Role of Mitochondria Transfer in Infertility: A Commentary
Abstract
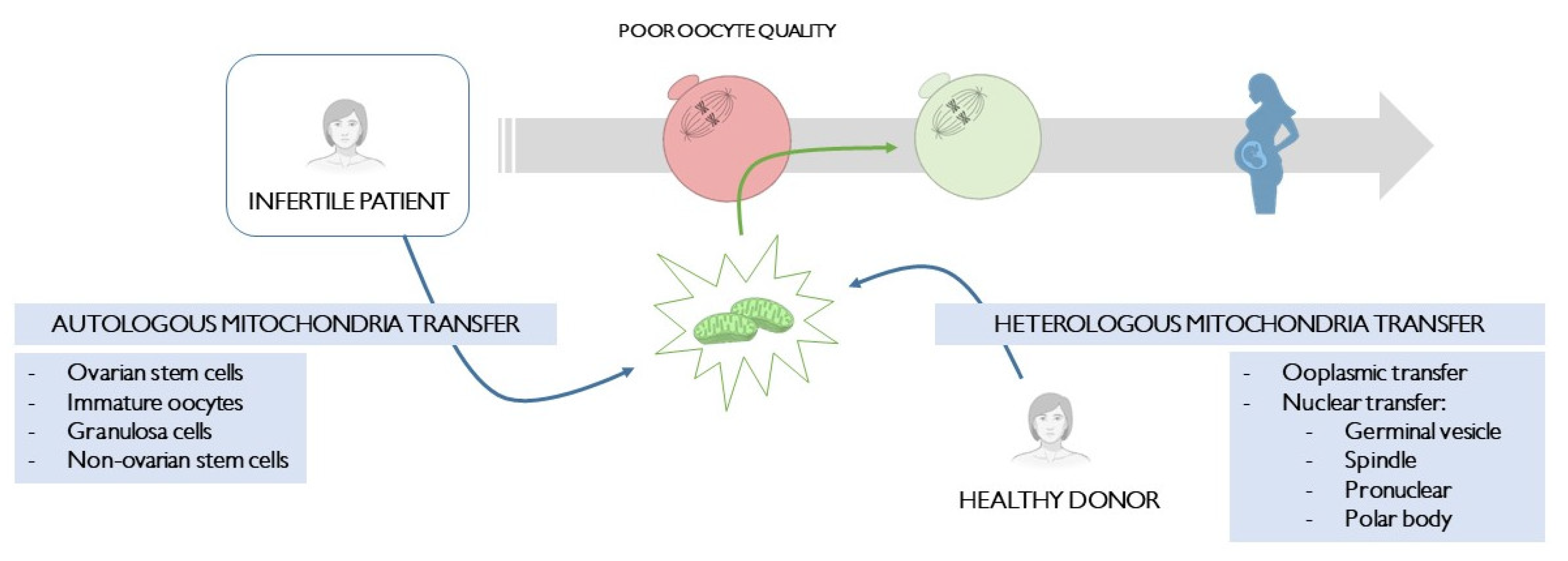
:1. Introduction
2. Heterologous Mitochondria Transfer
2.1. Ooplasmic Transfer (Cytotransfer)
2.2. Nuclear Transfer
2.2.1. Germinal Vesicle Transfer
2.2.2. Spindle Transfer
2.2.3. Pronuclear Transfer
2.2.4. Polar Body Transfer
3. Autologous Mitochondria Transfer
3.1. Ovarian Stem Cells
3.2. Immature Oocytes
3.3. Granulosa Cells
3.4. Non-Ovarian Stem Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Varela, C.; Herraiz, S.; Labarta, E. Mitochondrial enrichment in infertile patients: A review of different mitochondrial replacement therapies. Ther. Adv. Reprod. Health 2021, 15, 26334941211023544. [Google Scholar] [CrossRef] [PubMed]
- Paull, D.; Emmanuele, V.; Weiss, K.A.; Treff, N.; Stewart, L.; Hua, H.; Zimmer, M.; Kahler, D.J.; Goland, R.S.; Noggle, S.A.; et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 2013, 493, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Tuppen, H.A.; Greggains, G.D.; Harbottle, S.J.; Murphy, J.L.; Cree, L.M.; Murdoch, A.P.; Chinnery, P.F.; Taylor, R.W.; Lightowlers, R.N.; et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 2010, 465, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Sha, H.; Ji, D.; Zhang, H.L.; Chen, D.; Cao, Y.; Zhu, J. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell 2014, 157, 1591–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C. Why Do We Still Have a Maternally Inherited Mitochondrial DNA? Insights from Evolutionary Medicine. Annu. Rev. Biochem. 2007, 76, 781–821. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-Y.; Wang, D.H.; Zou, X.Y.; Xu, C.M. Mitochondrial functions on oocytes and preimplantation embryos. J. Zhejiang Univ. Sci. B 2009, 10, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Van Blerkom, J.; Sinclair, J.; Davis, P. Mitochondrial transfer between oocytes: Potential applications of mitochondrial donation and the issue of heteroplasmy. Hum. Reprod. 1998, 13, 2857–2868. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.W.; Grifo, J.A.; Krey, L.C.; Zhang, J. Reconstruction of mouse oocytes by germinal vesicle transfer: Maturity of host oocyte cytoplasm determines meiosis. Hum. Reprod. 1999, 14, 2357–2361. [Google Scholar] [CrossRef]
- Wakayama, T.; Yanagimachi, R. The First Polar Body Can Be Used for the Production of Normal Offspring in Mice 1. Biol. Reprod. 1998, 59, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Neupane, J.; Vandewoestyne, M.; Ghimire, S.; Lu, Y.; Qian, C.; Van Coster, R.; Gerris, J.; Deroo, T.; Deforce, D.; de Sutter, P.; et al. Assessment of nuclear transfer techniques to prevent the transmission of heritable mitochondrial disorders without compromising embryonic development competence in mice. Mitochondrion 2014, 18, 27–33. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, J.; Meng, T.; Guo, L.; Dong, M.; Fan, L.; Ouyang, Y.; Wang, G.; Sun, Q.; Ou, X.; et al. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging 2017, 9, 2480–2488. [Google Scholar] [CrossRef] [Green Version]
