Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Maintenance
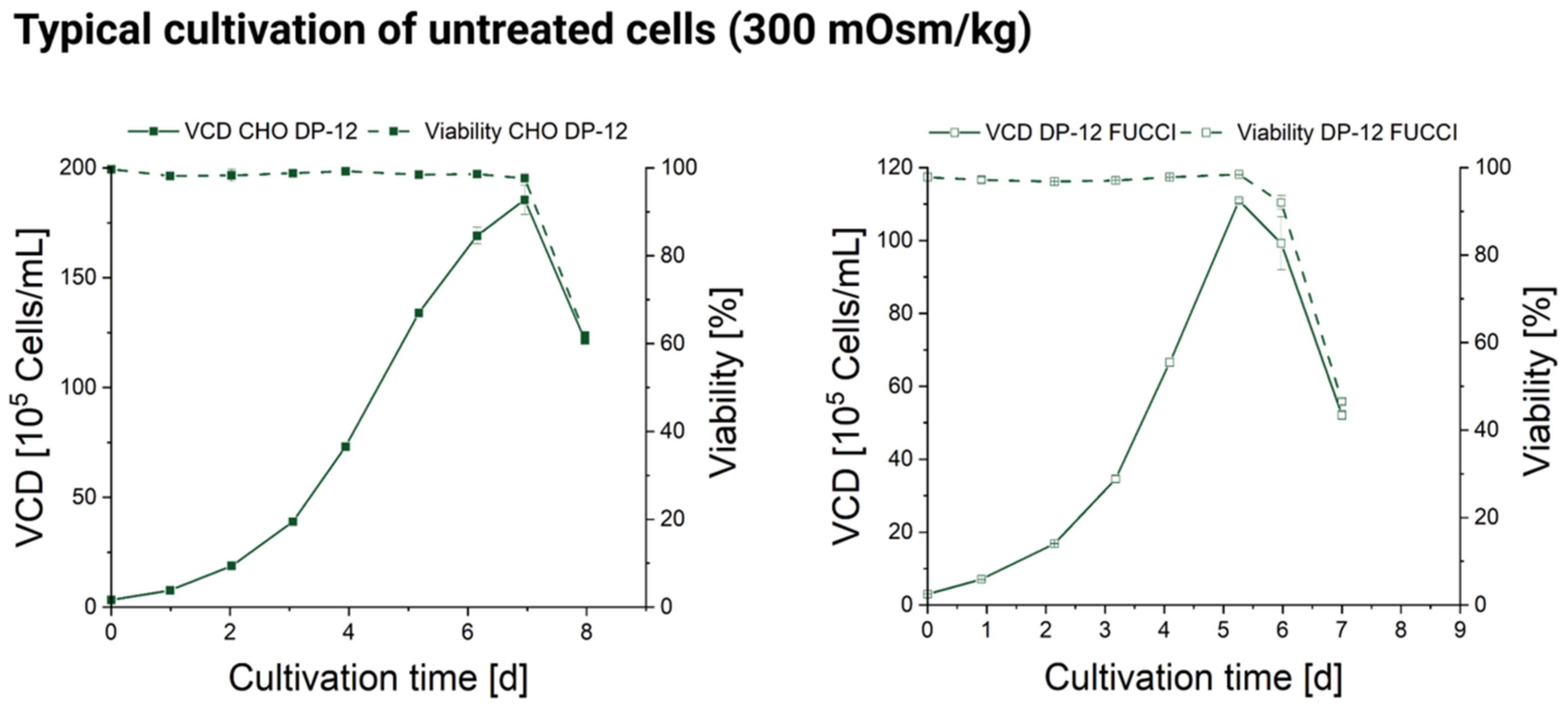
2.2. Stage Batch Cultivation in Shakers
2.3. Microfluidic Single-Cell Cultivation
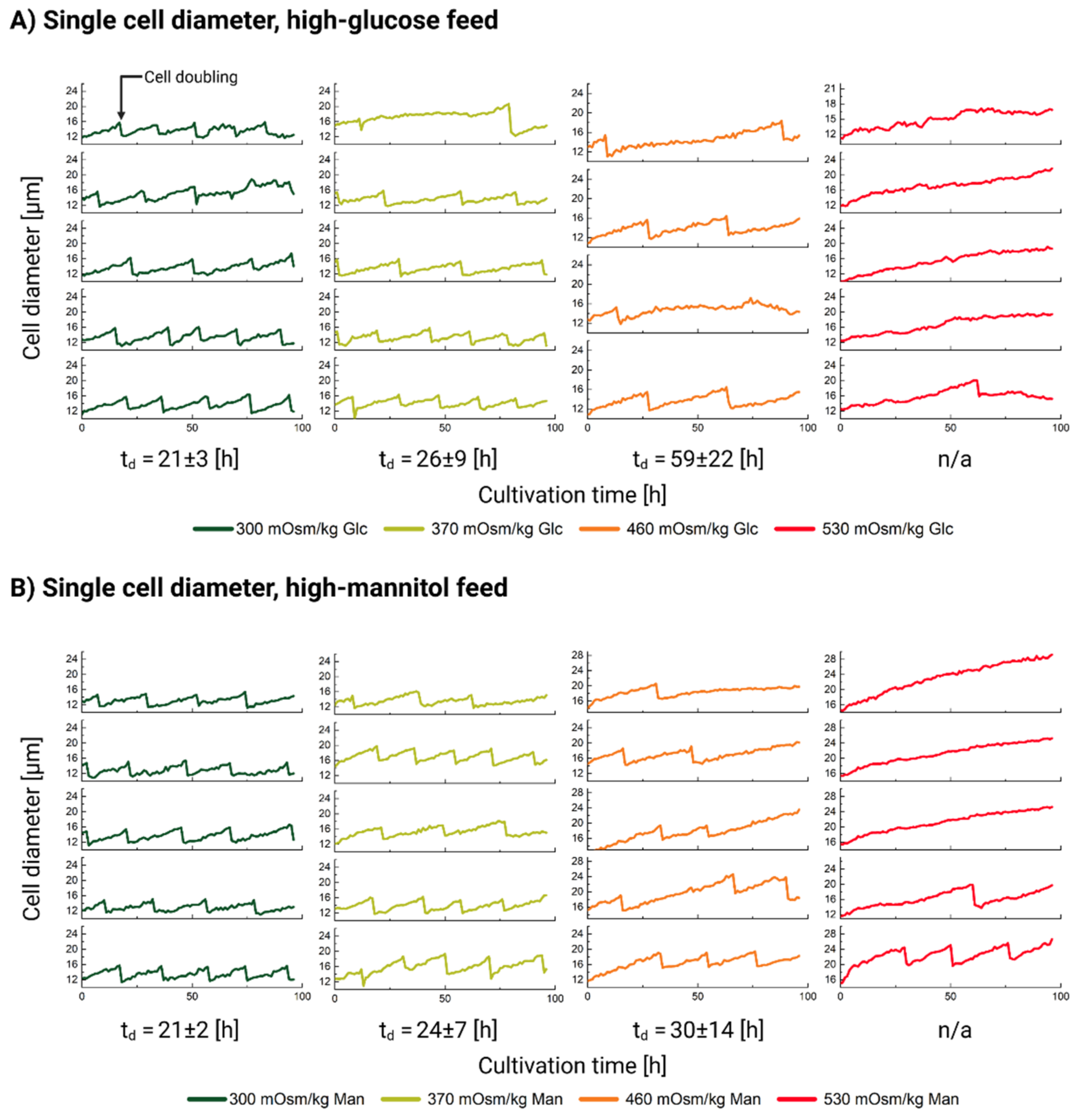
2.4. Single-Cell Diameter Analysis
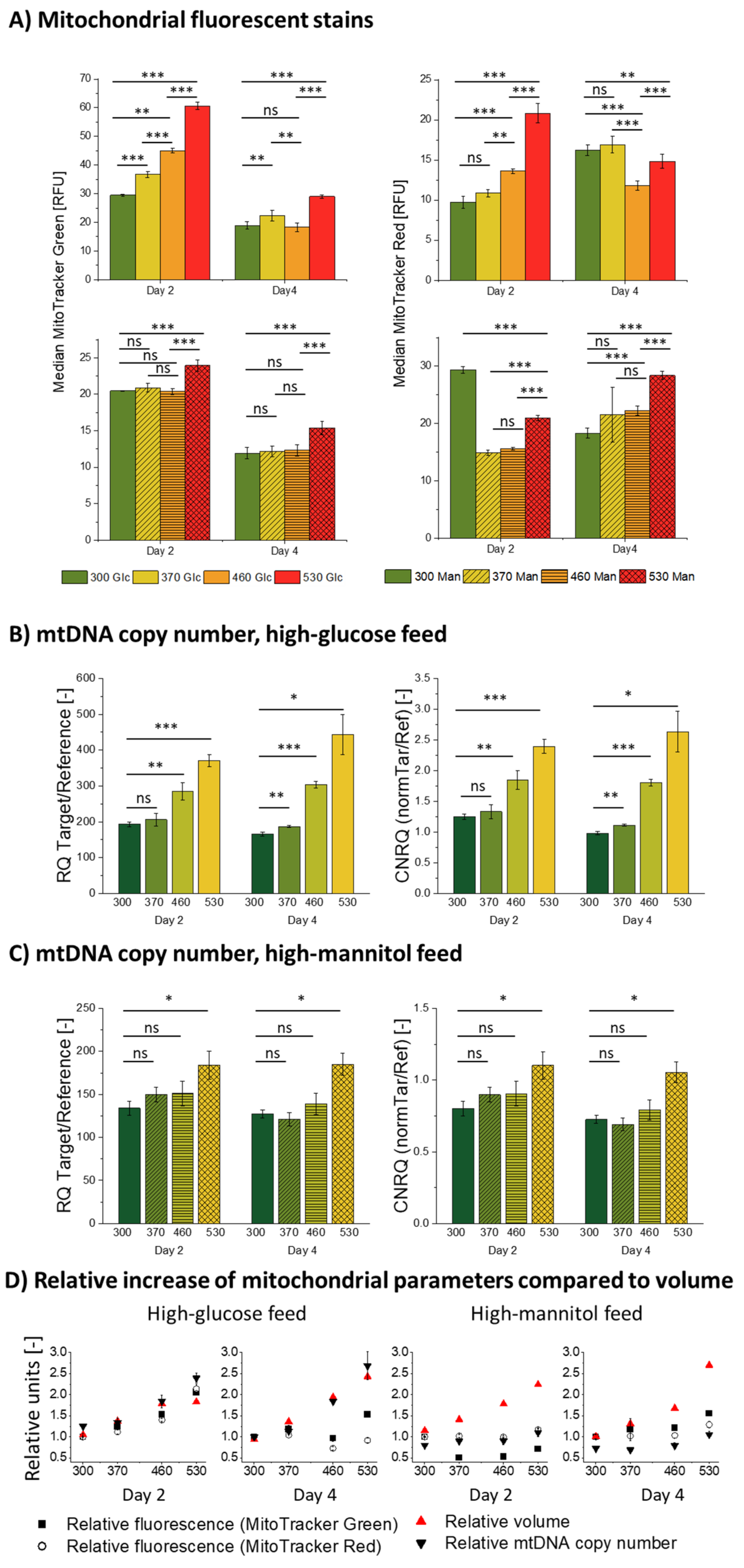
2.5. Mitochondrial Mass Assessment
2.6. Mitochondrial Activity Assessment
2.7. Relative mtDNA Copy-Number Analysis via qPCR
2.7.1. DNA Extraction
2.7.2. Primers
2.7.3. qPCR Settings and Experiment
2.7.4. Data Analysis
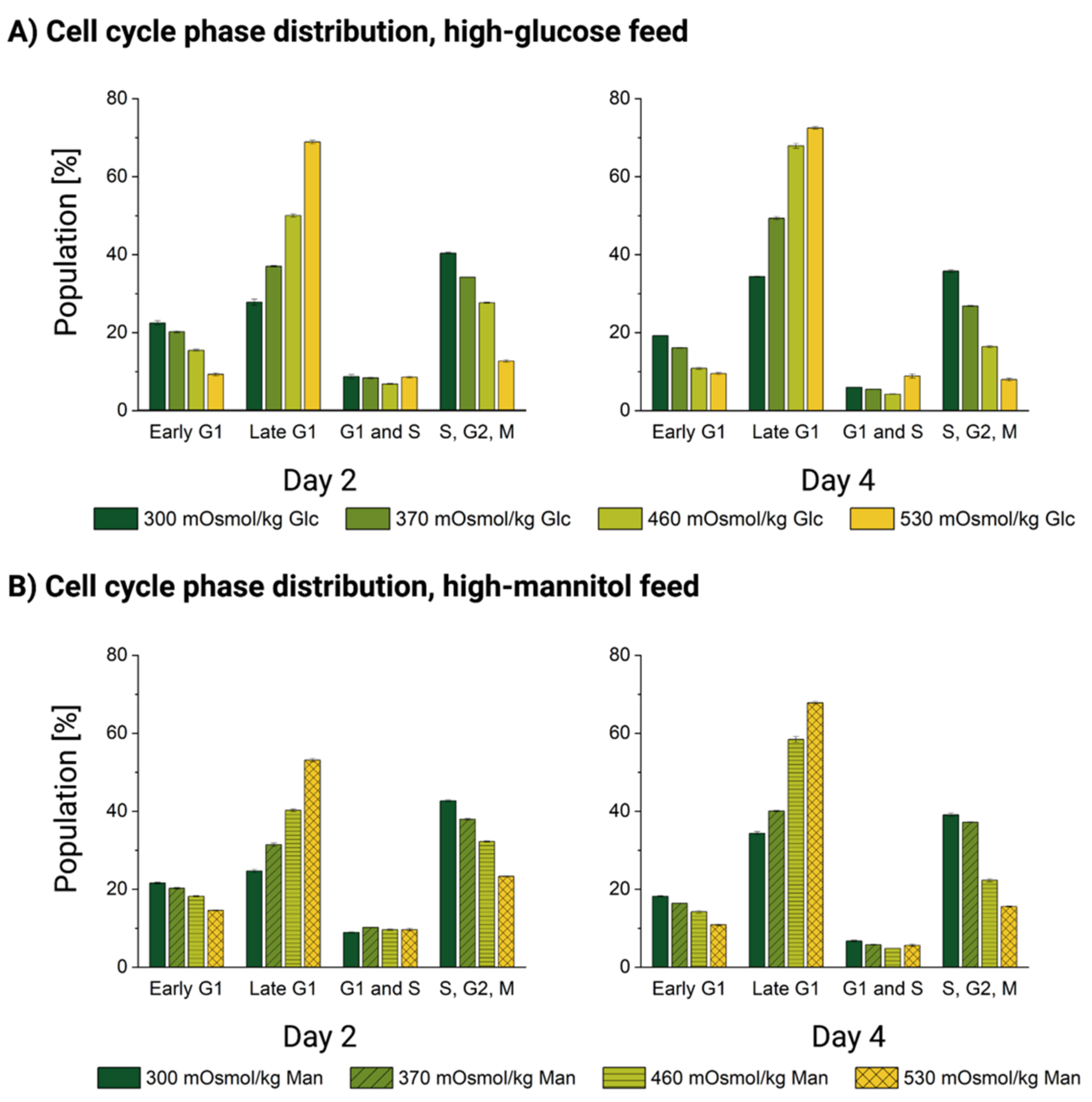
2.8. FUCCI-Transduced CHO DP-12 Cells-Based Cell Cycle Assessment
2.9. Flow Cytometry
2.10. Statistical Analysis
2.11. Growth Rate and Doubling Time (td) Calculation
3. Results and Discussion
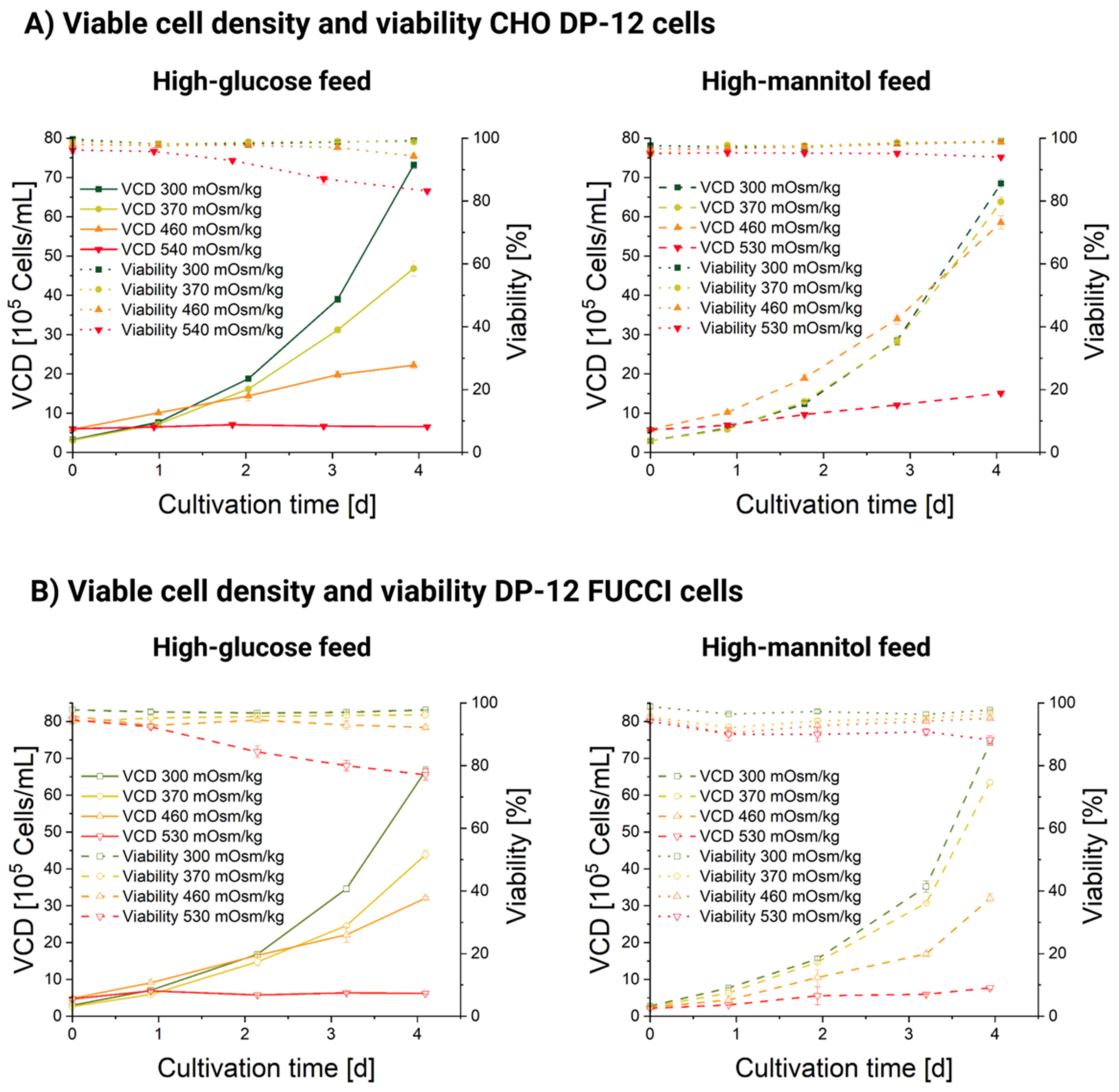
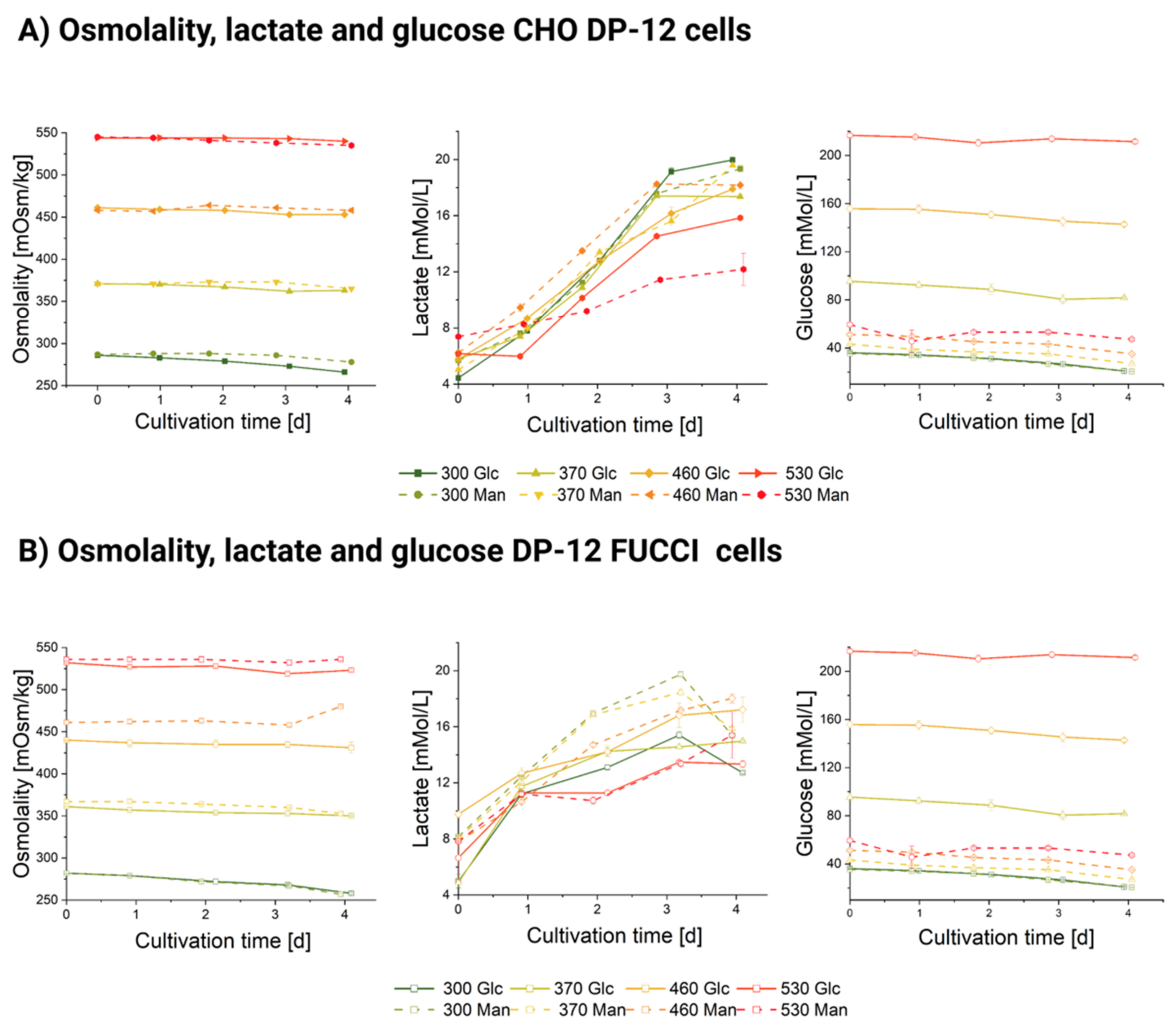
3.1. Influence of Hyperosmolality on Cell Growth
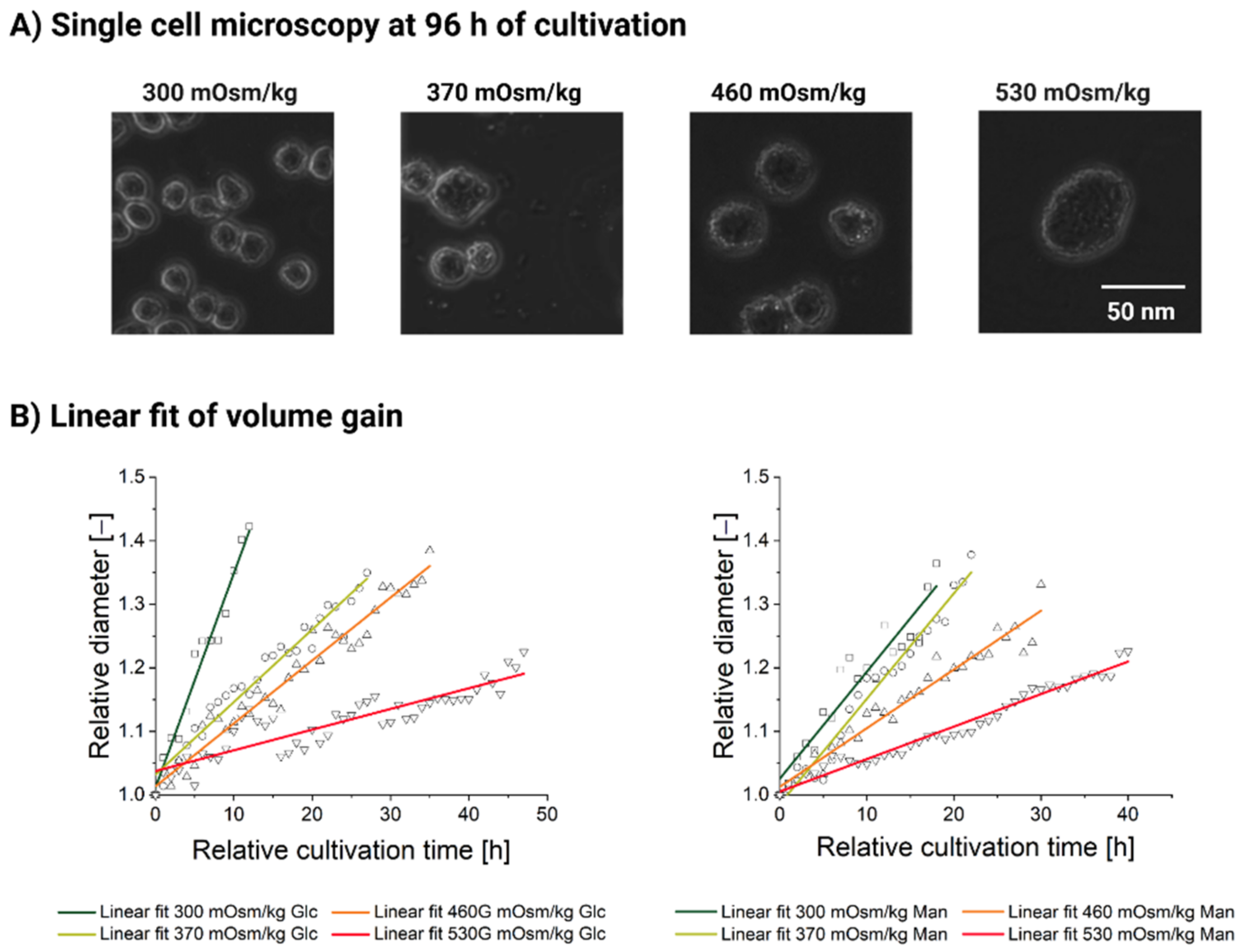
3.2. Influence of Hyperosmolality on Cell Diameter
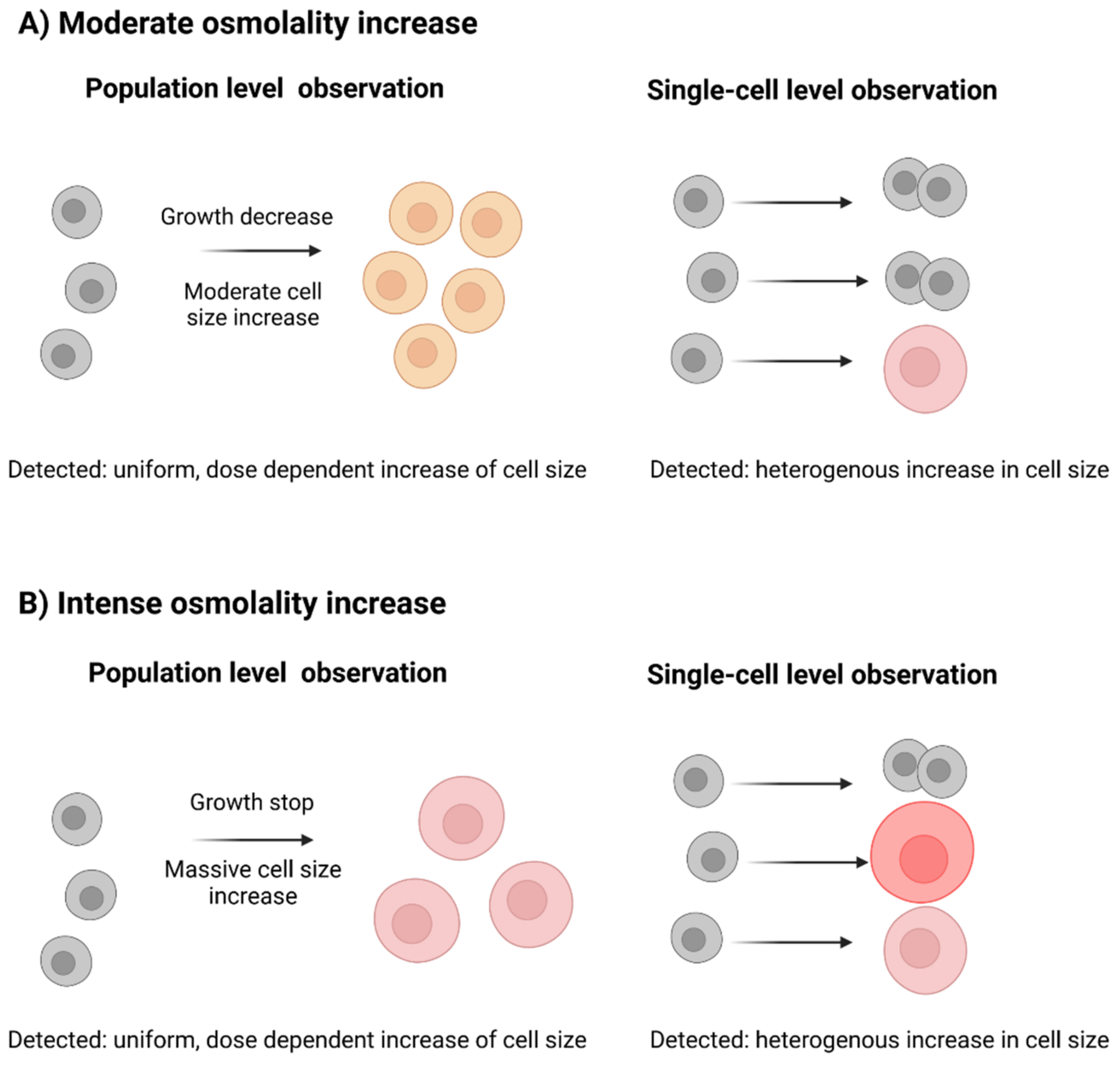
3.2.1. Populational Observation of Cell Diameter Dynamics
3.2.2. Single-Cell Analysis Reveals Heterogeneity in Response to Hyperosmolality
3.3. Influence of Hyperosmolality on Mitochondria
3.4. Cells Accumulate in the Late G1 Phase without Entering Mitosis upon Oversupplemented Feed Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Intercept | Intercept | Slope | Slope | Statistics | |
|---|---|---|---|---|---|
| Value | Std Error | Value | Std Error | Adj. R-Square | |
| Linear fit parameters, high-glucose feed | |||||
| 300 mOsm/kg | 1.01148 | 0.01283 | 0.03374 | 0.00181 | 0.96636 |
| 370 mOsm/kg | 1.03204 | 0.00772 | 0.01142 | 4.90 × 10−4 | 0.95248 |
| 460 mOsm/kg | 1.01358 | 0.00742 | 0.00991 | 3.64 × 10−4 | 0.9547 |
| 530 mOsm/kg | 1.03775 | 0.00653 | 0.00326 | 2.39 × 10−4 | 0.79675 |
| Linear fit parameters, high-mannitol feed | |||||
| 300 mOsm/kg | 1.02563 | 0.01405 | 0.01684 | 0.00133 | 0.89788 |
| 370 mOsm/kg | 1.05082 | 0.00945 | 0.01661 | 8.97 × 10−4 | 0.94995 |
| 460 mOsm/kg | 1.01276 | 0.00705 | 0.00924 | 4.04 × 10−4 | 0.94572 |
| 530 mOsm/kg | 1.00536 | 0.00343 | 0.00512 | 1.48 × 10−4 | 0.96777 |
References
- Sharker, S.M.; Rahman, M.A. Review of the current methods of Chinese hamster ovary (CHO) cells cultivation for production of therapeutic protein. Curr. Drug Discov. Technol. 2020, 18, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, P.; Junne, S. Scale-up and scale-down methodologies for bioreactors. In Bioreactors; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 323–354. [Google Scholar]
