Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors
Abstract
1. Introduction
1.1. Cancer Cachexia Overview
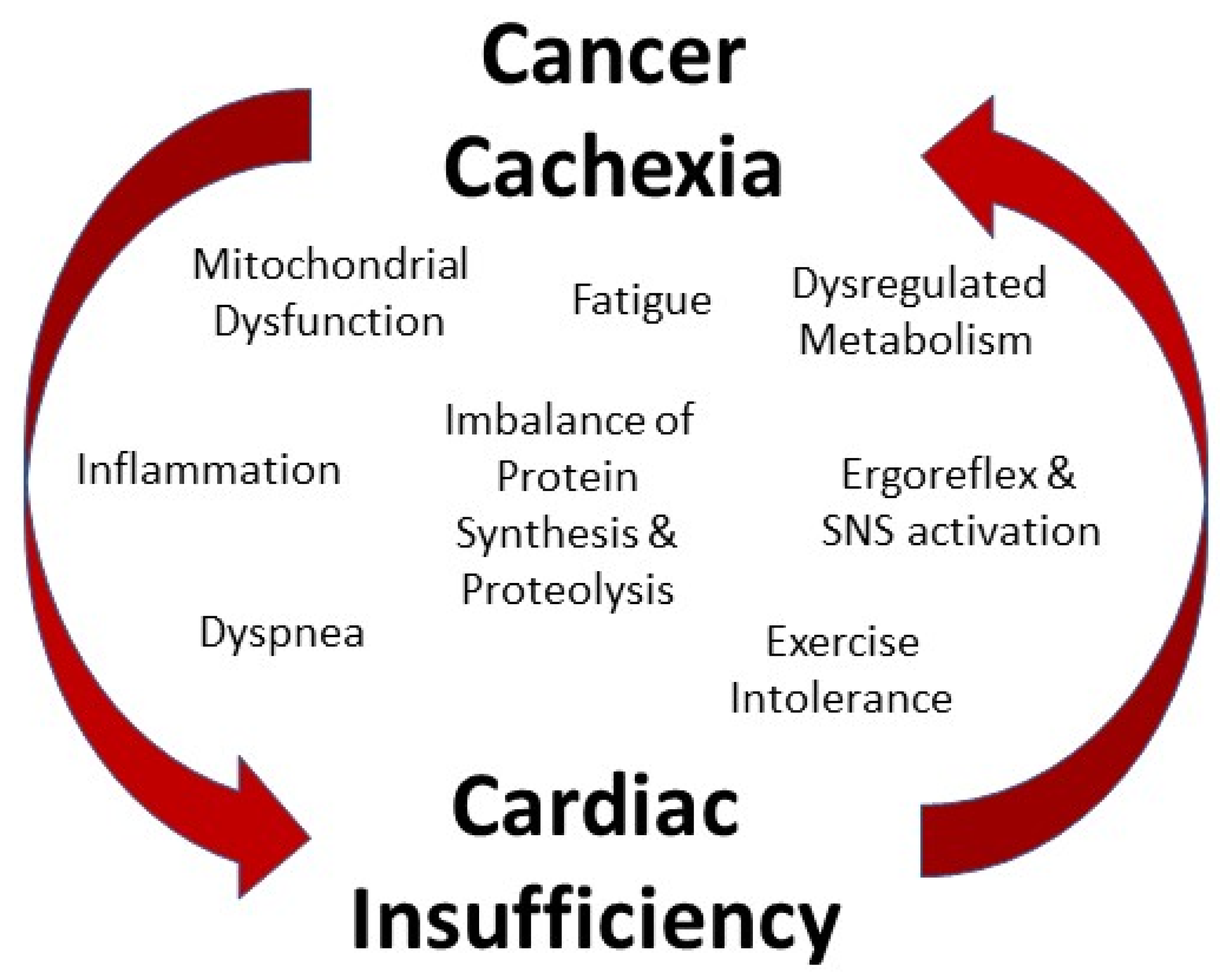
1.2. Cancer Cachexia and the Heart
2. Functional Remodeling
2.1. Clinical Observations of Cardiac Functional Changes
2.2. Mechanistic Contributors to Cardiac Functional Changes
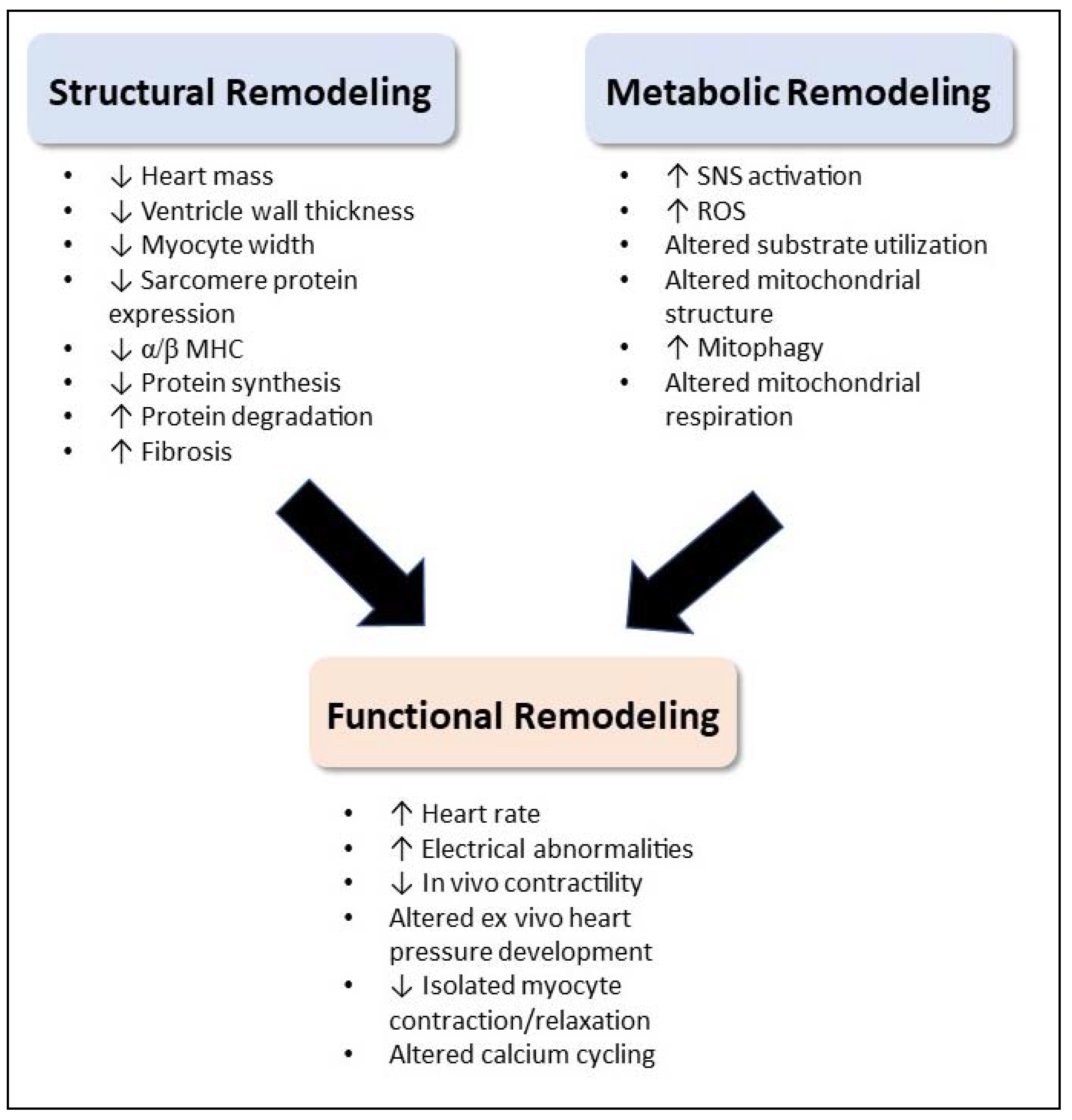
3. Structural Remodeling
3.1. Clinical Observations of Cardiac Structural Changes