- Li, G.P.; Chen, D.Y.; Lian, L.; Sun, Q.Y.; Wang, M.K.; Liu, J.L.; Li, J.S.; Han, Z.M. Viable rabbits derived from reconstructed oocytes by germinal vesicle transfer after intracytoplasmic sperm injection (ICSI). Mol. Reprod. Dev. 2001, 58, 180–185. [Google Scholar] [CrossRef]
- Cohen, J.; Scott, R.; Alikani, M.; Schimmel, T.; Munné, S.; Levron, J.; Wu, L.; Brenner, C.; Warner, C.; Willadsen, S. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod. 1998, 4, 269–280. [Google Scholar] [CrossRef]
- Eyre-Walker, A. Mitochondrial Replacement Therapy: Are Mito-nuclear. Genetics 2017, 205, 1365–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Scott, R.; Schimmel, T.; Levron, J.; Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 1997, 350, 186–187. [Google Scholar] [CrossRef]
- Brenner, C.A.; Barritt, J.A.; Willadsen, S.; Cohen, J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil. Steril. 2000, 74, 573–578. [Google Scholar] [CrossRef]
- Huang, C.-C.; Cheng, T.-C.; Chang, H.-H.; Chang, C.-C.; Chen, C.-I.; Liu, J.; Lee, M.-S. Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into metaphase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil. Steril. 1999, 72, 702–706. [Google Scholar] [CrossRef]
- Dale, B.; Wilding, M.; Botta, G.; Rasile, M.; Marino, M.; Di Matteo, L.; De Placido, G.; Izzo, A. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility. Hum. Reprod. 2001, 16, 1469–1472. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Pascale, C.; Jackson, M.; Szvetecz, M.A.; Cohen, J. A limited survey-based uncontrolled follow-up study of children born after ooplasmic transplantation in a single centre. Reprod. Biomed. Online 2016, 33, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Sobek, A.; Tkadlec, E.; Klaskova, E.; Prochazka, M. Cytoplasmic Transfer Improves Human Egg Fertilization and Embryo Quality: An Evaluation of Sibling Oocytes in Women with Low Oocyte Quality. Reprod. Sci. 2021, 28, 1362–1369. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.-W.; Krey, L.; Liu, H.; Meng, L.; Blaszczyk, A.; Adler, A.; Grifo, J. In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil. Steril. 1999, 71, 726–731. [Google Scholar] [CrossRef]
