IGF2: Development, Genetic and Epigenetic Abnormalities
Abstract
:1. Introduction
2. Structural and Regulation Aspects
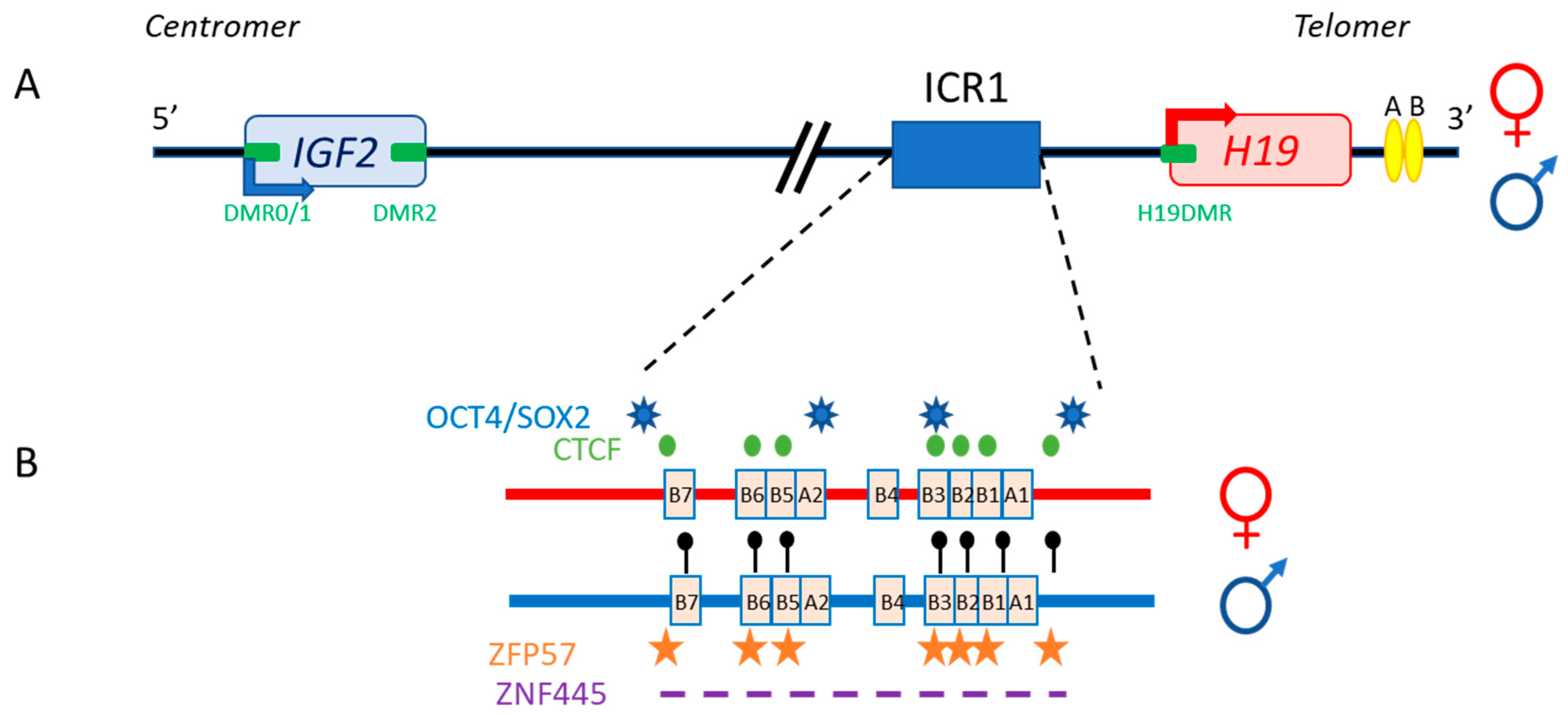
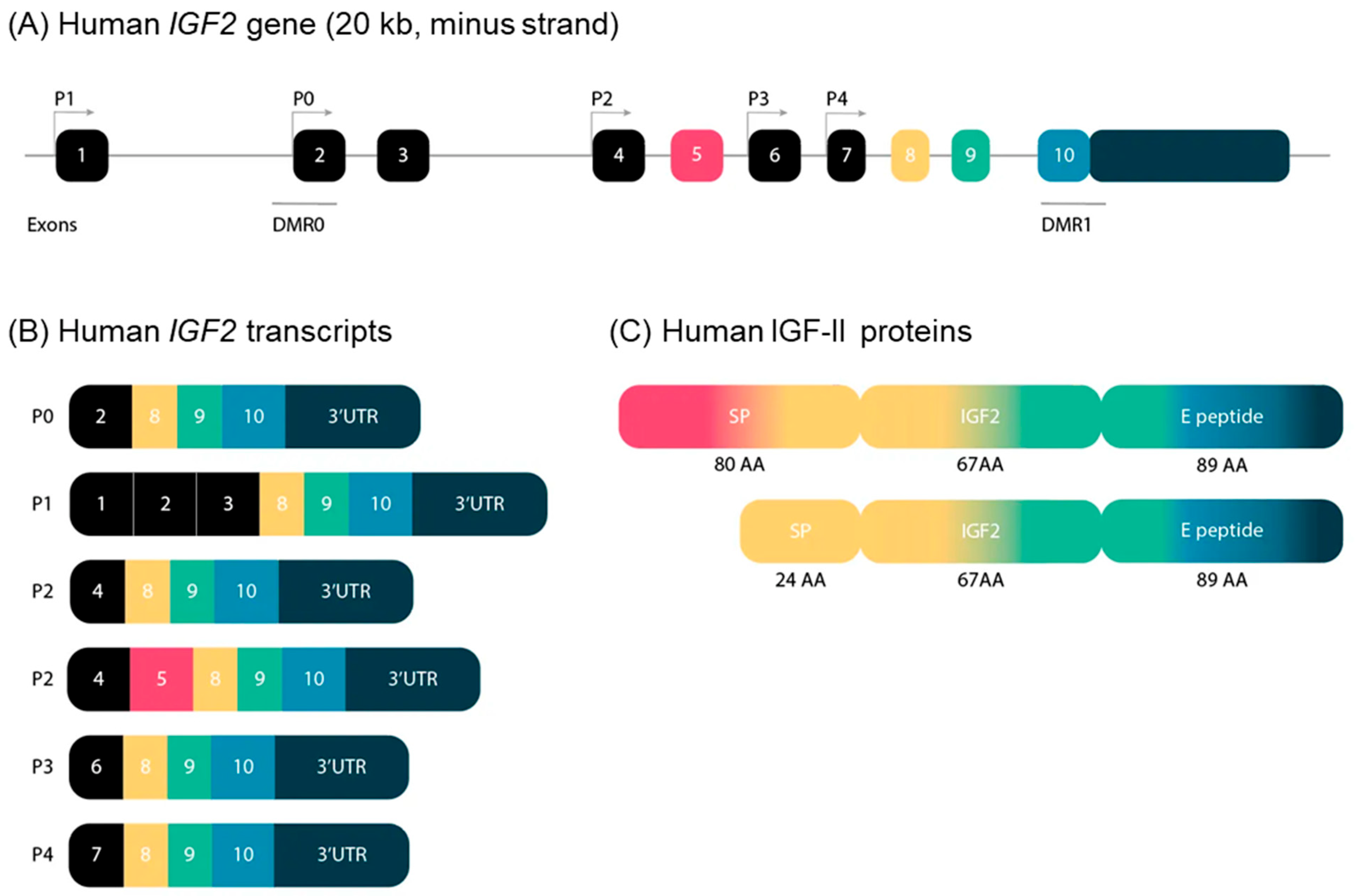
2.1. Main Characteristics, Linear Organization
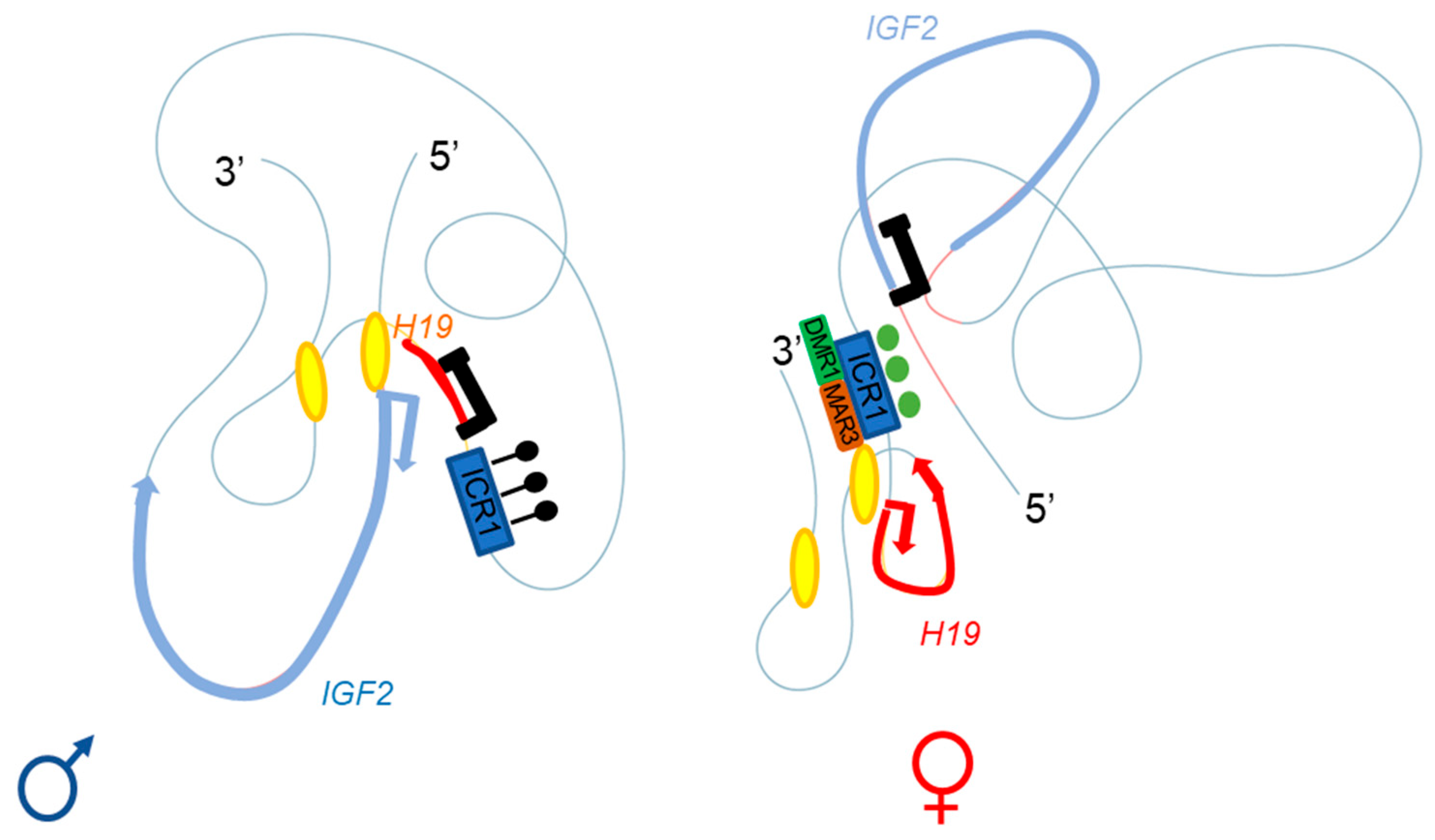
2.2. Three-Dimensional Organization
2.3. Trans-Regulation Mechanisms
3. Physiological Roles
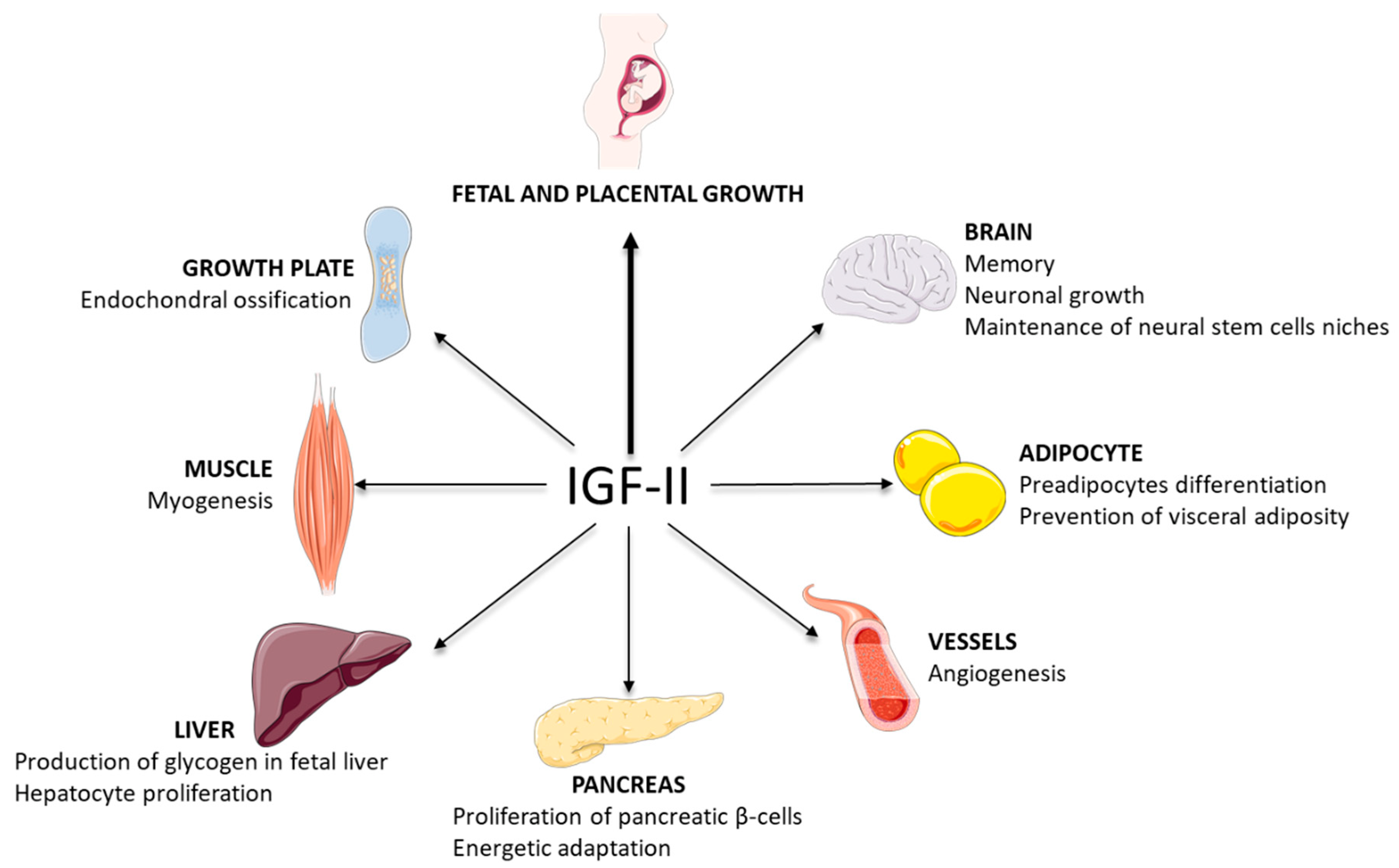
3.1. IGF-II: A Key Factor in Development
3.2. Some Roles of IGF-II in Tissues
4. Pathological Aspects
4.1. Silver–Russell Syndrome (SRS)
4.2. Temple Syndrome (TS14)
4.3. Wilms’ Tumors and Beckwith–Wiedemann Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Constância, M.; Hemberger, M.; Hughes, J.; Dean, W.; Ferguson-Smith, A.; Fundele, R.; Stewart, F.; Kelsey, G.; Fowden, A.; Sibley, C.; et al. Placental-Specific IGF-II Is a Major Modulator of Placental and Fetal Growth. Nature 2002, 417, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Giabicani, E.; Chantot-Bastaraud, S.; Bonnard, A.; Rachid, M.; Whalen, S.; Netchine, I.; Brioude, F. Roles of Type 1 Insulin-Like Growth Factor (IGF) Receptor and IGF-II in Growth Regulation: Evidence from a Patient Carrying Both an 11p Paternal Duplication and 15q Deletion. Front. Endocrinol. 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Berkel, H. Insulin-like Growth Factors and Cancer. J. State Med. Soc. 1999, 151, 218–223. [Google Scholar]
- Hakuno, F.; Takahashi, S.-I. IGF1 Receptor Signaling Pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef] [Green Version]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2013, 59, 240–249. [Google Scholar] [CrossRef]
- Chao, W.; D’Amore, P.A. IGF2: Epigenetic Regulation and Role in Development and Disease. Cytokine Growth Factor Rev. 2008, 19, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Henderson, S.T.; Brierley, G.V.; Surinya, K.H.; Priebe, I.K.; Catcheside, D.E.A.; Wallace, J.C.; Forbes, B.E.; Cosgrove, L.J. Delineation of the IGF-II C Domain Elements Involved in Binding and Activation of the IR-A, IR-B and IGF-IR. Growth Horm. IGF Res. 2015, 25, 20–27. [Google Scholar] [CrossRef]
- LeRoith, D.; Werner, H.; Beitner-Johnson, D.; Roberts, C.T. Molecular and Cellular Aspects of the Insulin-like Growth Factor I Receptor. Endocr. Rev. 1995, 16, 143–163. [Google Scholar] [CrossRef]
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Goldfine, I.D.; Belfiore, A.; Vigneri, R. Insulin Receptor Isoform A, a Newly Recognized, High-Affinity Insulin-like Growth Factor II Receptor in Fetal and Cancer Cells. Mol. Cell. Biol. 1999, 19, 3278–3288. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.; Nørgaard-Pedersen, D.; Brandt, J.; Pettersson, I.; Slaaby, R. IGF1 and IGF2 Specificities to the Two Insulin Receptor Isoforms Are Determined by Insulin Receptor Amino Acid 718. PLoS ONE 2017, 12, e0178885. [Google Scholar] [CrossRef] [Green Version]
- Tong, P.Y.; Tollefsen, S.E.; Kornfeld, S. The Cation-Independent Mannose 6-Phosphate Receptor Binds Insulin-like Growth Factor II. J. Biol. Chem. 1988, 263, 2585–2588. [Google Scholar] [CrossRef]
- Brown, J.; Delaine, C.; Zaccheo, O.J.; Siebold, C.; Gilbert, R.J.; van Boxel, G.; Denley, A.; Wallace, J.C.; Hassan, A.B.; Forbes, B.E.; et al. Structure and Functional Analysis of the IGF-II/IGF2R Interaction. EMBO J. 2008, 27, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, D.O.; Edman, J.C.; Standring, D.N.; Fried, V.A.; Smith, M.C.; Roth, R.A.; Rutter, W.J. Insulin-like Growth Factor II Receptor as a Multifunctional Binding Protein. Nature 1987, 329, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Structural and Functional Analysis of Insulin-like Growth Factors. Br. Med. Bull. 1989, 45, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Rozek, L.M.; Czech, M.P. Direct Demonstration of Rapid Insulin-like Growth Factor II Receptor Internalization and Recycling in Rat Adipocytes. Insulin Stimulates 125I-Insulin-like Growth Factor II Degradation by Modulating the IGF-II Receptor Recycling Process. J. Biol. Chem. 1985, 260, 9435–9442. [Google Scholar] [CrossRef]
- Yu, X.-W.; Pandey, K.; Katzman, A.C.; Alberini, C.M. A Role for CIM6P/IGF2 Receptor in Memory Consolidation and Enhancement. eLife 2020, 9, e54781. [Google Scholar] [CrossRef]
- Stern, S.A.; Chen, D.Y.; Alberini, C.M. The Effect of Insulin and Insulin-like Growth Factors on Hippocampus- and Amygdala-Dependent Long-Term Memory Formation. Learn. Mem. 2014, 21, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A Critical Role for IGF-II in Memory Consolidation and Enhancement. Nature 2011, 469, 491–497. [Google Scholar] [CrossRef]
