Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Study Protocol
2.3. Blood-Perfused Human Micro-Flow Chamber
2.4. Flow Cytometric Analysis of Neutrophils from Human Whole Blood
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
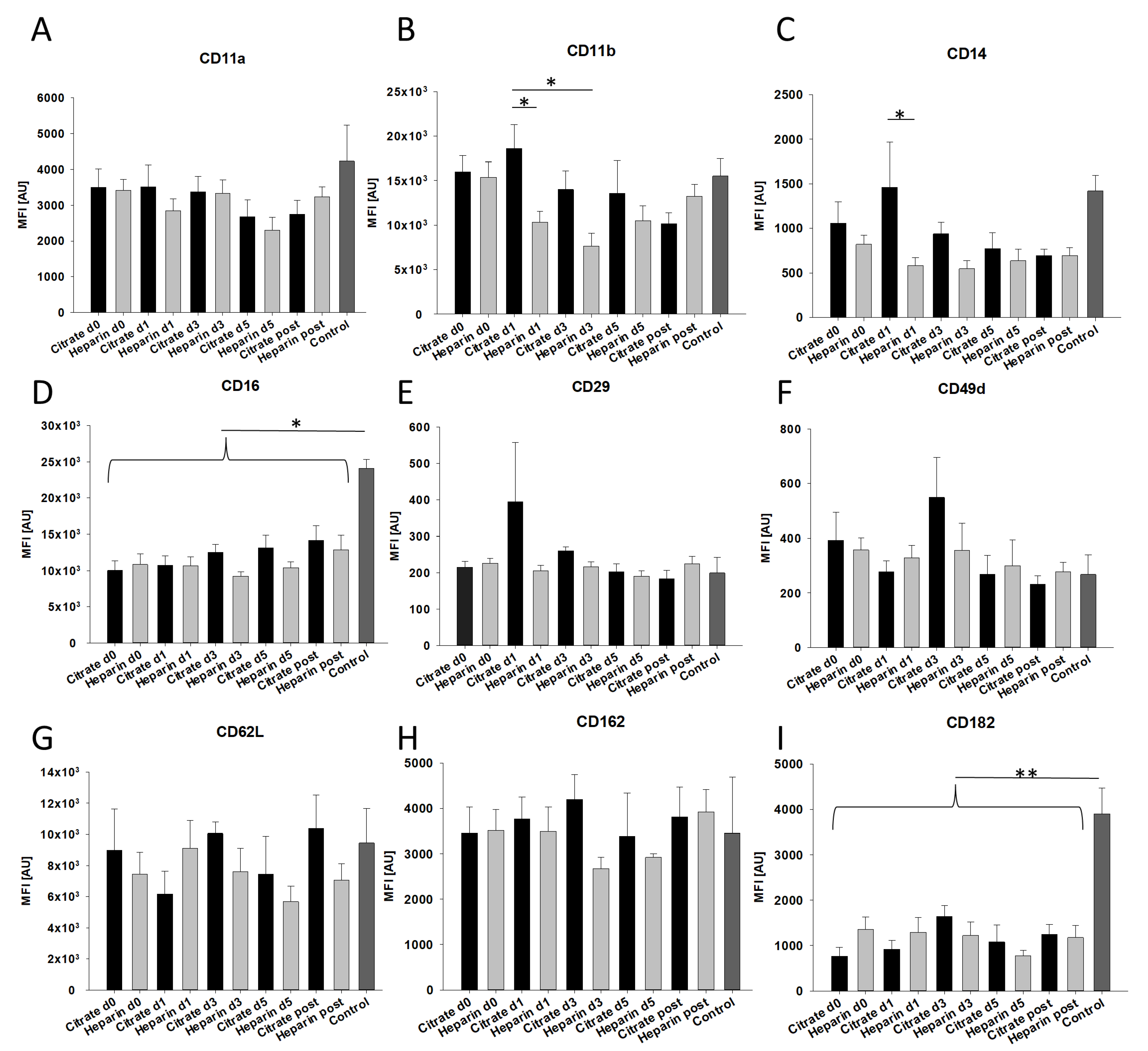
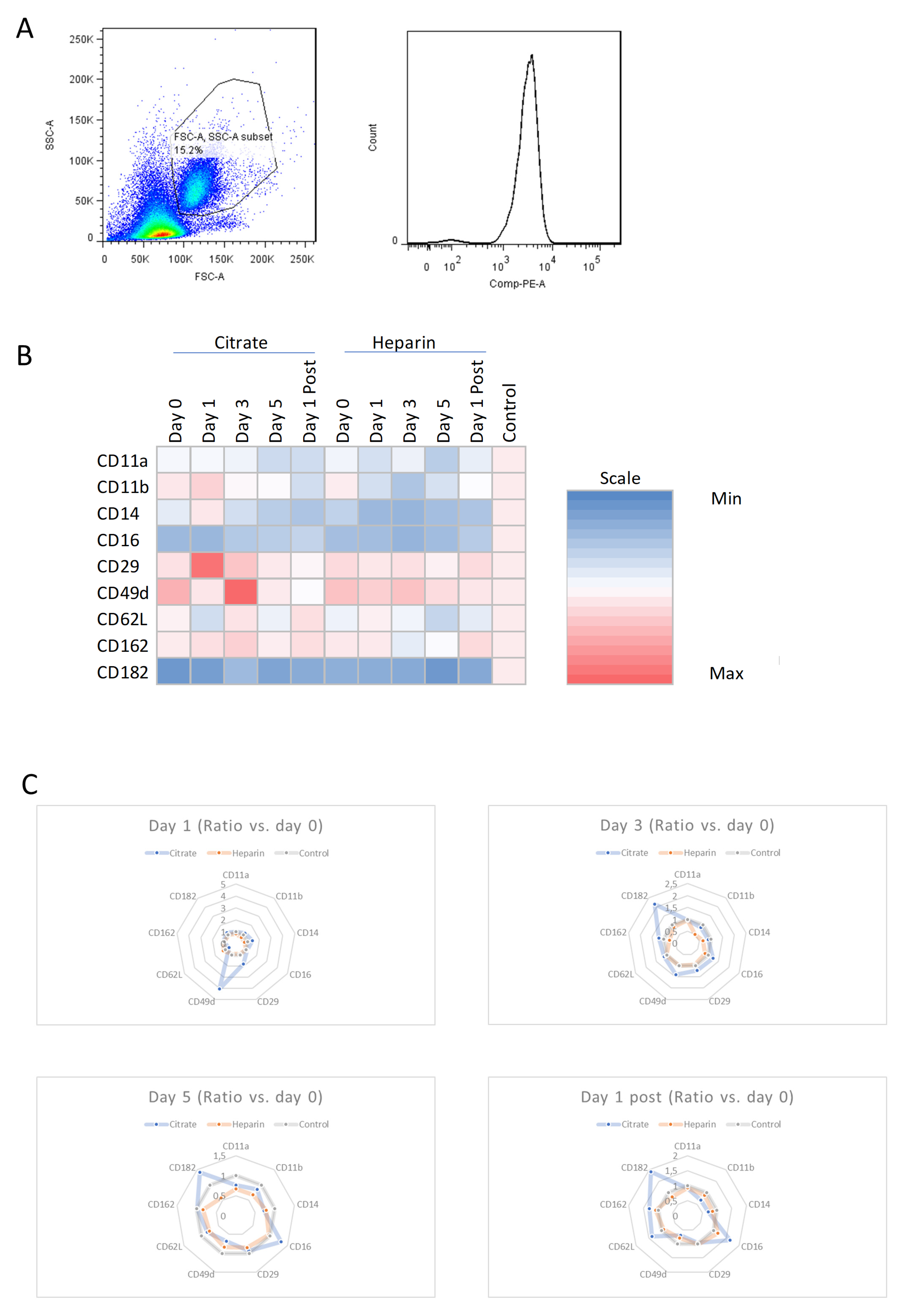
3.2. Effects of AKI and Mode of Anticoagulation on Neutrophil Surface Expression Characteristics
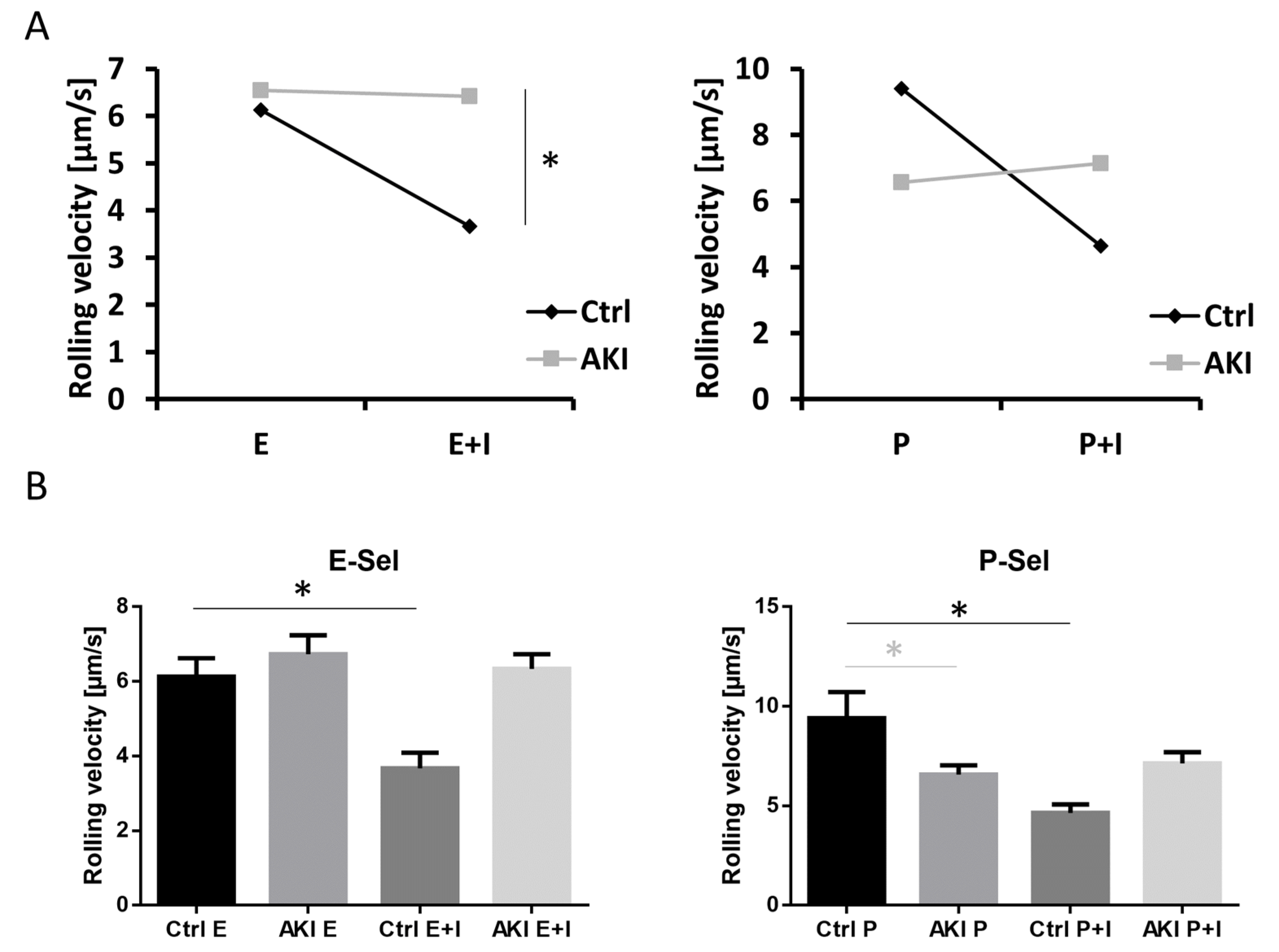
3.3. Selectin-Mediated Slow Leukocyte Rolling Is Abolished in Patients with AKI
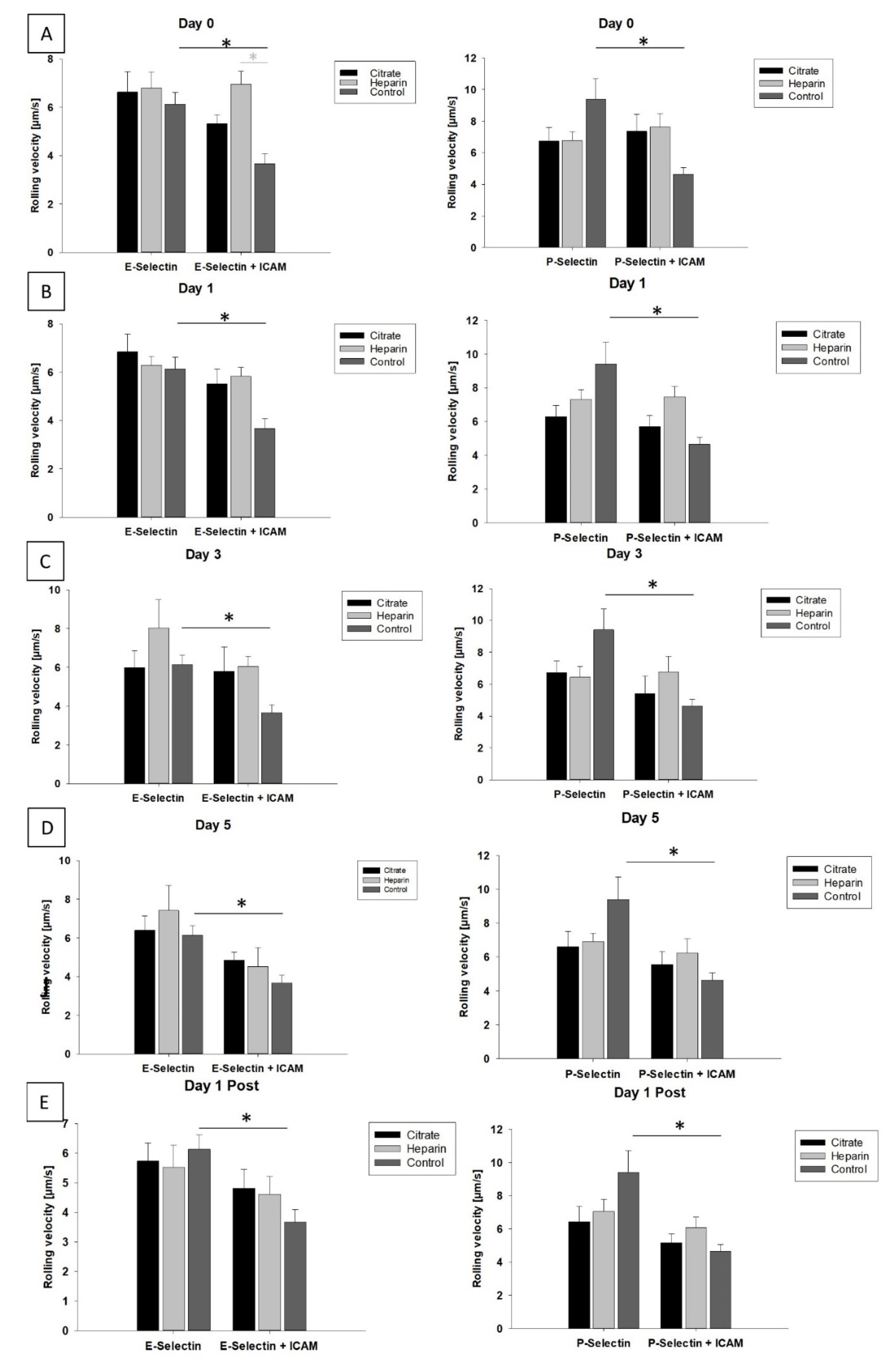
3.4. Mode of Anticoagulation and Duration of CVVH Have No Impact on Slow Leukocyte Rolling
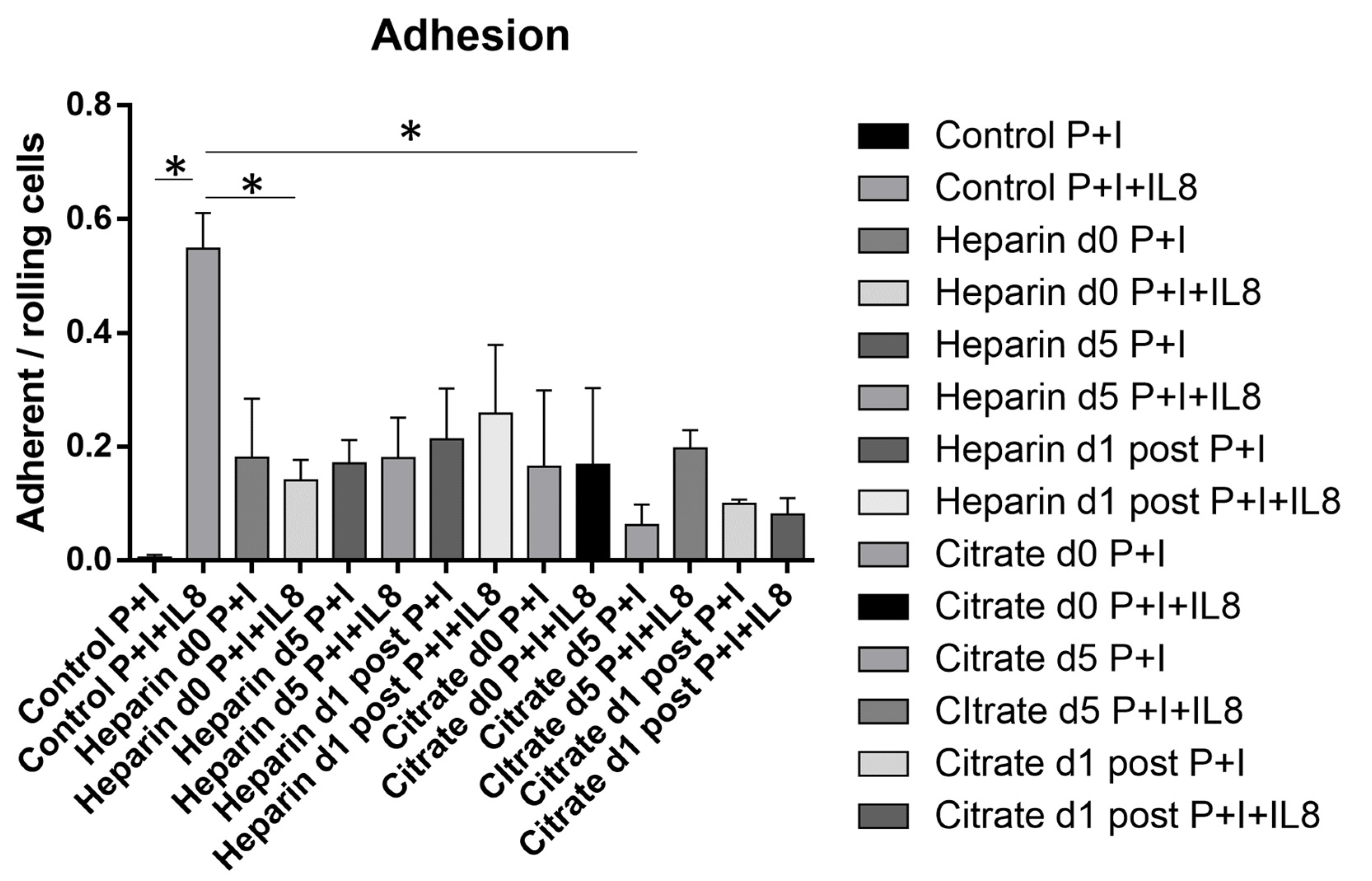
3.5. Chemokine-Induced Arrest Is Diminished in AKI Patients Independent of Mode of Anticoagulation or Duration of CVVH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, J.; Li, C.; Cheng, G.; Huang, L.; Bai, Z.; Fang, C. The efficacy of renal replacement therapy strategies for septic-acute kidney injury: A PRISMA-compliant network meta-analysis. Medicine 2019, 98, e15257. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstadt, H.; Boanta, A.; Gerss, J.; Meersch, M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients with Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group; Bagshaw, S.M.; Wald, R.; Adhikari, N.K.J.; Bellomo, R.; et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N. Engl. J. Med. 2020, 383, 240–251. [Google Scholar] [CrossRef]