- Darbandi, S.; Darbandi, M.; Agarwal, A.; Khorshid, H.R.K.; Sadeghi, M.R.; Esteves, S.C.; Sengupta, P.; Dutta, S.; Fathi, Z.; Zeraati, H.; et al. Comparing four laboratory three-parent techniques to construct human aged non-surrounded nucleolus germinal vesicle oocytes: A case-control study. Int. J. Reprod. Biomed. 2020, 18, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nagayoshi, M.; Awata, S.; Himeno, N.; Tanaka, I.; Watanabe, S.; Kusunoki, H. Metaphase II karyoplast transfer from human in-vitro matured oocytes to enuclueated mature oocytes. Reprod. Biomed. Online 2009, 19, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Borges, N. First registered pilot trial to validate the safety and effectiveness of maternal spindle transfer to overcome infertility associated with poor oocyte quality. Fertil. Sterlility 2020, 114, e71–e72. [Google Scholar]
- Hyslop, L.A.; Blakeley, P.; Craven, L.; Richardson, J.; Fogarty, N.M.E.; Fragouli, E.; Lamb, M.; Wamaitha, S.E.; Prathalingam, N.; Zhang, Q.; et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature 2016, 534, 383–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhuang, G.; Zeng, Y.; Grifo, J.; Acosta, C.; Shu, Y.; Liu, H. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod. Biomed. Online 2016, 33, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; O’neil, R.C.; Marti Gutierrez, N.; Hariharan, M.; Zhang, Z.Z.; He, Y.; Cinnioglu, C.; Kayali, R.; Kang, E.; Lee, Y.; et al. Functional Human Oocytes Generated by Transfer of Polar Body Genomes. Cell Stem Cell 2017, 20, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.P.; Lu, C.F.; Gong, F.; Xie, P.Y.; Hu, L.; Zhang, S.J.; Lu, G.X.; Lin, G. Polar body transfer restores the developmental potential of oocytes to blastocyst stage in a case of repeated embryo fragmentation. J. Assist. Reprod. Genet. 2017, 34, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Guggilla, R.R.; Gansemans, Y.; Van Der Jeught, M.; Boel, A.; Popovic, M.; Stamatiadis, P.; Ferrer-Buitrago, M.; Thys, V.; Van Coster, R.; et al. Comparative analysis of different nuclear transfer techniques to prevent the transmission of mitochondrial DNA variants. Mol. Hum. Reprod. 2019, 25, 797–810. [Google Scholar] [CrossRef]
- Barritt, J.A.; Cohen, J.; Brenner, C.A. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod. Biomed. Online 2000, 1, 96–100. [Google Scholar] [CrossRef]
- Barritt, J.A.; Brenner, C.A.; Malter, H.E.; Cohen, J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod. 2001, 16, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Zoon, K. Letter to Sponsors/Researchers—Human Cells Used in Therapy Involving the Transfer of Genetic Material By Means Other Than the Union of Gamete Nuclei. 2001. Available online: https://wayback.archive-it.org/7993/20170404210748/https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm105852.htm (accessed on 18 May 2022).