- Oh, Y.; Müller, H.L.; Lee, D.Y.; Fielder, P.J.; Rosenfeld, R.G. Characterization of the Affinities of Insulin-like Growth Factor (IGF)-Binding Proteins 1-4 for IGF-I, IGF-II, IGF-I/Insulin Hybrid, and IGF-I Analogs. Endocrinology 1993, 132, 1337–1344. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like Growth Factors and Their Binding Proteins: Biological Actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of IGF-Binding Proteins in Regulating IGF Responses to Changes in Metabolism. J. Mol. Endocrinol. 2018, 61, T139–T169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, B.E.; McCarthy, P.; Norton, R.S. Insulin-Like Growth Factor Binding Proteins: A Structural Perspective. Front. Endocrin. 2012, 3, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, L.A. 40 Years of IGF1: IGF-Binding Proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, R.C. Characterization of the Acid-Labile Subunit of the Growth Hormone-Dependent Insulin-like Growth Factor Binding Protein Complex. J. Clin. Endocrinol. Metab. 1988, 67, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Frystyk, J.; Teran, E.; Gude, M.F.; Bjerre, M.; Hjortebjerg, R. Pregnancy-Associated Plasma Proteins and Stanniocalcin-2—Novel Players Controlling IGF-I Physiology. Growth Horm. IGF Res. 2020, 53–54, 101330. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Blat, C.; Villaudy, J.; Binoux, M. In Vivo Proteolysis of Serum Insulin-like Growth Factor (IGF) Binding Protein-3 Results in Increased Availability of IGF to Target Cells. J. Clin. Investig. 1994, 93, 2286–2290. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.B.; Oxvig, C.; Overgaard, M.T.; Sottrup-Jensen, L.; Gleich, G.J.; Hays, L.G.; Yates, J.R.; Conover, C.A. The Insulin-like Growth Factor (IGF)-Dependent IGF Binding Protein-4 Protease Secreted by Human Fibroblasts Is Pregnancy-Associated Plasma Protein-A. Proc. Natl. Acad. Sci. USA 1999, 96, 3149–3153. [Google Scholar] [CrossRef] [Green Version]
- Oxvig, C. The Role of PAPP-A in the IGF System: Location, Location, Location. J. Cell Commun. Signal. 2015, 9, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Barrios, V.; Chowen, J.A.; Martín-Rivada, Á.; Guerra-Cantera, S.; Pozo, J.; Yakar, S.; Rosenfeld, R.G.; Pérez-Jurado, L.A.; Suárez, J.; Argente, J. Pregnancy-Associated Plasma Protein (PAPP)-A2 in Physiology and Disease. Cells 2021, 10, 3576. [Google Scholar] [CrossRef]
- Dauber, A.; Muñoz-Calvo, M.T.; Barrios, V.; Domené, H.M.; Kloverpris, S.; Serra-Juhé, C.; Desikan, V.; Pozo, J.; Muzumdar, R.; Martos-Moreno, G.Á.; et al. Mutations in Pregnancy-Associated Plasma Protein A2 Cause Short Stature Due to Low IGF-I Availability. EMBO Mol. Med. 2016, 8, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Han, V.K.; D’Ercole, A.J.; Lund, P.K. Cellular Localization of Somatomedin (Insulin-like Growth Factor) Messenger RNA in the Human Fetus. Science 1987, 236, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental Phenotype and the Insulin-like Growth Factors: Resource Allocation to Fetal Growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Hoffman, A.R. Promoter-Specific Imprinting of the Human Insulin-like Growth Factor-II Gene. Nature 1994, 371, 714–717. [Google Scholar] [CrossRef]
- Toogood, A.A.; Jones, J.; O’Neill, P.A.; Thorner, M.O.; Shalet, S.M. The Diagnosis of Severe Growth Hormone Deficiency in Elderly Patients with Hypothalamic-Pituitary Disease: Diagnosis of GH Deficiency in the Elderly. Clin. Endocrinol. 1998, 48, 569–576. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Efstratiadis, A.; Robertsen, E.J. A Growth-Deficiency Phenotype in Heterozygous Mice Carrying an Insulin-like Growth Factor II Gene Disrupted by Targeting. Nature 1990, 345, 78–80. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Robertson, E.J.; Efstratiadis, A. Parental Imprinting of the Mouse Insulin-like Growth Factor II Gene. Cell 1991, 64, 849–859. [Google Scholar] [CrossRef]
- Baral, K.; Rotwein, P. The Insulin-like Growth Factor 2 Gene in Mammals: Organizational Complexity within a Conserved Locus. PLoS ONE 2019, 14, e0219155. [Google Scholar] [CrossRef]
- De Pagter-Holthuizen, P.; Jansen, M.; van Schaik, F.M.A.; van der Kammen, R.; Oosterwijk, C.; Van den Brande, J.L.; Sussenbach, J.S. The Human Insulin-like Growth Factor II Gene Contains Two Development-Specific Promoters. FEBS Lett. 1987, 214, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Zapf, J.; Futo, E.; Peter, M.; Froesch, E.R. Can “Big” Insulin-like Growth Factor II in Serum of Tumor Patients Account for the Development of Extrapancreatic Tumor Hypoglycemia? J. Clin. Investig. 1992, 90, 2574–2584. [Google Scholar] [CrossRef] [Green Version]