3.2. Mechanistic Contributors to Cardiac Structural Changes
4. Metabolic Remodeling
4.1. Clinical Observations of Cardiac Metabolic Changes
4.2. Mechanistic Contributors to Cardiac Metabolic Changes
4.2.1. Substrate Utilization
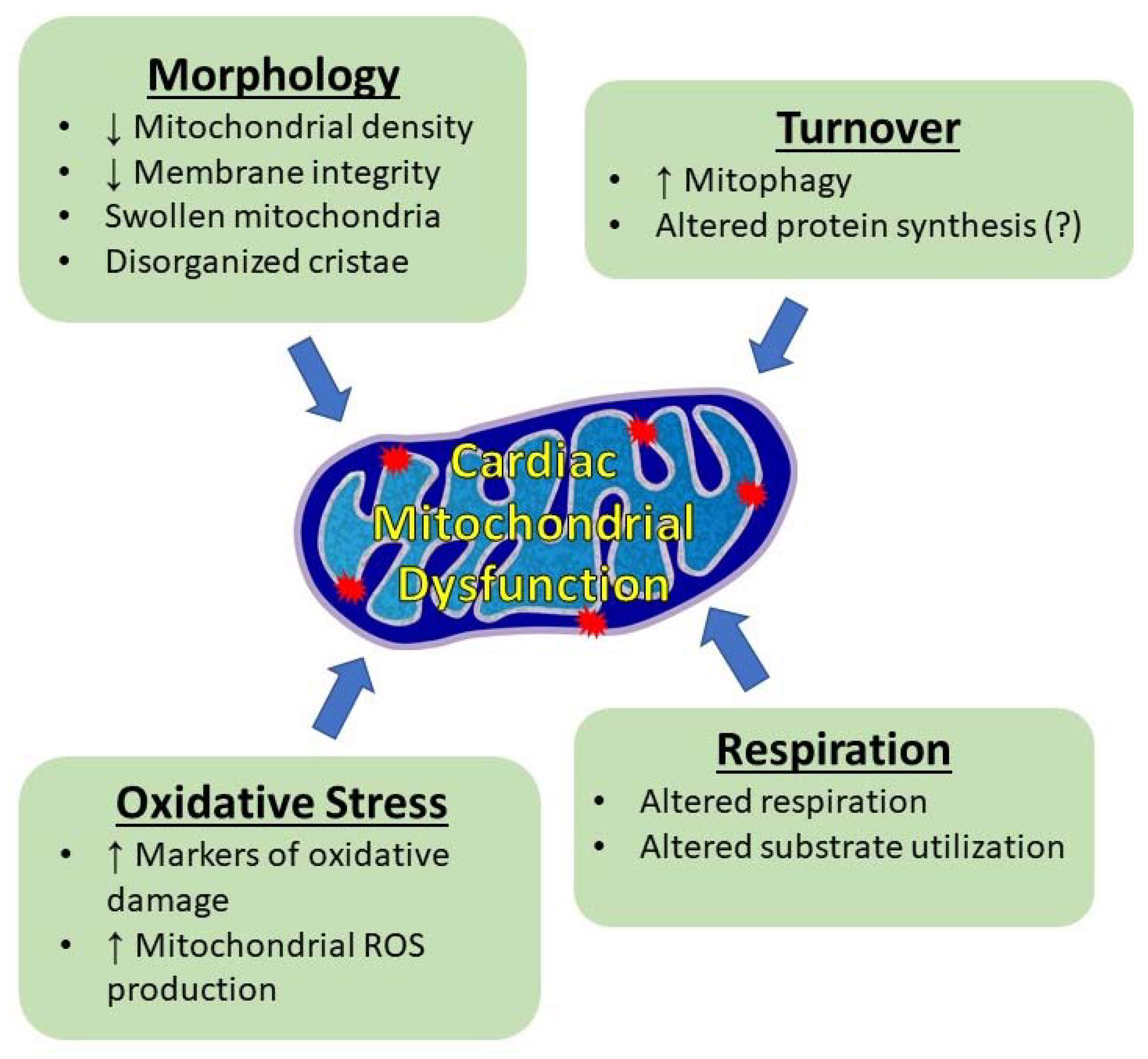
4.2.2. Mitochondrial Morphology
4.2.3. Mitochondrial Protein Synthesis and Mitophagy
4.2.4. Mitochondrial Respiration
4.2.5. Oxidative Stress
4.2.6. Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anker, M.S.; Holcomb, R.; Muscaritoli, M.; von Haehling, S.; Haverkamp, W.; Jatoi, A.; Morley, J.E.; Strasser, F.; Landmesser, U.; Coats, A.J.S.; et al. Orphan Disease Status of Cancer Cachexia in the USA and in the European Union: A Systematic Review. J. Cachexia Sarcopenia Muscle 2019, 10, 22–34. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in Cancer Patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Sun, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 20 October 2021).