- Joannidis, M.; Oudemans-van Straaten, H.M. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit. Care 2007, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Schilder, L.; Nurmohamed, S.A.; ter Wee, P.M.; Paauw, N.J.; Girbes, A.R.; Beishuizen, A.; Beelen, R.H.; Groeneveld, A.B. Citrate confers less filter-induced complement activation and neutrophil degranulation than heparin when used for anticoagulation during continuous venovenous haemofiltration in critically ill patients. BMC Nephrol. 2014, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; MacLeod, A.M.; et al. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Liu, C.; Mao, Z.; Kang, H.J.; Hu, J.; Zhou, F.H. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit. Care 2016, 20, 144. [Google Scholar] [CrossRef] [Green Version]
- Zarbock, A.; Kullmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mulling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients with Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef]
- Lehner, G.F.; Harler, U.; Feistritzer, C.; Haller, V.M.; Hasslacher, J.; Bellmann, R.; Joannidis, M. Hemofiltration induces generation of leukocyte-derived CD31+/CD41-microvesicles in sepsis. Ann. Intensive Care 2017, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Margraf, A.; Lowell, C.A.; Zarbock, A. Neutrophils in acute inflammation—Current concepts and translational implications. Blood 2021, 139, 2130–2144. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Ley, K.; Zarbock, A. Neutrophil Recruitment: From Model Systems to Tissue-Specific Patterns. Trends Immunol. 2019, 40, 613–634. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Kullmar, M.; Wempe, C.; Kindgen-Milles, D.; Kluge, S.; Slowinski, T.; Marx, G.; Gerss, J.; Zarbock, A.; SepNet Critical Care Trials Group. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill patients with acute kidney injury (RICH) trial: Study protocol for a multicentre, randomised controlled trial. BMJ Open 2019, 9, e024411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossaint, J.; Berger, C.; Van Aken, H.; Scheld, H.H.; Zahn, P.K.; Rukosujew, A.; Zarbock, A. Cardiopulmonary bypass during cardiac surgery modulates systemic inflammation by affecting different steps of the leukocyte recruitment cascade. PLoS ONE 2012, 7, e45738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwano, Y.; Spelten, O.; Zhang, H.; Ley, K.; Zarbock, A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 2010, 116, 617–624. [Google Scholar] [CrossRef] [Green Version]
- McAvoy, E.F.; McDonald, B.; Parsons, S.A.; Wong, C.H.; Landmann, R.; Kubes, P. The role of CD14 in neutrophil recruitment within the liver microcirculation during endotoxemia. J. Immunol. 2011, 186, 2592–2601. [Google Scholar] [CrossRef] [Green Version]
- Dransfield, I.; Buckle, A.M.; Savill, J.S.; McDowall, A.; Haslett, C.; Hogg, N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J. Immunol. 1994, 153, 1254–1263. [Google Scholar]