- Rotwein, P. Large-Scale Analysis of Variation in the Insulin-like Growth Factor Family in Humans Reveals Rare Disease Links and Common Polymorphisms. J. Biol. Chem. 2017, 292, 9252–9261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.; Constancia, M.; Zubair, M.; Bailleul, B.; Feil, R.; Sasaki, H.; Reik, W. Multiple Imprinted Sense and Antisense Transcripts, Differential Methylation and Tandem Repeats in a Putative Imprinting Control Region Upstream of Mouse Igf2. Proc. Natl. Acad. Sci. USA 1997, 94, 12509–12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Aitchison, A.; Gregory, R.; Allen, N.D.; Feil, R. Parental Allele-Specific Chromatin Configuration in a Boundary-Imprinting-Control Element Upstream of the Mouse H19 Gene. Mol. Cell. Biol. 1999, 19, 2556–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.E.; Corces, V.G. CTCF: Master Weaver of the Genome. Cell 2009, 137, 1194–1211. [Google Scholar] [CrossRef] [Green Version]
- Takai, D.; Gonzales, F.A.; Tsai, Y.C.; Thayer, M.J.; Jones, P.A. Large Scale Mapping of Methylcytosines in CTCF-Binding Sites in the Human H19 Promoter and Aberrant Hypomethylation in Human Bladder Cancer. Hum. Mol. Genet. 2001, 10, 2619–2626. [Google Scholar] [CrossRef] [Green Version]
- Abi Habib, W.; Azzi, S.; Brioude, F.; Steunou, V.; Thibaud, N.; Das Neves, C.; Le Jule, M.; Chantot-Bastaraud, S.; Keren, B.; Lyonnet, S.; et al. Extensive Investigation of the IGF2/H19 Imprinting Control Region Reveals Novel OCT4/SOX2 Binding Site Defects Associated with Specific Methylation Patterns in Beckwith-Wiedemann Syndrome. Hum. Mol. Genet. 2014, 23, 5763–5773. [Google Scholar] [CrossRef] [Green Version]
- Kurukuti, S.; Tiwari, V.K.; Tavoosidana, G.; Pugacheva, E.; Murrell, A.; Zhao, Z.; Lobanenkov, V.; Reik, W.; Ohlsson, R. CTCF Binding at the H19 Imprinting Control Region Mediates Maternally Inherited Higher-Order Chromatin Conformation to Restrict Enhancer Access to Igf2. Proc. Natl. Acad. Sci. USA 2006, 103, 10684–10689. [Google Scholar] [CrossRef] [Green Version]
- Demars, J.; Shmela, M.E.; Rossignol, S.; Okabe, J.; Netchine, I.; Azzi, S.; Cabrol, S.; Le Caignec, C.; David, A.; Le Bouc, Y.; et al. Analysis of the IGF2/H19 Imprinting Control Region Uncovers New Genetic Defects, Including Mutations of OCT-Binding Sequences, in Patients with 11p15 Fetal Growth Disorders. Hum. Mol. Genet. 2010, 19, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Weth, O.; Renkawitz, R. CTCF Function Is Modulated by Neighboring DNA Binding Factors. Biochem. Cell Biol. 2011, 89, 459–468. [Google Scholar] [CrossRef]
- Zimmerman, D.L.; Boddy, C.S.; Schoenherr, C.S. Oct4/Sox2 Binding Sites Contribute to Maintaining Hypomethylation of the Maternal Igf2/H19 Imprinting Control Region. PLoS ONE 2013, 8, e81962. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, R.; Okamura, E.; Matsuzaki, H.; Fukamizu, A.; Tanimoto, K. Sox-Oct Motifs Contribute to Maintenance of the Unmethylated H19 ICR in YAC Transgenic Mice. Hum. Mol. Genet. 2013, 22, 4627–4637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abi Habib, W.; Brioude, F.; Azzi, S.; Salem, J.; Das Neves, C.; Personnier, C.; Chantot-Bastaraud, S.; Keren, B.; Le Bouc, Y.; Harbison, M.D.; et al. 11p15 ICR1 Partial Deletions Associated with IGF2/H19 DMR Hypomethylation and Silver-Russell Syndrome. Hum. Mutat. 2017, 38, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Quenneville, S.; Verde, G.; Corsinotti, A.; Kapopoulou, A.; Jakobsson, J.; Offner, S.; Baglivo, I.; Pedone, P.V.; Grimaldi, G.; Riccio, A.; et al. In Embryonic Stem Cells, ZFP57/KAP1 Recognize a Methylated Hexanucleotide to Affect Chromatin and DNA Methylation of Imprinting Control Regions. Mol. Cell 2011, 44, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Coluccio, A.; Thorball, C.W.; Planet, E.; Shi, H.; Offner, S.; Turelli, P.; Imbeault, M.; Ferguson-Smith, A.C.; Trono, D. ZNF445 Is a Primary Regulator of Genomic Imprinting. Genes Dev. 2019, 33, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Monk, D.; Sanches, R.; Arnaud, P.; Apostolidou, S.; Hills, F.A.; Abu-Amero, S.; Murrell, A.; Friess, H.; Reik, W.; Stanier, P.; et al. Imprinting of IGF2 P0 Transcript and Novel Alternatively Spliced INS-IGF2 Isoforms Show Differences between Mouse and Human. Hum. Mol. Genet. 2006, 15, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Dekker, J.; Rippe, K.; Dekker, M.; Kleckner, N. Capturing Chromosome Conformation. Science 2002, 295, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Arney, K.L. H19 and Igf2—Enhancing the Confusion? Trends Genet. 2003, 19, 17–23. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Jeong, S.; Rong, Q.; Park, K.-Y.; Chung, J.H.; Pfeifer, K. Analysis of the H19ICR Insulator. Mol. Cell. Biol. 2007, 27, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Llères, D.; Moindrot, B.; Pathak, R.; Piras, V.; Matelot, M.; Pignard, B.; Marchand, A.; Poncelet, M.; Perrin, A.; Tellier, V.; et al. CTCF Modulates Allele-Specific Sub-TAD Organization and Imprinted Gene Activity at the Mouse Dlk1-Dio3 and Igf2-H19 Domains. Genome Biol. 2019, 20, 272. [Google Scholar] [CrossRef] [Green Version]
- Rovina, D.; La Vecchia, M.; Cortesi, A.; Fontana, L.; Pesant, M.; Maitz, S.; Tabano, S.; Bodega, B.; Miozzo, M.; Sirchia, S.M. Profound Alterations of the Chromatin Architecture at Chromosome 11p15.5 in Cells from Beckwith-Wiedemann and Silver-Russell Syndromes Patients. Sci. Rep. 2020, 10, 8275. [Google Scholar] [CrossRef]