- Nienow, A.W.; Scott, W.H.; Hewitt, C.J.; Thomas, C.R.; Lewis, G.; Amanullah, A.; Kiss, R.; Meier, S.J. Scale-down studies for assessing the impact of different stress parameters on growth and product quality during animal cell culture. Chem. Eng. Res. Des. 2013, 91, 2265–2274. [Google Scholar] [CrossRef] [Green Version]
- Brunner, M.; Braun, P.; Doppler, P.; Posch, C.; Behrens, D.; Herwig, C.; Fricke, J. The impact of pH inhomogeneities on CHO cell physiology and fed-batch process performance–two-compartment scale-down modelling and intracellular pH excursion. Biotechnol. J. 2017, 12, 1600633. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, X.; Xia, Z.; Huang, Z.; Zhang, Y.; Wang, Y.; Fu, Q.; Zheng, C. The effect of hyperosmolality application time on production, quality, and biopotency of monoclonal antibodies produced in CHO cell fed-batch and perfusion cultures. Appl. Microbiol. Biotechnol. 2019, 103, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Koo, T.Y.; Lee, G.M. Enhanced interferon—Production by CHO cells through elevated osmolality and reduced culture temperature. Biotechnol. Prog. 2009, 25, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.K.; Hwang, S.-J.; Jeon, C.-J.; Lee, G.M.; Yoon, S.K. Use of NaCl prevents aggregation of recombinant COMPangiopoietin-1 in Chinese hamster ovary cells. J. Biotechnol. 2009, 143, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Lee, G.M. Response of recombinant Chinese hamster ovary cells to hyperosmotic pressure: Effect of bcl-2 overexpression. J. Biotechnol. 2002, 95, 237–248. [Google Scholar] [CrossRef]
- Ryu, J.S.; Lee, M.S.; Lee, G.M. Effects of cloned gene dosage on the response of recombinant CHO cells to hyperosmotic pressure in regard to cell growth and antibody production. Biotechnol. Prog. 2001, 17, 993–999. [Google Scholar] [CrossRef]
- deZengotita, V.M.; Schmelzer, A.E.; Miller, W.M. Characterization of hybridoma cell responses to elevated pCO2 and osmolality: Intracellular pH, cell size, apoptosis, and metabolism. Biotechnol. Bioeng. 2002, 77, 369–380. [Google Scholar] [CrossRef]
- Romanova, N.; Niemann, T.; Greiner, J.F.W.; Kaltschmidt, B.; Kaltschmidt, C.; Noll, T. Hyperosmolality in CHO culture: Effects on cellular behavior and morphology. Biotechnol. Bioeng. 2021, 118, 2348–2359. [Google Scholar] [CrossRef]