- Golay, J.; Valgardsdottir, R.; Musaraj, G.; Giupponi, D.; Spinelli, O.; Introna, M. Human neutrophils express low levels of FcgammaRIIIA, which plays a role in PMN activation. Blood 2019, 133, 1395–1405. [Google Scholar] [CrossRef]
- Boras, M.; Volmering, S.; Bokemeyer, A.; Rossaint, J.; Block, H.; Bardel, B.; Van Marck, V.; Heitplatz, B.; Kliche, S.; Reinhold, A.; et al. Skap2 is required for beta2 integrin-mediated neutrophil recruitment and functions. J. Exp. Med. 2017, 214, 851–874. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Rossaint, J.; Spelten, O.; Kassens, N.; Mueller, H.; Van Aken, H.K.; Singbartl, K.; Zarbock, A. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011, 80, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Mishra, H.K.; Long, C.; Bahaie, N.S.; Walcheck, B. Regulation of CXCR2 expression and function by a disintegrin and metalloprotease-17 (ADAM17). J. Leukoc. Biol. 2015, 97, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, C.; Hosseinkhani, M.R.; Wang, Y.; Sriramarao, P.; Walcheck, B. ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J. Leukoc. Biol. 2012, 92, 667–672. [Google Scholar] [CrossRef]
- Kermarrec, N.; Selloum, S.; Plantefeve, G.; Chosidow, D.; Paoletti, X.; Lopez, A.; Mantz, J.; Desmonts, J.M.; Gougerot-Pocidalo, M.A.; Chollet-Martin, S. Regulation of peritoneal and systemic neutrophil-derived tumor necrosis factor-alpha release in patients with severe