- Goud, A.P.; Goud, P.T.; Van Oostveldt, P.; Diamond, M.P.; Dhont, M. Dynamic changes in microtubular cytoskeleton of human postmature oocytes revert after ooplasm transfer. Fertil. Steril. 2004, 81, 323–331. [Google Scholar] [CrossRef]
- Chiang, T.; Schultz, R.M.; Lampson, M.A. Meiotic origins of maternal age-related aneuploidy. Biol. Reprod. 2012, 86, 3. [Google Scholar] [CrossRef] [PubMed]
- Cree, L.; Loi, P. Mitochondrial replacement: From basic research to assisted reproductive technology portfolio tool-technicalities and possible risks. Mol. Hum. Reprod. 2015, 21, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Woodward, J.; Sanchis, D.M.; Ma, H.; Gutierrez, N.M.; Tippner-Hedges, R.; Kang, E.; Lee, H.S.; et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 2013, 493, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Reznichenko, A.S.; Huyser, C.; Pepper, M.S. Mitochondrial transfer: Implications for assisted reproductive technologies. Appl. Transl. Genomics 2016, 11, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Steuerwald, N.; Barritt, J.A.; Adler, R.; Malter, H.; Schimmel, T.; Cohen, J.; Brenner, C.A. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 2000, 8, 209–215. [Google Scholar] [CrossRef]
- Kong, L.H.; Liu, Z.; Li, H.; Zhu, L.; Chen, S.M.; Chen, S.L.; Xing, F.Q. Mitochondria transfer from self-granular cells to improve embryos’ quality. Zhonghua Fu Chan Ke Za Zhi 2004, 39, 105–107. [Google Scholar]
- Labarta, E.; de los Santos, M.J.; Herraiz, S.; Escribá, M.J.; Marzal, A.; Buigues, A.; Pellicer, A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization—a randomized pilot study. Fertil. Steril. 2019, 111, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Fakih, M.H. The AUGMENT Treatment: Physician Reported Outcomes of the Initial Global Patient Experience. J. Fertil. Vitr. IVF-Worldwide, Reprod. Med. Genet. Stem Cell Biol. 2015, 3, 1000154. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Baltaci, V.; Sonmezer, M.; Turan, V.; Unsal, E.; Baltaci, A.; Aktuna, S.; Moy, F. Oogonial Precursor Cell-Derived Autologous Mitochondria Injection to Improve Outcomes in Women with Multiple IVF Failures Due to Low Oocyte Quality: A Clinical Translation. Reprod. Sci. 2015, 22, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.R.; Hsieh, R.H.; Au, H.K.; Yen, Y.H.; Chang, S.J.; Cheng, Y.F. Mitochondria transfer (MIT) into oocyte from autologous cumulus granulosa cells (cGCs). Fertil. Steril. 2004, 82, S53. [Google Scholar] [CrossRef]
- White, Y.A.R.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Tilly, J.L.; Niikura, Y.; Rueda, B.R. The current status of evidence for and against postnatal oogenesis in mammals:A case of ovarian optimism versus pessimism? Biol. Reprod. 2009, 80, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Woods, D.C.; Tilly, J.L. Autologous Germline Mitochondrial Energy Transfer (AUGMENT) in Human Assisted Reproduction. Semin. Reprod. Med. 2015, 33, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.G.; Pors, S.E.; Andersen, C.Y. Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum. Reprod. 2017, 32, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Wai, T.; Teoli, D.; Shoubridge, E.A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008, 40, 1484–1488. [Google Scholar] [CrossRef]
- Qi, L.; Chen, X.; Wang, J.; Lv, B.; Zhang, J.; Ni, B.; Xue, Z. Mitochondria: The panacea to improve oocyte quality? Ann. Transl. Med. 2019, 7, 789. [Google Scholar] [CrossRef]