- Alhuthali, S.; Kotidis, P.; Kontoravdi, C. Osmolality effects on CHO cell growth, cell volume, antibody productivity and glycosylation. Int. J. Mol. Sci. 2021, 22, 3290. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, T.R.; Shen, D.; Khattak, S.F.; Li, Z.J.; Sharfstein, S.T. Observations of cell size dynamics under osmotic stress. Cytom. Part A 2011, 79A, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Dalm, C.; Wijffels, R.H.; Martens, D.E. Metabolic characterization of a CHO cell size increase phase in fed-batch cultures. Appl. Microbiol. Biotechnol. 2017, 101, 8101–8113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopman, W.J.H.; Verkaart, S.; Visch, H.J.; Vries, S.V.E.-D.; Nijtmans, L.G.J.; Smeitink, J.A.M.; Willems, P.H.G.M. Human NADH:ubiquinone oxidoreductase deficiency: Radical changes in mitochondrial morphology? Am. J. Physiol. Cell Physiol. 2007, 293, C22–C29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.H.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H2O2 signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Moreira, J.D.V.; De Staercke, L.; Martínez-Basilio, P.C.; Gauthier-Thibodeau, S.; Montégut, L.; Schwartz, L.; Jolicoeur, M. Hyperosmolarity triggers the Warburg effect in Chinese hamster ovary cells and reveals a reduced mitochondria horsepower. Metabolites 2021, 11, 344. [Google Scholar] [CrossRef]
- Eisner, V.; Picard, M.; Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018, 20, 755–765. [Google Scholar] [CrossRef]
- Grauso, M.; Lan, A.; Andriamihaja, M.; Bouillaud, F.; Blachier, F. Hyperosmolar environment and intestinal epithelial cells: Impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro. Sci. Rep. 2019, 9, 11360. [Google Scholar] [CrossRef]
- Küper, C.; Beck, F.-X.; Neuhofer, W. Osmoadaptation of mammalian cells—an orchestrated network of protective genes. Curr. Genom. 2007, 8, 209–218. [Google Scholar] [CrossRef]
- Badsha, M.B.; Kurata, H.; Onitsuka, M.; Oga, T.; Omasa, T. Metabolic analysis of antibody producing Chinese hamster ovary cell culture under different stresses conditions. J. Biosci. Bioeng. 2016, 122, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Kiehl, T.R.; Khattak, S.F.; Li, Z.J.; He, A.; Kayne, P.S.; Patel, V.; Neuhaus, I.M.; Sharfstein, S.T. Transcriptomic responses to sodium chloride-induced osmotic stress: A study of industrial fed-batch CHO cell cultures. Biotechnol. Prog. 2010, 26, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.I.; Cui, K.; Kitchaev, D.A.; Zhao, K.; Burg, M.B. DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc. Natl. Acad. Sci. USA 2011, 108, 20796–20801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kultz, D.; Chakravarty, D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1999–2004. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Alsayyari, A.A.; Dalm, C.; Hageman, J.; Wijffels, R.H.; Martens, D.E. Transcriptome analysis of CHO cell size increase during a fed-batch process. Biotechnol. J. 2019, 14, 1800156. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Abou-Mohamed, G.; Caldwell, R.W.; Caldwell, R.B. High glucose-induced tyrosine nitration in endothelial cells: Role of eNOS uncoupling and aldose reductase activation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3135. [Google Scholar] [CrossRef]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.-J.; De Caterina, R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 2016, 15, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Wijdicks, E.F.M. Finding the right osmotic agent: Why mannitol prevailed. Neurocrit. Care 2021, 1–4. [Google Scholar] [CrossRef]
- Altschuler, S.J.; Wu, L.F. Cellular heterogeneity: Do differences make a difference? Cell 2010, 141, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Delvigne, F.; Goffin, P. Microbial heterogeneity affects bioprocess robustness: Dynamic single-cell analysis contributes to understanding of microbial populations. Biotechnol. J. 2013, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Kuchemüller, K.B.; Steinmetz, T.; Koopmann, K.S.; Pörtner, R. Model-assisted design of experiments as a concept for knowledge-based bioprocess development. Bioprocess Biosyst. Eng. 2019, 42, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Kuang, B.; Yoon, S. Characterization of dynamic regulation in Chinese hamster ovary (CHO) cell cultures in the late exponential phase. Biochem. Eng. J. 2021, 167, 107897. [Google Scholar] [CrossRef]
- Schmitz, J.; Täuber, S.; Westerwalbesloh, C.; Lieres, E.; Noll, T.; Grünberger, A. Development and application of a cultivation platform for mammalian suspension cell lines with single-cell resolution. Biotechnol. Bioeng. 2020, 118, 992–1005. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Cottet-Rousselle, C.; Ronot, X.; Leverve, X.; Mayol, J.-F. Cytometric assessment of mitochondria using fluorescent probes. Cytom. Part A 2011, 79A, 405–425. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, T.; Fan, J.; Li, Z.; Jiang, N.; Wang, J.; Dou, B.; Sun, S.; Song, F.; Peng, X. A BODIPY-based fluorescent dye for mitochondria in living cells, with low cytotoxicity and high photostability. Org. Biomol Chem. 2013, 11, 555–558. [Google Scholar] [CrossRef]