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Zheng, H.; Jiang, W.; Wang, Y.; Jiang, Z.; Xu, J. Serum IL-35 Levels Is a New Candidate Biomarker of Cancer-related Cachexia in Stage IV Non-small Cell Lung Cancer. Thorac. Cancer 2022, 13, 716–723. [Google Scholar] [CrossRef]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E.R. Do Patients with Weight Loss Have a Worse Outcome When Undergoing Chemotherapy for Lung Cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Wallengren, O.; Lundholm, K.; Bosaeus, I. Diagnostic Criteria of Cancer Cachexia: Relation to Quality of Life, Exercise Capacity and Survival in Unselected Palliative Care Patients. Supportive Care Cancer 2013, 21, 1569–1577. [Google Scholar] [CrossRef]
- Gourin, C.G.; Couch, M.E.; Johnson, J.T. Effect of Weight Loss on Short-Term Outcomes and Costs of Care after Head and Neck Cancer Surgery. Ann. Otol. Rhinol. Laryngol. 2014, 123, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.T.; van Doren, B.A.; Roy, D.; Noone, J.M.; Zacherle, E.; Blanchette, C.M. Cachexia among US Cancer Patients. J. Med. Econ. 2016, 19, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Arai, H.; Inui, A. The Regulatory Approval of Anamorelin for Treatment of Cachexia in Patients with Non-Small Cell Lunger Cancer, Gastric Cancer, and Colorectal Cancer in Japan: Facts and Numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Dhanapal, R.; Saraswathi, T.; Rajkumar, G. Cancer Cachexia. J. Oral Maxillofac. Pathol. 2011, 15, 257. [Google Scholar] [CrossRef]
- Prado, C.M.; Laviano, A.; Gillis, C.; Sung, A.D.; Gardner, M.; Yalcin, S.; Dixon, S.; Newman, S.M.; Bastasch, M.D.; Sauer, A.C.; et al. Examining Guidelines and New Evidence in Oncology Nutrition: A Position Paper on Gaps and Opportunities in Multimodal Approaches to Improve Patient Care. Supportive Care Cancer 2022, 30, 3073–3083. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.M.; Baracos, V.E. A Viscerally Driven Cachexia Syndrome in Patients with Advanced Colorectal Cancer: Contributions of Organ and Tumor Mass to Whole-Body Energy Demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor Induces Muscle Wasting in Mice through Releasing Extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef]
- Friesen, D.E.; Baracos, V.E.; Tuszynski, J.A. Modeling the Energetic Cost of Cancer as a Result of Altered Energy Metabolism: Implications for Cachexia. Theor. Biol. Med. Model. 2015, 12, 17. [Google Scholar] [CrossRef]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 Axis Is a Critical Driver of Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and Myostatin in Human Cancer Cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.; Wigmore, S.J. Systemic Inflammation, Cachexia and Prognosis in Patients with Cancer. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial Degeneration Precedes the Development of Muscle Atrophy in Progression of Cancer Cachexia in Tumour-Bearing Mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 Induces Fat Loss in Cancer Cachexia by Promoting White Adipose Tissue Lipolysis and Browning. Lipids Health Dis. 2018, 17, 14. [Google Scholar] [CrossRef]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-Derived PTH-Related Protein Triggers Adipose Tissue Browning and Cancer Cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef]