peritonitis: Role of tumor necrosis factor-alpha converting enzyme cleavage. Crit. Care Med. 2005, 33, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Palau, V.; Pascual, J.; Soler, M.J.; Riera, M. Role of ADAM17 in kidney disease. Am. J. Physiol. Ren. Physiol. 2019, 317, F333–F342. [Google Scholar] [CrossRef]
- Nomellini, V.; Brubaker, A.L.; Mahbub, S.; Palmer, J.L.; Gomez, C.R.; Kovacs, E.J. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging Dis. 2012, 3, 234–247. [Google Scholar] [PubMed]
- Beyrau, M.; Bodkin, J.V.; Nourshargh, S. Neutrophil heterogeneity in health and disease: A revitalized avenue in inflammation and immunity. Open Biol. 2012, 2, 120134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.C.; Grooteman, M.P.; van Houte, A.J.; Schoorl, M.; van Limbeek, J.; Nube, M.J. Low polymorphonuclear cell degranulation during citrate anticoagulation: A comparison between citrate and heparin dialysis. Nephrol. Dial. Transplant. 1997, 12, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.V.; Williams, R.N.; Paterson, C.A. The effect of sodium citrate on the stimulation of polymorphonuclear leukocytes. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1257–1261. [Google Scholar]
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef]
- Immler, R.; Simon, S.I.; Sperandio, M. Calcium signalling and related ion channels in neutrophil recruitment and function. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12964. [Google Scholar] [CrossRef] [PubMed]