- Bai, R.-K.; Wong, L.-J.C. A BODIPY-based fluorescent dye for mitochondria in living cells, with low cytotoxicity and high photostability. J. Mol. Diagn. 2005, 7, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Venegas, V.; Halberg, M.C. Measurement of mitochondrial DNA copy number. Methods Mol. Biol. 2012, 837, 327–335. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Svec, D.; Tichopad, A.; Novosadova, V.; Pfaffl, M.W.; Kubista, M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomol. Detect. Quantif. 2015, 3, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauklin, S.; Vallier, L. The cell-cycle state of stem cells determines cell fate propensity. Cell 2013, 155, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartley, F.; Walker, T.; Chung, V.; Morten, K. Mechanisms driving the lactate switch in Chinese hamster ovary cells. Biotechnol. Bioeng. 2018, 115, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Kaeidi, A.; Hajializadeh, Z.; Jahandari, F.; Fatemi, I. Leptin attenuates oxidative stress and neuronal apoptosis in hyperglycemic condition. Fundam. Clin. Pharmacol. 2018, 33, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Pfizenmaier, J.; Matuszczyk, J.-C.; Takors, R. Changes in intracellular ATP-content of CHO cells as response to hyperosmolality. Biotechnol. Prog. 2015, 31, 1212–1216. [Google Scholar] [CrossRef]
- Eveloff, J.L.; Warnock, D.G. Activation of ion transport systems during cell volume regulation. Am. J. Physiol. Ren. Physiol. 1987, 252, F1–F10. [Google Scholar] [CrossRef]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 377–383. [Google Scholar] [CrossRef]
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 2001, 532, 3–16. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, Y.-G.; Kim, J.Y.; Lee, G.M. Hyperosmotic stress induces autophagy and apoptosis in recombinant Chinese hamster ovary cell culture. Biotechnol. Bioeng. 2010, 105, 1187–1192. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.R.; Kim, Y.-G.; Lee, G.M. Understanding of decreased sialylation of fc-fusion protein in hyperosmotic recombinant Chinese hamster ovary cell culture: N -glycosylation gene expression and n -linked glycan antennary profile. Biotechnol. Bioeng. 2017, 114, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Takagi, M.; Qu, Y.; Gao, P.; Yoshida, T. Metabolic flux change in hybridoma cells under high osmotic pressure. J. Biosci. Bioeng. 1999, 87, 255–257. [Google Scholar] [CrossRef]