- Kliewer, K.L.; Ke, J.Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose Tissue Lipolysis and Energy Metabolism in Early Cancer Cachexia in Mice. Cancer Biol. Ther. 2015, 16, 886–897. [Google Scholar] [CrossRef]
- Cao, D.; Wu, G.; Zhang, B.; Quan, Y.; Wei, J.; Jin, H.; Jiang, Y.; Yang, Z. Resting Energy Expenditure and Body Composition in Patients with Newly Detected Cancer. Clin. Nutr. 2010, 29, 72–77. [Google Scholar] [CrossRef]
- Falconer, J.S.; Fearon, K.C.H.; Plester, C.E.; Ross, J.A.; Carter, D.C. Cytokines, the Acute-Phase Response, and Resting Energy Expenditure in Cachectic Patients with Pancreatic Cancer. Ann. Surg. 1994, 219, 325–331. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Smith, K.L.; Tisdale, M.J. Increased Protein Degradation and Decreased Protein Synthesis in Skeletal Muscle during Cancer Cachexia. Br. J. Cancer 1993, 67, 680–685. [Google Scholar] [CrossRef]
- Sandri, M. Protein Breakdown in Cancer Cachexia. Semin. Cell Dev. Biol. 2016, 54, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Marzetti, E.; Rosa, F.; Pacelli, F. Skeletal Muscle Regeneration in Cancer Cachexia. Clin. Exp. Pharmacol. Physiol. 2016, 43, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-Tissue Communication in Cancer Cachexia. Nat. Rev. Endocrinol. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle Wasting and Cachexia in Heart Failure: Mechanisms and Therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.R.; Becher, H.; Fassbender, K.; Chu, Q.; Baracos, V.E. Concurrent Evolution of Cancer Cachexia and Heart Failure: Bilateral Effects Exist. J. Cachexia Sarcopenia Muscle 2014, 5, 95–104. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Beavers, C.J.; Rodgers, J.E.; Bagnola, A.J.; Beckie, T.M.; Campia, U.; di Palo, K.E.; Okwuosa, T.M.; Przespolewski, E.R.; Dent, S. Cardio-Oncology Drug Interactions: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e811–e838. [Google Scholar] [CrossRef]
- Kostakou, P.M.; Kouris, N.T.; Kostopoulos, V.S.; Damaskos, D.S.; Olympios, C.D. Cardio-Oncology: A New and Developing Sector of Research and Therapy in the Field of Cardiology. Heart Fail. Rev. 2019, 24, 91–100. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of Death among Cancer Patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Ambrus, J.L.; Ambrus, C.M.; Mink, I.B.; Pickren, J.W. Causes of Death in Cancer Patients. J. Med. 1975, 6, 61–64. [Google Scholar]
- Inagaki, J.; Rodriguez, V.; Bodey, G.P. Causes of Death in Cancer Patients. Cancer 1974, 33, 568–573. [Google Scholar] [CrossRef]
- Kikuchi, K.; Poss, K.D. Cardiac Regenerative Capacity and Mechanisms. Annu. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.; Lim, S.M.; Lee, M.K.; Giovannucci, E.L.; Kim, J.H.; Kim, S.I.; Jeon, J.Y. Resting Heart Rate as a Prognostic Factor for Mortality in Patients with Breast Cancer. Breast Cancer Res. Treat. 2016, 159, 375–384. [Google Scholar] [CrossRef]
- Anker, M.S.; Ebner, N.; Hildebrandt, B.; Springer, J.; Sinn, M.; Riess, H.; Anker, S.D.; Landmesser, U.; Haverkamp, W.; von Haehling, S. Resting Heart Rate Is an Independent Predictor of Death in Patients with Colorectal, Pancreatic, and Non-Small Cell Lung Cancer: Results of a Prospective Cardiovascular Long-Term Study. Eur. J. Heart Fail. 2016, 18, 1524–1534. [Google Scholar] [CrossRef]