- Abi Habib, W.; Brioude, F.; Edouard, T.; Bennett, J.T.; Lienhardt-Roussie, A.; Tixier, F.; Salem, J.; Yuen, T.; Azzi, S.; Le Bouc, Y.; et al. Genetic Disruption of the Oncogenic HMGA2-PLAG1-IGF2 Pathway Causes Fetal Growth Restriction. Genet. Med. 2018, 20, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voz, M.L.; Agten, N.S.; Van de Ven, W.J.; Kas, K. PLAG1, the Main Translocation Target in Pleomorphic Adenoma of the Salivary Glands, Is a Positive Regulator of IGF-II. Cancer Res. 2000, 60, 106–113. [Google Scholar] [PubMed]
- Van Dyck, F.; Declercq, J.; Braem, C.V.; Van de Ven, W.J.M. PLAG1, the Prototype of the PLAG Gene Family: Versatility in Tumour Development. Int. J. Oncol. 2007, 30, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensen, K.; Braem, C.; Declercq, J.; Van Dyck, F.; Dewerchin, M.; Fiette, L.; Denef, C.; Van de Ven, W.J.M. Targeted Disruption of the Murine Plag1 Proto-Oncogene Causes Growth Retardation and Reduced Fertility. Dev. Growth Differ. 2004, 46, 459–470. [Google Scholar] [CrossRef]
- Manfioletti, G.; Giancotti, V.; Bandiera, A.; Buratti, E.; Sautière, P.; Cary, P.; Crane-Robinson, C.; Coles, B.; Goodwin, G.H. CDNA Cloning of the HMGI-C Phosphoprotein, a Nuclear Protein Associated with Neoplastic and Undifferentiated Phenotypes. Nucleic Acids Res. 1991, 19, 6793–6797. [Google Scholar] [CrossRef]
- Zhou, X.; Benson, K.F.; Ashar, H.R.; Chada, K. Mutation Responsible for the Mouse Pygmy Phenotype in the Developmentally Regulated Factor HMGI-C. Nature 1995, 376, 771–774. [Google Scholar] [CrossRef]
- Klemke, M.; Müller, M.H.; Wosniok, W.; Markowski, D.N.; Nimzyk, R.; Helmke, B.M.; Bullerdiek, J. Correlated Expression of HMGA2 and PLAG1 in Thyroid Tumors, Uterine Leiomyomas and Experimental Models. PLoS ONE 2014, 9, e88126. [Google Scholar] [CrossRef]
- Astuti, D.; Morris, M.R.; Cooper, W.N.; Staals, R.H.J.; Wake, N.C.; Fews, G.A.; Gill, H.; Gentle, D.; Shuib, S.; Ricketts, C.J.; et al. Germline Mutations in DIS3L2 Cause the Perlman Syndrome of Overgrowth and Wilms Tumor Susceptibility. Nat. Genet. 2012, 44, 277–284. [Google Scholar] [CrossRef]
- Hunter, R.W.; Liu, Y.; Manjunath, H.; Acharya, A.; Jones, B.T.; Zhang, H.; Chen, B.; Ramalingam, H.; Hammer, R.E.; Xie, Y.; et al. Loss of Dis3l2 Partially Phenocopies Perlman Syndrome in Mice and Results in Up-Regulation of Igf2 in Nephron Progenitor Cells. Genes Dev. 2018, 32, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Varrault, A.; Gueydan, C.; Delalbre, A.; Bellmann, A.; Houssami, S.; Aknin, C.; Severac, D.; Chotard, L.; Kahli, M.; Le Digarcher, A.; et al. Zac1 Regulates an Imprinted Gene Network Critically Involved in the Control of Embryonic Growth. Dev. Cell 2006, 11, 711–722. [Google Scholar] [CrossRef]
- Gabory, A.; Ripoche, M.-A.; Le Digarcher, A.; Watrin, F.; Ziyyat, A.; Forné, T.; Jammes, H.; Ainscough, J.F.X.; Surani, M.A.; Journot, L.; et al. H19 Acts as a Trans Regulator of the Imprinted Gene Network Controlling Growth in Mice. Development 2009, 136, 3413–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Adhami, H.; Evano, B.; Le Digarcher, A.; Gueydan, C.; Dubois, E.; Parrinello, H.; Dantec, C.; Bouschet, T.; Varrault, A.; Journot, L. A Systems-Level Approach to Parental Genomic Imprinting: The Imprinted Gene Network Includes Extracellular Matrix Genes and Regulates Cell Cycle Exit and Differentiation. Genome Res. 2015, 25, 353–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whipple, A.J.; Breton-Provencher, V.; Jacobs, H.N.; Chitta, U.K.; Sur, M.; Sharp, P.A. Imprinted Maternally Expressed MicroRNAs Antagonize Paternally Driven Gene Programs in Neurons. Mol. Cell 2020, 78, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Eggermann, T.; Davies, J.H.; Tauber, M.; van den Akker, E.; Hokken-Koelega, A.; Johansson, G.; Netchine, I. Growth Restriction and Genomic Imprinting-Overlapping Phenotypes Support the Concept of an Imprinting Network. Genes 2021, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Geoffron, S.; Abi Habib, W.; Chantot-Bastaraud, S.; Dubern, B.; Steunou, V.; Azzi, S.; Afenjar, A.; Busa, T.; Pinheiro Canton, A.; Chalouhi, C.; et al. Chromosome 14q32.2 Imprinted Region Disruption as an Alternative Molecular Diagnosis of Silver-Russell Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 2436–2446. [Google Scholar] [CrossRef] [Green Version]
- Wakeling, E.L.; Brioude, F.; Lokulo-Sodipe, O.; O’Connell, S.M.; Salem, J.; Bliek, J.; Canton, A.P.M.; Chrzanowska, K.H.; Davies, J.H.; Dias, R.P.; et al. Diagnosis and Management of Silver-Russell Syndrome: First International Consensus Statement. Nat. Rev. Endocrinol. 2017, 13, 105–124. [Google Scholar] [CrossRef]