- Presley, A.D.; Fuller, K.M.; Arriaga, E.A. MitoTracker green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. 2003, 793, 141–150. [Google Scholar] [CrossRef]
- Yao, C.-H.; Wang, R.; Wang, Y.; Kung, C.-P.; Weber, J.D.; Patti, G.J. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. eLife 2019, 8, e41351. [Google Scholar] [CrossRef]
- Bogdan, E.D.; Stuard, W.L.; Titone, R.; Robertson, D.M. IGFBP-3 mediates metabolic homeostasis during hyperosmolar stress in the corneal epithelium. Investig. Ophtalmol. Vis. Sci. 2021, 62, 11. [Google Scholar] [CrossRef]
- Kong, J.-G.; Park, J.-B.; Lee, D.; Park, E.-Y. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 2015, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflam. 2009, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Aluksanasuwan, S.; Plumworasawat, S.; Malaitad, T.; Chaiyarit, S.; Thongboonkerd, V. High glucose induces phosphorylation and oxidation of mitochondrial proteins in renal tubular cells: A proteomics approach. Sci. Rep. 2020, 10, 5843. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Romanova, N.; Schelletter, L.; Hoffrogge, R.; Noll, T. Hyperosmolality in CHO cell culture: Effects on the proteome. Appl. Microbiol. Biotechnol. 2022, 106, 2569–2586. [Google Scholar] [CrossRef]
- Russell, J.W.; Golovoy, D.; Vincent, A.M.; Mahendru, P.; Olzmann, J.A.; Mentzer, A.; Feldman, E.L. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002, 16, 1738–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassanayaka, S.; Readnower, R.D.; Salabei, J.K.; Long, B.W.; Aird, A.L.; Zheng, Y.-T.; Muthusamy, S.; Facundo, H.T.; Hill, B.G.; Jones, S.P. High glucose induces mitochondrial dysfunction independently of protein o-GlcNAcylation. Biochem. J. 2015, 467, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Tien, T.; Zhang, J.; Muto, T.; Kim, D.; Sarthy, V.P.; Roy, S. High glucose induces mitochondrial dysfunction in retinal müller cells: Implications for diabetic retinopathy. Investig. Ophtalmol. Vis. Sci. 2017, 58, 2915. [Google Scholar] [CrossRef] [PubMed]