- Krisher, R.L.; Prather, R.S. A role for the Warburg effect in preimplantation embryo development: Metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 2012, 79, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Houghton, F.D. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation 2006, 74, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yang, Y.; Zhou, J.; Yan, G.; Liu, M.; Xu, L.; Li, Z.; Jiang, R.; Diao, Z.; Zhen, X.; et al. Mitochondrial transfer from aged adipose-derived stem cells does not improve the quality of aged oocytes in C57BL/6 mice. Mol. Reprod. Dev. 2019, 86, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y. Adenosine Triphosphate Content in Human Unfertilized Oocytes, Undivided Zygotes and Embryos Unsuitable for Transfer or Cryopreservation. J. Int. Med. Res. 2012, 40, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, M.; Alfarawati, S.; Hurd, D.; Paolucci, M.; Shovelton, J.; Fragouli, E.; Wells, D. Simultaneous assessment of aneuploidy, polymorphisms, and mitochondrial DNA content in human polar bodies and embryos with the use of a novel microarray platform. Fertil. Steril. 2014, 102, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
| Study Name | Type of Mitochondria Transfer | n | Patients’ Etiology | Main Outcome |
|---|---|---|---|---|
| Cohen, 1997 [15] | Ooplasmic transfer | 1 patient | History of impaired embryo development | First human birth using this approach |
| Cohen, 1998 [13] | 8 cycles | Repeated implantation failure | Improved results using the injection technique vs. electrofusion. One healthy infant and ongoing pregnancy in the injection group (total n = 5) vs. no pregnancy in the electrofusion group (n = 3) | |
| Brenner, 2000 [16] | 23 cycles | Twelve clinical pregnancies and overall improved embryo development. Proven mtDNA heteroplasmy in the offspring. | ||
| Huang, 1999 [17] | 9 cycles | Five healthy infants after ooplasmic transfer from tripronucleated zygotes | ||
| Dale, 2001 [18] | 1 patient | Birth of healthy twins | ||
| Chen, 2016 [19] | 33 cycles | Follow-up study of 17 healthy infants from 13 couples [13,15,16]. Limited study with high bias, but overall unaffected healthy offspring. | ||
| Sobek, 2021 [20] | 125 cycles Ooplasmic transfer vs. control in sibling oocytes | Low ovarian function | Increased fertilization and embryo development rates. A reduction in fertilization rates with age was observed in the control group but not in the ooplasmic transfer group. 28 healthy infants in the ooplasmic transfer group. | |
| Zhang, 1999 [21] | GV transfer | 60 GVs | Advanced maternal age | 12 GVs were successfully removed, transferred, and fused into previously enucleated oocytes from young patients. 7 of these matured to a metaphase II oocyte, similar maturation rate to the non-manipulated GVs. |
| Darbandi, 2020 [22] | 10 GVs | 0% fusion rate | ||
| Tanaka, 2009 [23] | Spindle transfer | 31 MII spindle transfer group 98 MII control group | In vitro matured MII oocytes (model of aged oocytes) | 25/31 correctly fused (80.6%). Significantly higher number of oocytes developed to the blastocyst stage in the spindle transfer group (7 vs. 3 in the control group). |
| Zhang, 2017 [24] | 1 patient | History of pregnancy loss and asymptomatic carrier of a Leigh syndrome mutation | First human birth after spindle transfer | |
| Costa-Borges, 2020 [25] | 9 cycles | Age range 32–40 years. History of embryo developmental arrest | Preliminary results from a larger pilot study (n = 25). Applied successfully in 39/44 oocytes (88.6%). Of these, 76.9% (30/39) fertilized and 20 developed into good quality blastocysts (66.7%). Genetic analysis revealed 35% (7/20) of the embryos to be euploid and mtDNA carryover levels <1%. Two blastocysts were warmed and transferred, resulting in two pregnancies. | |
| Craven, 2010 [3] | PN transfer | 80 uni- and tripronucleated zygotes with PN transfer vs. 76 unmanipulated control group | Transfer of PN from abnormally fertilized zygotes discarded from IVF cycles | First PN transfer attempt in humans. Minimal mtDNA carryover and compatible with onward development to the blastocyst stage. |
| Hyslop, 2016 [26] | 523 MII | MII donated oocytes fertilized with donated sperm | Alternative approach based on transplanting pronuclei shortly after completion of meiosis rather than shortly before the first mitotic division. mtDNA carryover below 2%. Efficient development to the blastocyst stage with no detectable effect on aneuploidy or gene expression. | |
| Zhang, 2016 [27] | 1 patient | History of embryo developmental arrest | Viable pregnancy with normal karyotype and minimal mtDNA heteroplasmy | |