- Abi Habib, W.; Brioude, F.; Azzi, S.; Rossignol, S.; Linglart, A.; Sobrier, M.-L.; Giabicani, É.; Steunou, V.; Harbison, M.D.; Le Bouc, Y.; et al. Transcriptional Profiling at the DLK1/MEG3 Domain Explains Clinical Overlap between Imprinting Disorders. Sci. Adv. 2019, 5, eaau9425. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Ohashi, S.; Kizaki, Y.; Naito, J.; Horiguchi, K.; Harigaya, T. Expression Profiling of Mouse Placental Lactogen II and Its Correlative Genes Using a CDNA Microarray Analysis in the Developmental Mouse Placenta. J. Reprod. Dev. 2007, 53, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Coan, P.M.; Fowden, A.L.; Constancia, M.; Ferguson-Smith, A.C.; Burton, G.J.; Sibley, C.P. Disproportional Effects of Igf2 Knockout on Placental Morphology and Diffusional Exchange Characteristics in the Mouse. J. Physiol. 2008, 586, 5023–5032. [Google Scholar] [CrossRef]
- Sibley, C.P.; Coan, P.M.; Ferguson-Smith, A.C.; Dean, W.; Hughes, J.; Smith, P.; Reik, W.; Burton, G.J.; Fowden, A.L.; Constância, M. Placental-Specific Insulin-like Growth Factor 2 (Igf2) Regulates the Diffusional Exchange Characteristics of the Mouse Placenta. Proc. Natl. Acad. Sci. USA 2004, 101, 8204–8208. [Google Scholar] [CrossRef] [Green Version]
- Sandovici, I.; Georgopoulou, A.; Pérez-García, V.; Hufnagel, A.; López-Tello, J.; Lam, B.Y.H.; Schiefer, S.N.; Gaudreau, C.; Santos, F.; Hoelle, K.; et al. The Imprinted Igf2-Igf2r Axis Is Critical for Matching Placental Microvasculature Expansion to Fetal Growth. Dev. Cell 2022, 57, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only Humans Have Human Placentas: Molecular Differences between Mice and Humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Malassiné, A.; Frendo, J.L.; Evain-Brion, D. A Comparison of Placental Development and Endocrine Functions between the Human and Mouse Model. Hum. Reprod. Update 2003, 9, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazawa, K.; Kagami, M.; Nagai, T.; Kondoh, T.; Onigata, K.; Maeyama, K.; Hasegawa, T.; Hasegawa, Y.; Yamazaki, T.; Mizuno, S.; et al. Molecular and Clinical Findings and Their Correlations in Silver-Russell Syndrome: Implications for a Positive Role of IGF2 in Growth Determination and Differential Imprinting Regulation of the IGF2–H19 Domain in Bodies and Placentas. J. Mol. Med. 2008, 86, 1171–1181. [Google Scholar] [CrossRef]
- Harris, L.K.; Crocker, I.P.; Baker, P.N.; Aplin, J.D.; Westwood, M. IGF2 Actions on Trophoblast in Human Placenta Are Regulated by the Insulin-like Growth Factor 2 Receptor, Which Can Function as Both a Signaling and Clearance Receptor. Biol. Reprod. 2011, 84, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillot-Durand, L.; Brioude, F.; Beneteau, C.; Le Breton, F.; Massardier, J.; Michon, L.; Devouassoux-Shisheboran, M.; Allias, F. Placental Pathology in Beckwith-Wiedemann Syndrome According to Genotype/Epigenotype Subgroups. Fetal Pediatr. Pathol. 2018, 37, 387–399. [Google Scholar] [CrossRef]
- Armes, J.E.; McGown, I.; Williams, M.; Broomfield, A.; Gough, K.; Lehane, F.; Lourie, R. The Placenta in Beckwith-Wiedemann Syndrome: Genotype-Phenotype Associations, Excessive Extravillous Trophoblast and Placental Mesenchymal Dysplasia. Pathology 2012, 44, 519–527. [Google Scholar] [CrossRef]
- Morali, O.G.; Jouneau, A.; McLaughlin, K.J.; Thiery, J.P.; Larue, L. IGF-II Promotes Mesoderm Formation. Dev. Biol. 2000, 227, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cui, H.; Sandstedt, B.; Nordlinder, H.; Larsson, E.; Ekström, T.J. Expression Levels of the Insulin-like Growth Factor-II Gene (IGF2) in the Human Liver: Developmental Relationships of the Four Promoters. J. Endocrinol. 1996, 149, 117–124. [Google Scholar] [CrossRef]
- Barton, S.C.; Surani, M.A.; Norris, M.L. Role of Paternal and Maternal Genomes in Mouse Development. Nature 1984, 311, 374–376. [Google Scholar] [CrossRef]
- McGrath, J.; Solter, D. Inability of Mouse Blastomere Nuclei Transferred to Enucleated Zygotes to Support Development in Vitro. Science 1984, 226, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Baron, J. Evidence That Igf2 Down-Regulation in Postnatal Tissues and up-Regulation in Malignancies Is Driven by Transcription Factor E2f3. Proc. Natl. Acad. Sci. USA 2013, 110, 6181–6186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Lucas, M.; Viana da Silva, S.; Di Scala, M.; Garcia-Barroso, C.; González-Aseguinolaza, G.; Mulle, C.; Alberini, C.M.; Cuadrado-Tejedor, M.; Garcia-Osta, A. Insulin-like Growth Factor 2 Reverses Memory and Synaptic Deficits in APP Transgenic Mice. EMBO Mol. Med. 2014, 6, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, M.J.; Baumann, B.; Johannsen, S.; Vindedal, G.F.; Jensen, V.; Hvalby, Ø.C.; Sprengel, R.; Seither, J.; Maqbool, A.; Magnutzki, A.; et al. IκB Kinase/Nuclear Factor ΚB-Dependent Insulin-like Growth Factor 2 (Igf2) Expression Regulates Synapse Formation and Spine Maturation via Igf2 Receptor Signaling. J. Neurosci. 2012, 32, 5688–5703. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Feng, Q.; Chidambaram, S.; Testai, J.M.; Kumari, E.; Rothbard, D.E.; Constancia, M.; Sandovici, I.; Cominski, T.; Pang, K.; et al. Insulin-like Growth Factor II: An Essential Adult Stem Cell Niche Constituent in Brain and Intestine. Stem Cell Rep. 2019, 12, 816–830. [Google Scholar] [CrossRef] [Green Version]
