kelly, R.; Borsuk, R.; Asara, J.M.; Phillips, P.E. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: Implications for activation of the mechanistic target of rapamycin. Metabolomics 2015, 11, 1157–1174. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Niu, H.; Yan, N.; Sun, X.; Li, Z.; Chen, M.; Gao, C.; Li, X.; Wang, C. FRI0063 Rapamycin induces remission in patients with refractory rheumatoid arthritis. BMJ 2018, 77, 578. [Google Scholar] [CrossRef]
- Hao, Z.; Miao, M.; Guo, Y.; Zhang, X.; Li, X.; Gao, C.; Wang, X. AB0536 Rapamycin attenuates symptom and restores the balance of th17/treg in refractory primary sjogren’s syndrome. BMJ 2018, 77, 1425. [Google Scholar] [CrossRef]
- Su, T.I.K.; Khanna, D.; Furst, D.E.; Danovitch, G.; Burger, C.; Maranian, P.; Clements, P.J. Rapamycin versus methotrexate in early diffuse systemic sclerosis: Results from a randomized, single-blind pilot study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Choi, S.-C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group, U.K.P.D.S. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar]
- Gray, O.; McDonnell, G.V.; Forbes, R.B. Methotrexate for multiple sclerosis. Cochrane Database Syst. Rev. 2004, 2004, CD003208. [Google Scholar] [CrossRef]
- Matsushita, M.; Pearce, E.J. Disrupting metabolism to treat autoimmunity. Science 2018, 360, 377–378. [Google Scholar] [CrossRef]
- Kaminskas, E.; Nussey, A.C. Effects of Methotrexate and of Environmental Factors on Glycolysis and Metabolic Energy State In Cultured Ehrlich Ascites Carcinoma Cells1. Cancer Res. 1978, 38, 2989–2996. [Google Scholar]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.S.; Rao, V.T.S.; Durafourt, B.A.; Bedell, B.J.; Ludwin, S.K.; Bar-Or, A.; Antel, J.P. miR-155 as a multiple sclerosis–relevant regulator of myeloid cell polarization. Ann. Neurol. 2013, 74, 709–720. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Metabolic Modulators | Target |
|---|---|
| CARKL | PPP, NF-kB-regulated cytokines |
| Shikonin | Glycolysis (PKM2) |
| 3PO | Glycolysis (PFKFB3) |
| DEX | Glycolysis, HIF-1α |
| FX11 | LDHA |
| sDR5-Fc | HK2 and GLUT1 |
| FP7 | TLR-4 |
| SF2312 | ENO2 |
| Chetomin | HIF-1α |
| 2DG | Glycolysis |
| iGP-1 | GPD2 |
| 3-BrPA | Glycolysis (HK) |
| SCT | Glycolysis (PFK-1) |
| STF-31 and WZB117 | GLUT1 |
| Rapamycin | PI3K/Akt/mTOR |
| NAC | mTORC1 |
| Met + 2DG | mTOR/Glycolysis |
| Methotrexate | mTOR |
| DMF | Glycolysis (GAPDH) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadnezhad, L.; Shekarkar Azgomi, M.; La Manna, M.P.; Sireci, G.; Rizzo, C.; Badami, G.D.; Tamburini, B.; Dieli, F.; Guggino, G.; Caccamo, N. Metabolic Reprogramming of Innate Immune Cells as a Possible Source of New Therapeutic Approaches in Autoimmunity. Cells 2022, 11, 1663. https://doi.org/10.3390/cells11101663
Mohammadnezhad L, Shekarkar Azgomi M, La Manna MP, Sireci G, Rizzo C, Badami GD, Tamburini B, Dieli F, Guggino G, Caccamo N. Metabolic Reprogramming of Innate Immune Cells as a Possible Source of New Therapeutic Approaches in Autoimmunity. Cells. 2022; 11(10):1663. https://doi.org/10.3390/cells11101663
Chicago/Turabian StyleMohammadnezhad, Leila, Mojtaba Shekarkar Azgomi, Marco Pio La Manna, Guido Sireci, Chiara Rizzo, Giusto Davide Badami, Bartolo Tamburini, Francesco Dieli, Giuliana Guggino, and Nadia Caccamo. 2022. "Metabolic Reprogramming of Innate Immune Cells as a Possible Source of New Therapeutic Approaches in Autoimmunity" Cells 11, no. 10: 1663. https://doi.org/10.3390/cells11101663
APA StyleMohammadnezhad, L., Shekarkar Azgomi, M., La Manna, M. P., Sireci, G., Rizzo, C., Badami, G. D., Tamburini, B., Dieli, F., Guggino, G., & Caccamo, N. (2022). Metabolic Reprogramming of Innate Immune Cells as a Possible Source of New Therapeutic Approaches in Autoimmunity. Cells, 11(10), 1663. https://doi.org/10.3390/cells11101663