- Craddock, P.R.; Yawata, Y.; VanSanten, L.; Gilberstadt, S.; Silvis, S.; Jacob, H.S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N. Engl. J. Med. 1974, 290, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Morabito, S.; Pistolesi, V.; Tritapepe, L.; Zeppilli, L.; Polistena, F.; Fiaccadori, E.; Pierucci, A. Regional citrate anticoagulation in CVVH: A new protocol combining citrate solution with a phosphate-containing replacement fluid. Hemodial. Int. 2013, 17, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Thorlacius, H.; Raud, J.; Hedqvist, P.; Lindbom, L. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules In Vivo. Br. J. Pharmacol. 1997, 122, 906–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.A.; Lever, R.; Jones, N.A.; Page, C.P. Effects of heparin and related molecules upon neutrophil aggregation and elastase release In Vitro. Br. J. Pharmacol. 2003, 139, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, R.; Hoult, J.R.; Page, C.P. The effects of heparin and related molecules upon the adhesion of human polymorphonuclear leucocytes to vascular endothelium In Vitro. Br. J. Pharmacol. 2000, 129, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Cairo, M.S.; Allen, J.; Higgins, C.; Baehner, R.L.; Boxer, L.A. Synergistic effect of heparin and chemotactic factor on polymorphonuclear leukocyte aggregation and degranulation. Am. J. Pathol. 1983, 113, 67–74. [Google Scholar]