- Rotwein, P. The Complex Genetics of Human Insulin-like Growth Factor 2 Are Not Reflected in Public Databases. J. Biol. Chem. 2018, 293, 4324–4333. [Google Scholar] [CrossRef] [Green Version]
- van Dijk, M.A.; Holthuizen, P.E.; Sussenbach, J.S. Elements Required for Activation of the Major Promoter of the Human Insulin-like Growth Factor II Gene. Mol. Cell. Endocrinol. 1992, 88, 175–185. [Google Scholar] [CrossRef]
- Jin, I.H.; Sinha, G.; Yballe, C.; Vu, T.H.; Hoffman, A.R. The Human Insulin-like Growth Factor-II Promoter P1 Is Not Restricted to Liver: Evidence for Expression of P1 in Other Tissues and for a Homologous Promoter in Baboon Liver. Horm. Metab. Res. 1995, 27, 447–449. [Google Scholar] [CrossRef]
- Uchimura, T.; Hollander, J.M.; Nakamura, D.S.; Liu, Z.; Rosen, C.J.; Georgakoudi, I.; Zeng, L. An Essential Role for IGF2 in Cartilage Development and Glucose Metabolism during Postnatal Long Bone Growth. Development 2017, 144, 3533–3546. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jiang, W.; Huang, J.; He, B.-C.; Zuo, G.-W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.-Q.; Wagner, E.R.; et al. Insulin-like Growth Factor 2 (IGF-2) Potentiates BMP-9-Induced Osteogenic Differentiation and Bone Formation. J. Bone Miner. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef] [Green Version]
- Hamamura, K.; Zhang, P.; Yokota, H. IGF2-Driven PI3 Kinase and TGFbeta Signaling Pathways in Chondrogenesis. Cell Biol. Int. 2008, 32, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piecewicz, S.M.; Pandey, A.; Roy, B.; Xiang, S.H.; Zetter, B.R.; Sengupta, S. Insulin-like Growth Factors Promote Vasculogenesis in Embryonic Stem Cells. PLoS ONE 2012, 7, e32191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallinga, M.G.; Yetkin-Arik, B.; Kayser, R.P.; Vogels, I.M.C.; Nowak-Sliwinska, P.; Griffioen, A.W.; van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. IGF2 and IGF1R Identified as Novel Tip Cell Genes in Primary Microvascular Endothelial Cell Monolayers. Angiogenesis 2018, 21, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallinga, M.G.; Habani, Y.I.; Kayser, R.P.; Van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. IGF-Binding Proteins 3 and 4 Are Regulators of Sprouting Angiogenesis. Mol. Biol. Rep. 2020, 47, 2561–2572. [Google Scholar] [CrossRef] [Green Version]
- Alfares, M.N.; Perks, C.M.; Hamilton-Shield, J.P.; Holly, J.M.P. Insulin-like Growth Factor-II in Adipocyte Regulation: Depot-Specific Actions Suggest a Potential Role Limiting Excess Visceral Adiposity. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E1098–E1107. [Google Scholar] [CrossRef]
- Liang, L.; Guo, W.H.; Esquiliano, D.R.; Asai, M.; Rodriguez, S.; Giraud, J.; Kushner, J.A.; White, M.F.; Lopez, M.F. Insulin-Like Growth Factor 2 and the Insulin Receptor, But Not Insulin, Regulate Fetal Hepatic Glycogen Synthesis. Endocrinology 2010, 151, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-J.; Chen, F.; Liu, Q.-G.; Liu, C.-C.; Yao, H.; Yu, B.; Zhang, H.-B.; Yan, H.-X.; Ye, Y.; Chen, T.; et al. Insulin-like Growth Factor 2 Is a Key Mitogen Driving Liver Repopulation in Mice. Cell Death Dis. 2018, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Portela-Gomes, G.M.; Höög, A. Insulin-like Growth Factor II in Human Fetal Pancreas and Its Co-Localization with the Major Islet Hormones: Comparison with Adult Pancreas. J. Endocrinol. 2000, 165, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Hammerle, C.M.; Sandovici, I.; Brierley, G.V.; Smith, N.M.; Zimmer, W.E.; Zvetkova, I.; Prosser, H.M.; Sekita, Y.; Lam, B.Y.H.; Ma, M.; et al. Mesenchyme-Derived IGF2 Is a Major Paracrine Regulator of Pancreatic Growth and Function. PLoS Genet. 2020, 16, e1009069. [Google Scholar] [CrossRef]
- Sandovici, I.; Hammerle, C.M.; Virtue, S.; Vivas-Garcia, Y.; Izquierdo-Lahuerta, A.; Ozanne, S.E.; Vidal-Puig, A.; Medina-Gómez, G.; Constância, M. Autocrine IGF2 Programmes β-Cell Plasticity under Conditions of Increased Metabolic Demand. Sci. Rep. 2021, 11, 7717. [Google Scholar] [CrossRef]
- Wilson, E.M.; Rotwein, P. Control of MyoD Function during Initiation of Muscle Differentiation by an Autocrine Signaling Pathway Activated by Insulin-like Growth Factor-II. J. Biol. Chem. 2006, 281, 29962–29971. [Google Scholar] [CrossRef] [Green Version]
- Aboalola, D.; Han, V.K.M. Different Effects of Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor-2 on Myogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 8286248. [Google Scholar] [CrossRef] [Green Version]