- Cramer, L.; Hildebrandt, B.; Kung, T.; Wichmann, K.; Springer, J.; Doehner, W.; Sandek, A.; Valentova, M.; Stojakovic, T.; Scharnagl, H.; et al. Cardiovascular Function and Predictors of Exercise Capacity in Patients with Colorectal Cancer. J. Am. Coll Cardiol 2014, 64, 1310–1319. [Google Scholar] [CrossRef]
- Hyltander, A.; Daneryd, P.; Sandström, R.; Körner, U.; Lundholm, K. β-Adrenoceptor Activity and Resting Energy Metabolism in Weight Losing Cancer Patients. Eur. J. Cancer 2000, 36, 330–334. [Google Scholar] [CrossRef]
- Springer, J.; Tschirner, A.; Haghikia, A.; von Haehling, S.; Lal, H.; Grzesiak, A.; Kaschina, E.; Palus, S.; Pötsch, M.; von Websky, K.; et al. Prevention of Liver Cancer Cachexia-Induced Cardiac Wasting and Heart Failure. Eur. Heart J. 2014, 35, 932–941. [Google Scholar] [CrossRef]
- Cunha, G.J.L.; Rocha, B.M.L.; Menezes Falcão, L. Iron Deficiency in Chronic and Acute Heart Failure: A Contemporary Review on Intertwined Conditions. Eur. J. Intern. Med. 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Coats, A.J.S.; Clark, A.L.; Piepoli, M.; Volterrani, M.; Poole-Wilson, P.A. Symptoms and Quality of Life in Heart Failure: The Muscle Hypothesis. Br. Heart J. 1994, 72 (Suppl. S2), S36–S39. [Google Scholar] [CrossRef]
- Giannoni, A.; Aimo, A.; Mancuso, M.; Piepoli, M.F.; Orsucci, D.; Aquaro, G.D.; Barison, A.; De Marchi, D.; Taddei, C.; Cameli, M.; et al. Autonomic, Functional, Skeletal Muscle, and Cardiac Abnormalities are Associated with Increased Ergoreflex Sensitivity in Mitochondrial Disease. Eur. J. Heart Fail. 2017, 19, 1701–1709. [Google Scholar] [CrossRef]
- Anker, M.S.; Sanz, A.P.; Zamorano, J.L.; Mehra, M.R.; Butler, J.; Riess, H.; Coats, A.J.S.; Anker, S.D. Advanced Cancer Is Also a Heart Failure Syndrome: A Hypothesis. Eur. J. Heart Fail. 2021, 23, 140–144. [Google Scholar] [CrossRef]
- Anker, M.S.; Haehling, S.; Coats, A.J.S.; Riess, H.; Eucker, J.; Porthun, J.; Butler, J.; Karakas, M.; Haverkamp, W.; Landmesser, U.; et al. Ventricular Tachycardia, Premature Ventricular Contractions, and Mortality in Unselected Patients with Lung, Colon, or Pancreatic Cancer: A Prospective Study. Eur. J. Heart Fail. 2021, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.R.; Becher, H.; Butts, C.; Basappa, N.S.; Smylie, M.; Joy, A.A.; Sangha, R.; Gallivan, A.; Kavsak, P.; Chu, Q.; et al. Rapid Atrophy of Cardiac Left Ventricular Mass in Patients with Non-Small Cell Carcinoma of the Lung. J. Cachexia Sarcopenia Muscle 2019, 10, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.M.; Gigli, L.; Tagliasacchi, M.I.; di Iorio, C.; Carbone, F.; Nencioni, A.; Montecucco, F.; Brunelli, C. Update on Cardiotoxicity of Anti-Cancer Treatments. Eur. J. Clin. Investig. 2016, 46, 264–284. [Google Scholar] [CrossRef]
- Kazemi-Bajestani, S.M.R.; Becher, H.; Butts, C.; Basappa, N.S.; Smylie, M.; Joy, A.A.; Sangha, R.; Gallivan, A.; Chu, Q.; Baracos, V.E. Undiagnosed Cardiac Deficits in Non-Small Cell Carcinoma Patients in the Candidate Population for Anti-Cachexia Clinical Trials. Supportive Care Cancer 2019, 27, 1551–1561. [Google Scholar] [CrossRef]
- Barkhudaryan, A.; Scherbakov, N.; Springer, J.; Doehner, W. Cardiac Muscle Wasting in Individuals with Cancer Cachexia. ESC Heart Fail. 2017, 4, 458–467. [Google Scholar] [CrossRef]
- Cai, Q.; Mao, Y.; Yang, Q.; Wen, H.; Lv, Y.; Zhang, R. Are Left Ventricular Muscle Area and Radiation Attenuation Associated with Overall Survival in Advanced Pancreatic Cancer Patients Treated with Chemotherapy? Clin. Radiol. 2020, 75, 238-E1–238-E9. [Google Scholar] [CrossRef]