- Alcántar-Fernández, J.; González-Maciel, A.; Reynoso-Robles, R.; Andrade, M.E.P.; Hernández-Vázquez, A.D.J.; Velázquez-Arellano, A.; Miranda-Rós, J. High-glucose diets induce mitochondrial dysfunction in caenorhabditis elegans. PLoS ONE 2019, 14, e0226652. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Zhang, T.; Chi, Y.; Liu, M. Effects of transcription factor EB on oxidative stress and apoptosis induced by high glucose in podocytes. Int. J. Mol. Med. 2019, 44, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [Green Version]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker green and CMX-rosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytom. Part A 2004, 61, 162–169. [Google Scholar] [CrossRef]
- Doherty, E.; Perl, A. Measurement of mitochondrial mass by flow cytometry during oxidative stress. React. Oxyg. Species 2017, 4, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Keij, J.F.; Bell-Prince, C.; Steinkamp, J.A. Staining of mitochondrial membranes with 10-nonyl acridine orange MitoFluor green, and MitoTracker green is affected by mitochondrial membrane potential altering drugs. Cytometry 2000, 39, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Hayashi, H.; Yoshida, T. The effect of osmolarity on metabolism and morphology in adhesion and suspension Chinese hamster ovary cells producing tissue plasminogen activator. Cytotechnology 2000, 32, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell 2019, 176, 1083.e18–1097.e18. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Qin, S.; Townsend, D.; Schulte, B.A.; Tew, K.D.; Wang, G.Y. Oxidative stress induces senescence in breast cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 514, 1204–1209. [Google Scholar] [CrossRef]
- Aryan, N.; Betts-Obregon, B.S.; Perry, G.; Tsin, A.T. Oxidative stress induces senescence in cultured RPE cells. Open Neurol. J. 2016, 10, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Brandl, A.; Meyer, M.; Bechmann, V.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp. Cell Res. 2011, 317, 1541–1547. [Google Scholar] [CrossRef]
- Campisi, J. Replicative senescence: An old lives tale? Cell 1996, 84, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Unger, M.W.; Hartwell, L.H. Control of cell division in saccharomyces cerevisiae by methionyl-tRNA. Proc. Natl. Acad. Sci. USA 1976, 73, 1664–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Sato, Y.; Higashiyama, T.; Sasaki, N. Live imaging reveals the dynamics and regulation of mitochondrial nucleoids during the cell cycle