- Erbay, E.; Park, I.-H.; Nuzzi, P.D.; Schoenherr, C.J.; Chen, J. IGF-II Transcription in Skeletal Myogenesis Is Controlled by MTOR and Nutrients. J. Cell Biol. 2003, 163, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Luo, W.; Abdalla, B.A.; Ouyang, H.; Yu, J.; Hu, F.; Nie, Q.; Zhang, X. MiRNA-223 Upregulated by MYOD Inhibits Myoblast Proliferation by Repressing IGF2 and Facilitates Myoblast Differentiation by Inhibiting ZEB1. Cell Death Dis. 2017, 8, e3094. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, Y.; Chen, J. IGF-II Is Regulated by MicroRNA-125b in Skeletal Myogenesis. J. Cell Biol. 2011, 192, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The Insulin-like Growth Factor System and Its Pleiotropic Functions in Brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef] [Green Version]
- Cline, B.H.; Steinbusch, H.W.; Malin, D.; Revishchin, A.V.; Pavlova, G.V.; Cespuglio, R.; Strekalova, T. The Neuronal Insulin Sensitizer Dicholine Succinate Reduces Stress-Induced Depressive Traits and Memory Deficit: Possible Role of Insulin-like Growth Factor 2. BMC Neurosci. 2012, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Bannerman, D.M.; Rawlins, J.N.P.; McHugh, S.B.; Deacon, R.M.J.; Yee, B.K.; Bast, T.; Zhang, W.-N.; Pothuizen, H.H.J.; Feldon, J. Regional Dissociations within the Hippocampus—Memory and Anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Fromer, M.; Roussos, P.; Sieberts, S.K.; Johnson, J.S.; Kavanagh, D.H.; Perumal, T.M.; Ruderfer, D.M.; Oh, E.C.; Topol, A.; Shah, H.R.; et al. Gene Expression Elucidates Functional Impact of Polygenic Risk for Schizophrenia. Nat. Neurosci. 2016, 19, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, Y.; Banno, Y.; Shimizu, Y.; Ando, S.; Hasegawa, H.; Adachi, K.; Iwamoto, T. Reduced Adult Hippocampal Neurogenesis and Working Memory Deficits in the Dgcr8-Deficient Mouse Model of 22q11.2 Deletion-Associated Schizophrenia Can Be Rescued by IGF2. J. Neurosci. 2013, 33, 9408–9419. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Luo, T.; Zhao, Y.; Jiang, S.-Z.; Xiong, J.-W.; Zhan, J.-Q.; Yu, B.; Yan, K.; Wei, B. Altered Insulin-like Growth Factor-2 Signaling Is Associated with Psychopathology and Cognitive Deficits in Patients with Schizophrenia. PLoS ONE 2020, 15, e0226688. [Google Scholar] [CrossRef] [Green Version]
- Allodi, I.; Comley, L.; Nichterwitz, S.; Nizzardo, M.; Simone, C.; Benitez, J.A.; Cao, M.; Corti, S.; Hedlund, E. Differential Neuronal Vulnerability Identifies IGF-2 as a Protective Factor in ALS. Sci. Rep. 2016, 6, 25960. [Google Scholar] [CrossRef] [Green Version]
- García-Huerta, P.; Troncoso-Escudero, P.; Wu, D.; Thiruvalluvan, A.; Cisternas-Olmedo, M.; Henríquez, D.R.; Plate, L.; Chana-Cuevas, P.; Saquel, C.; Thielen, P.; et al. Insulin-like Growth Factor 2 (IGF2) Protects against Huntington’s Disease through the Extracellular Disposal of Protein Aggregates. Acta Neuropathol. 2020, 140, 737–764. [Google Scholar] [CrossRef]
- Osborn, T.M.; Beagan, J.; Isacson, O. Increased Motor Neuron Resilience by Small Molecule Compounds That Regulate IGF-II Expression. Neurobiol. Dis. 2018, 110, 218–230. [Google Scholar] [CrossRef]
- Steinmetz, A.B.; Stern, S.A.; Kohtz, A.S.; Descalzi, G.; Alberini, C.M. Insulin-Like Growth Factor II Targets the MTOR Pathway to Reverse Autism-Like Phenotypes in Mice. J. Neurosci. 2018, 38, 1015–1029. [Google Scholar] [CrossRef]
- Patti, G.; De Mori, L.; Tortora, D.; Severino, M.; Calevo, M.; Russo, S.; Napoli, F.; Confalonieri, L.; Schiavone, M.; Thiabat, H.F.; et al. Cognitive Profiles and Brain Volume Are Affected in Patients with Silver–Russell Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, e1478–e1488. [Google Scholar] [CrossRef]
- Azzi, S.; Salem, J.; Thibaud, N.; Chantot-Bastaraud, S.; Lieber, E.; Netchine, I.; Harbison, M.D. A Prospective Study Validating a Clinical Scoring System and Demonstrating Phenotypical-Genotypical Correlations in Silver-Russell Syndrome. J. Med. Genet. 2015, 52, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Begemann, M.; Zirn, B.; Santen, G.; Wirthgen, E.; Soellner, L.; Büttel, H.-M.; Schweizer, R.; van Workum, W.; Binder, G.; Eggermann, T. Paternally Inherited IGF2 Mutation and Growth Restriction. N. Engl. J. Med. 2015, 373, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Gicquel, C.; Rossignol, S.; Cabrol, S.; Houang, M.; Steunou, V.; Barbu, V.; Danton, F.; Thibaud, N.; Le Merrer, M.; Burglen, L.; et al. Epimutation of the Telomeric Imprinting Center Region on Chromosome 11p15 in Silver-Russell Syndrome. Nat. Genet. 2005, 37, 1003–1007. [Google Scholar] [CrossRef]
- Netchine, I.; Rossignol, S.; Dufourg, M.-N.; Azzi, S.; Rousseau, A.; Perin, L.; Houang, M.; Steunou, V.; Esteva, B.; Thibaud, N.; et al. 11p15 Imprinting Center Region 1 Loss of Methylation Is a Common and Specific Cause of Typical Russell-Silver Syndrome: Clinical Scoring System and Epigenetic-Phenotypic Correlations. J. Clin. Endocrinol. Metab. 2007, 92, 3148–3154. [Google Scholar] [CrossRef]