- Hyltander, A.; Körner, U.; Lundholm, K.G. Evaluation of Mechanisms behind Elevated Energy Expenditure in Cancer Patients with Solid Tumours. Eur. J. Clin. Investig. 1993, 23, 46–52. [Google Scholar] [CrossRef]
- Heckmann, M.B.; Totakhel, B.; Finke, D.; Anker, M.S.; Müller-Tidow, C.; Haberkorn, U.; Katus, H.A.; Lehmann, L.H. Evidence for a Cardiac Metabolic Switch in Patients with Hodgkin’s Lymphoma. ESC Heart Fail. 2019, 6, 824–829. [Google Scholar] [CrossRef]
- Devine, R.D.; Eichenseer, C.M.; Wold, L.E. Minocycline Attenuates Cardiac Dysfunction in Tumor-Burdened Mice. J. Mol. Cell. Cardiol. 2016, 100, 35–42. [Google Scholar] [CrossRef]
- Stevens, S.C.W.; Velten, M.; Youtz, D.J.; Clark, Y.; Jing, R.; Reiser, P.J.; Bicer, S.; Devine, R.D.; McCarthy, D.O.; Wold, L.E. Losartan Treatment Attenuates Tumor-Induced Myocardial Dysfunction. J. Mol. Cell. Cardiol. 2015, 85, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Oeing, C.U.; Rohm, M.; Baysal-Temel, E.; Lehmann, L.H.; Bauer, R.; Volz, H.C.; Boutros, M.; Sohn, D.; Sticht, C.; et al. Ataxin-10 Is Part of a Cachexokine Cocktail Triggering Cardiac Metabolic Dysfunction in Cancer Cachexia. Mol. Metab. 2016, 5, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.G.; Tobias, G.C.; Paixão, A.O.; Dourado, P.M.; Voltarelli, V.A.; Brum, P.C. Exercise Training Delays Cardiac Remodeling in a Mouse Model of Cancer Cachexia. Life Sci. 2020, 260, 118392. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Crawford, D.; Hutchinson, K.R.; Youtz, D.J.; Lucchesi, P.A.; Velten, M.; McCarthy, D.O.; Wold, L.E. Myocardial Dysfunction in an Animal Model of Cancer Cachexia. Life Sci. 2011, 88, 406–410. [Google Scholar] [CrossRef]
- Tian, M.; Asp, M.L.; Nishijima, Y.; Belury, M.A. Evidence for Cardiac Atrophic Remodeling in Cancer-Induced Cachexia in Mice. Int. J. Oncol. 2011, 39, 1321–1326. [Google Scholar] [CrossRef]
- Tian, M.; Nishijima, Y.; Asp, M.L.; Stout, M.B.; Reiser, P.J.; Belury, M.A. Cardiac Alterations in Cancer-Induced Cachexia in Mice. Int. J. Oncol. 2010, 37, 347–353. [Google Scholar] [CrossRef]
- Smuder, A.J.; Roberts, B.M.; Wiggs, M.P.; Kwon, O.S.; Yoo, J.K.; Christou, D.D.; Fuller, D.D.; Szeto, H.H.; Judge, A.R. Pharmacological Targeting of Mitochondrial Function and Reactive Oxygen Species Production Prevents Colon 26 Cancer-Induced Cardiorespiratory Muscle Weakness. Oncotarget 2020, 11, 3502–3514. [Google Scholar] [CrossRef]
- Berent, T.E.; Dorschner, J.M.; Meyer, T.; Craig, T.A.; Wang, X.; Kunz, H.; Jatoi, A.; Lanza, I.R.; Chen, H.; Kumar, R. Impaired Cardiac Performance, Protein Synthesis, and Mitochondrial Function in Tumor-Bearing Mice. PLoS ONE 2019, 14, e0226440. [Google Scholar] [CrossRef]
- Toledo, M.; Springer, J.; Busquets, S.; Tschirner, A.; López-Soriano, F.J.; Anker, S.D.; Argilés, J.M. Formoterol in the Treatment of Experimental Cancer Cachexia: Effects on Heart Function. J. Cachexia Sarcopenia Muscle 2014, 5, 315–320. [Google Scholar] [CrossRef]
- Musolino, V.; Palus, S.; Tschirner, A.; Drescher, C.; Gliozzi, M.; Carresi, C.; Vitale, C.; Muscoli, C.; Doehner, W.; von Haehling, S.; et al. Megestrol Acetate Improves Cardiac Function in a Model of Cancer Cachexia-Induced Cardiomyopathy by Autophagic Modulation. J. Cachexia Sarcopenia Muscle 2016, 7, 555–566. [Google Scholar] [CrossRef]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma Mice Model for Studying Cancer-Induced Cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef] [PubMed]