- Huang, S.; Sandholm, K.; Jonsson, N.; Nilsson, A.; Wieslander, A.; Grundstrom, G.; Hancock, V.; Ekdahl, K.N. Low concentrations of citrate reduce complement and granulocyte activation in vitro in human blood. Clin. Kidney J. 2015, 8, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Singbartl, K.; Miller, L.; Ruiz-Velasco, V.; Kellum, J.A. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2016, 44, e492–e501. [Google Scholar] [CrossRef] [Green Version]
| Regional Citrate | Systemic Heparin | Control | |
|---|---|---|---|
| Number of individual patients | 16 | 20 | 4 |
| Age (Mean ± SEM) | 62.29 ± 4.43 | 65.90 ± 2.97 | 32.67 ± 4.98 |
| Gender (% female) | 50.00% | 45.00% | 25% |
| 90-d mortality | 37.50% | 30.00% | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margraf, A.; Liu, C.; Küllmar, M.; Meersch, M.; Rossaint, J.; Zarbock, A. Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation. Cells 2022, 11, 1815. https://doi.org/10.3390/cells11111815
Margraf A, Liu C, Küllmar M, Meersch M, Rossaint J, Zarbock A. Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation. Cells. 2022; 11(11):1815. https://doi.org/10.3390/cells11111815
Chicago/Turabian StyleMargraf, Andreas, Chang Liu, Mira Küllmar, Melanie Meersch, Jan Rossaint, and Alexander Zarbock. 2022. "Analysis of Leukocyte Recruitment in Continuous Veno-Venous Hemofiltration with Regional Citrate vs. Systemic Heparin Anticoagulation" Cells 11, no. 11: 1815. https://doi.org/10.3390/cells11111815