| Ma, 2017 [28] | PB1 transfer | 32 oocytes in PB1T group vs. 21 in the control group 11 women | Healthy volunteers | Oocytes supported the formation of de novo meiotic spindles and, after fertilization with sperm, meiosis completion and formation of normal diploid zygotes. Lower blastocyst formation rates in the PB1T group in comparison to the control group |
| Zhang, 2017 [29] | PB1 and PB2 transfer | 1 patient | Repeated embryo fragmentation of maternal origin | PB1T but not PB2T into enucleated in vitro matured donor MII oocytes successfully generate normal fertilized zygotes with high efficiency for developing into blastocysts |
| Tang, 2019 [30] | PB2 transfer | 134 oocytes | In vitro matured oocytes and in vivo matured oocytes with smooth endoplasmic reticulum aggregate, both donated from young women | Novel strategy for PB2 transfer. Unaltered blastocyst quality in the PB2T and control groups and similar euploidy rates |
| Study Name | Type of Mitochondria Transfer | n | Patients’ Etiology | Main Outcome |
|---|---|---|---|---|
| Fakih, 2015 [43] | Ovarian stem cells (AUGMENT®) | 59 + 34 patients (2 different clinics) | Poor oocyte and embryo quality | Poor study design with high bias. Increased pregnancy rates in comparison to the historic IVF success rates in the same patients |
| Oktay, 2015 [44] | 16 patients | 2 or more previous IVF attempts failure, and poor oocyte and embryo quality | Poor study design with high bias. Higher fertilization rates (78.3% vs. 47.9%; p = 0.036) and better embryo quality (3.1% vs. 2.3%; p = 0.082) than the results obtained in previous cycles from the same patients. | |
| Labarta, 2019 [42] | 57 patients | Previous IVF failures and well-documented poor embryo quality | Intrapatient and intracycle comparison design. Significantly lower day 5 blastocyst formation rate in the AUGMENT group. No statistically significant differences in any other variable studied. | |
| Kong, 2004 [41] | Granulosa cells | 18 patients | A previous failed IVF treatment or order than 37 years | Similar fertilization rates (74.4% vs. 76.8% in the control group; p > 0.05). Significantly higher good quality embryo rate in mitochondria transfer group (59.4% vs. 34.9% in the control group; p < 0.05). There were 7 clinical pregnancies in the 18 cases. |
| Tzeng, 2004 [45] | 71 cycles vs. 81 historic cycles in the same patient group | A previous failed IVF treatment | Significantly higher pregnancy rates (35.2% vs. 6.2% in the historic control group; p < 0.05) and lower miscarriage rates (15.4% vs. 100% in the historic control group; p < 0.05). Significantly higher day 3 embryo quality. Twenty live births. Oocytes following this technique had a propensity to cleave faster, as well as lower apoptosis and fragmentation rates. |
| Type of Mitochondria Transfer | Clinically Used in Infertile Patients (Yes/No) | Has Showed Promising Results (Yes/No) | Live Birth/s (Yes/No) |
|---|---|---|---|
| Ooplasmic transfer | Yes | Yes | Yes |
| Germinal vesicle transfer | Yes | Yes | No |
| Spindle transfer | Yes | Yes | Yes |
| Pronuclear transfer | Yes | Yes | Yes |
| First polar body transfer | Yes | Yes | No |
| Second polar body transfer | Yes | No | No |
| Ovarian stem cells | Yes | No | Yes |
| Immature oocytes | No | - | - |
| Granulosa cells | Yes | Yes | Yes |
| Non-ovarian stem cells | No | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Varela, C.; Labarta, E. Role of Mitochondria Transfer in Infertility: A Commentary. Cells 2022, 11, 1867. https://doi.org/10.3390/cells11121867
Rodríguez-Varela C, Labarta E. Role of Mitochondria Transfer in Infertility: A Commentary. Cells. 2022; 11(12):1867. https://doi.org/10.3390/cells11121867
Chicago/Turabian StyleRodríguez-Varela, Cristina, and Elena Labarta. 2022. "Role of Mitochondria Transfer in Infertility: A Commentary" Cells 11, no. 12: 1867. https://doi.org/10.3390/cells11121867
APA StyleRodríguez-Varela, C., & Labarta, E. (2022). Role of Mitochondria Transfer in Infertility: A Commentary. Cells, 11(12), 1867. https://doi.org/10.3390/cells11121867