- Masunaga, Y.; Inoue, T.; Yamoto, K.; Fujisawa, Y.; Sato, Y.; Kawashima-Sonoyama, Y.; Morisada, N.; Iijima, K.; Ohata, Y.; Namba, N.; et al. IGF2 Mutations. J. Clin. Endocrinol. Metab. 2020, 105, 116–125. [Google Scholar] [CrossRef]
- Brioude, F.; Oliver-Petit, I.; Blaise, A.; Praz, F.; Rossignol, S.; Le Jule, M.; Thibaud, N.; Faussat, A.-M.; Tauber, M.; Le Bouc, Y.; et al. CDKN1C Mutation Affecting the PCNA-Binding Domain as a Cause of Familial Russell Silver Syndrome. J. Med. Genet. 2013, 50, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lokulo-Sodipe, O.; Ballard, L.; Child, J.; Inskip, H.M.; Byrne, C.D.; Ishida, M.; Moore, G.E.; Wakeling, E.L.; Fenwick, A.; Mackay, D.J.G.; et al. Phenotype of Genetically Confirmed Silver-Russell Syndrome beyond Childhood. J. Med. Genet. 2020, 57, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Steen, M.; Lem, A.J.; van der Kaay, D.C.M.; Bakker-van Waarde, W.M.; van der Hulst, F.J.P.C.M.; Neijens, F.S.; Noordam, C.; Odink, R.J.; Oostdijk, W.; Schroor, E.J.; et al. Metabolic Health in Short Children Born Small for Gestational Age Treated with Growth Hormone and Gonadotropin-Releasing Hormone Analog: Results of a Randomized, Dose-Response Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal Nutrition and Cardiovascular Disease in Adult Life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Temple, I.K.; Cockwell, A.; Hassold, T.; Pettay, D.; Jacobs, P. Maternal Uniparental Disomy for Chromosome 14. J. Med. Genet. 1991, 28, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Kagami, M.; Nagasaki, K.; Kosaki, R.; Horikawa, R.; Naiki, Y.; Saitoh, S.; Tajima, T.; Yorifuji, T.; Numakura, C.; Mizuno, S.; et al. Temple Syndrome: Comprehensive Molecular and Clinical Findings in 32 Japanese Patients. Genet. Med. 2017, 19, 1356–1366. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.; Cowell, J.; Robertson, M.E.; Priestley, L.M.; Wadey, R.; Hopkins, B.; Pritchard, J.; Bell, G.I.; Rall, L.B.; Graham, C.F. Insulin-like Growth Factor-II Gene Expression in Wilms’ Tumour and Embryonic Tissues. Nature 1985, 317, 260–262. [Google Scholar] [CrossRef]
- Rainier, S.; Johnson, L.A.; Dobry, C.J.; Ping, A.J.; Grundy, P.E.; Feinberg, A.P. Relaxation of Imprinted Genes in Human Cancer. Nature 1993, 362, 747–749. [Google Scholar] [CrossRef]
- Ogawa, O.; Eccles, M.R.; Szeto, J.; McNoe, L.A.; Yun, K.; Maw, M.A.; Smith, P.J.; Reeve, A.E. Relaxation of Insulin-like Growth Factor II Gene Imprinting Implicated in Wilms’ Tumour. Nature 1993, 362, 749–751. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sullivan, M.J.; Ogawa, O.; Reeve, A.E. Epigenetic Changes Encompassing the IGF2/H19 Locus Associated with Relaxation of IGF2 Imprinting and Silencing of H19 in Wilms Tumor. Proc. Natl. Acad. Sci. USA 1995, 92, 2159–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert Consensus Document: Clinical and Molecular Diagnosis, Screening and Management of Beckwith-Wiedemann Syndrome: An International Consensus Statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.M.; Vansenne, F.; Kadouch, D.J.M.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.M.; Maher, E.R.; Hennekam, R.C. Phenotype, Cancer Risk, and Surveillance in Beckwith-Wiedemann Syndrome Depending on Molecular Genetic Subgroups. Am. J. Med. Genet. Part A 2016, 170, 2248–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horii, T.; Morita, S.; Hino, S.; Kimura, M.; Hino, Y.; Kogo, H.; Nakao, M.; Hatada, I. Successful Generation of Epigenetic Disease Model Mice by Targeted Demethylation of the Epigenome. Genome Biol. 2020, 21, 77. [Google Scholar] [CrossRef]
- Giabicani, E.; Pham, A.; Sélénou, C.; Sobrier, M.-L.; Andrique, C.; Lesieur, J.; Linglart, A.; Poliard, A.; Chaussain, C.; Netchine, I. Dental Pulp Stem Cells as a Promising Model to Study Imprinting Diseases. Int. J. Oral Sci. 2022, 14, 19. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sélénou, C.; Brioude, F.; Giabicani, E.; Sobrier, M.-L.; Netchine, I. IGF2: Development, Genetic and Epigenetic Abnormalities. Cells 2022, 11, 1886. https://doi.org/10.3390/cells11121886
Sélénou C, Brioude F, Giabicani E, Sobrier M-L, Netchine I. IGF2: Development, Genetic and Epigenetic Abnormalities. Cells. 2022; 11(12):1886. https://doi.org/10.3390/cells11121886
Chicago/Turabian StyleSélénou, Céline, Frédéric Brioude, Eloïse Giabicani, Marie-Laure Sobrier, and Irène Netchine. 2022. "IGF2: Development, Genetic and Epigenetic Abnormalities" Cells 11, no. 12: 1886. https://doi.org/10.3390/cells11121886
APA StyleSélénou, C., Brioude, F., Giabicani, E., Sobrier, M.-L., & Netchine, I. (2022). IGF2: Development, Genetic and Epigenetic Abnormalities. Cells, 11(12), 1886. https://doi.org/10.3390/cells11121886