in FUCCI2-HeLa cells. Sci. Rep. 2017, 7, 11257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chainiaux, F.; de Magalhaes, J.P.; Eliaers, F.; Remacle, J.; Toussaint, O. UVB-induced premature senescence of human diploid skin fibroblasts. Int. J. Biochem. Cell Biol. 2002, 34, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Pande, P.; Mathur, M.; Shukla, N.; Ralhan, R. pRb and p16 protein alterations in human oral tumorigenesis. Oral Oncol. 1998, 34, 396–403. [Google Scholar] [CrossRef]

| Condition | Spent Medium * | TCX6D w/6 mM Gln | 80% High-Glucose or High-Mannitol Feed + 20% TCX6D w/6 mM Gln |
|---|---|---|---|
| 300 mOsm/kg (control) ** | 50% v/v | 50% v/v | - |
| 370 mOsm/kg | 50% v/v | 16.65% v/v | 33.3% v/v |
| 460 mOsm/kg | 50% v/v | 33.3% v/v | 16.65% v/v |
| 530 mOsm/kg | 50% v/v | - | 50% v/v |
| Primer | Sequence (5′ to 3′) | Genome Region |
|---|---|---|
| mt16srRNA (for) | TATCCTGACCGTGCAAAGGTA | 1999–2134 bp |
| mt16srRNA (rev) | AGGTTATTCCAGCCTCTTCACTG | |
| refB2m (for) | CTTGGGCTCCTTCAGAGTGG | 178,645–178,693 bp |
| refB2m (rev) | TGGACAAAGTCGAGCTGTCA |
| Cultivation | Doubling Times according to Osmolality Stage and Reagent [h] | |||
|---|---|---|---|---|
| 300 mOsm/kg | 370 mOsm/kg | 460 mOsm/kg | 530 mOsm/kg | |
| CHO DP-12 Man | 21.37 | 21.7 | 28.78 | 67.98 |
| CHO DP-12 Glc | 21.12 | 24.42 | 50.27 | 862.02 |
| DP-12 FUCCI Man | 19.3 | 19.67 | 24.26 | 48.54 |
| DP-12 FUCCI Glc | 21.68 | 24.24 | 35.76 | 195.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, N.; Schmitz, J.; Strakeljahn, M.; Grünberger, A.; Bahnemann, J.; Noll, T. Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response. Cells 2022, 11, 1763. https://doi.org/10.3390/cells11111763
Romanova N, Schmitz J, Strakeljahn M, Grünberger A, Bahnemann J, Noll T. Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response. Cells. 2022; 11(11):1763. https://doi.org/10.3390/cells11111763
Chicago/Turabian StyleRomanova, Nadiya, Julian Schmitz, Marie Strakeljahn, Alexander Grünberger, Janina Bahnemann, and Thomas Noll. 2022. "Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response" Cells 11, no. 11: 1763. https://doi.org/10.3390/cells11111763
APA StyleRomanova, N., Schmitz, J., Strakeljahn, M., Grünberger, A., Bahnemann, J., & Noll, T. (2022). Single-Cell Analysis of CHO Cells Reveals Clonal Heterogeneity in Hyperosmolality-Induced Stress Response. Cells, 11(11), 1763. https://doi.org/10.3390/cells11111763