- Mühlfeld, C.; Das, S.K.; Heinzel, F.R.; Schmidt, A.; Post, H.; Schauer, S.; Papadakis, T.; Kummer, W.; Hoefler, G. Cancer Induces Cardiomyocyte Remodeling and Hypoinnervation in the Left Ventricle of the Mouse Heart. PLoS ONE 2011, 6, e20424. [Google Scholar] [CrossRef] [PubMed]
- Law, M.L.; Metzger, J.M. Cardiac Myocyte Intrinsic Contractility and Calcium Handling Deficits Underlie Heart Organ Dysfunction in Murine Cancer Cachexia. Sci. Rep. 2021, 11, 23627. [Google Scholar] [CrossRef] [PubMed]
- Drott, C.; Waldenström, A.; Lundholm, K. Cardiac Sensitivity and Responsiveness to β-Adrenergic Stimulation in Experimental Cancer and Undernutrition. J. Mol. Cell. Cardiol. 1987, 19, 675–683. [Google Scholar] [CrossRef]
- Drott, C.; Ekman, L.; Holm, S.; Waldenström, A.; Lundholm, K. Effects of Tumor-Load and Malnutrition on Myocardial Function in the Isolated Working Rat Heart. J. Mol. Cell. Cardiol. 1986, 18, 1165–1176. [Google Scholar] [CrossRef]
- Liao, R.; Podesser, B.K.; Lim, C.C. The Continuing Evolution of the Langendorff and Ejecting Murine Heart: New Advances in Cardiac Phenotyping. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, 156–167. [Google Scholar] [CrossRef]
- Burch, G.E.; Phillips, J.H.; Ansari, A. The Cachectic Heart. Dis. Chest 1968, 54, 403–409. [Google Scholar] [CrossRef]
- Sabbah, H.N. Silent Disease Progression in Clinically Stable Heart Failure. Eur. J. Heart Fail. 2017, 19, 469–478. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of Skeletal Muscle and Strength in the Elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef]
- Shum, A.M.Y.; Poljak, A.; Bentley, N.L.; Turner, N.; Tan, T.C.; Polly, P. Proteomic Profiling of Skeletal and Cardiac Muscle in Cancer Cachexia: Alterations in Sarcomeric and Mitochondrial Protein Expression. Oncotarget 2018, 9, 22001–22022. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.L.; Tanaka, A.; Sorescu, D.; Liu, H.; Jeong, E.-M.; Sturdy, M.; Walp, E.R.; Dudley, S.C.; Sutliff, R.L. Diastolic Dysfunction Is Associated with Cardiac Fibrosis in the Senescence-Accelerated Mouse. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H824–H831. [Google Scholar] [CrossRef] [PubMed]
- Cosper, P.F.; Leinwand, L.A. Cancer Causes Cardiac Atrophy and Autophagy in a Sexually Dimorphic Manner. Cancer Res. 2011, 71, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Lowes, B.D.; Gilbert, E.M.; Abraham, W.T.; Minobe, W.A.; Larrabee, P.; Ferguson, D.; Wolfel, E.E.; Lindenfeld, J.; Tsvetkova, T.; Robertson, A.D.; et al. Myocardial Gene Expression in Dilated Cardiomyopathy Treated with Beta-Blocking Agents. N. Engl. J. Med. 2002, 346, 1357–1365. [Google Scholar] [CrossRef]
- Miyata, S.; Minobe, W.; Bristow, M.R.; Leinwand, L.A. Myosin Heavy Chain Isoform Expression in the Failing and Nonfailing Human Heart. Circ. Res. 2000, 86, 386–390. [Google Scholar] [CrossRef]
- Herron, T.J.; Vandenboom, R.; Fomicheva, E.; Mundada, L.; Edwards, T.; Metzger, J.M. Calcium-Independent Negative Inotropy by β-Myosin Heavy Chain Gene Transfer in Cardiac Myocytes. Circ. Res. 2007, 100, 1182–1190. [Google Scholar] [CrossRef]
- Herron, T.J.; Devaney, E.; Mundada, L.; Arden, E.; Day, S.; Guerrero-Serna, G.; Turner, I.; Westfall, M.; Metzger, J.M. Ca2+ -independent Positive Molecular Inotropy for Failing Rabbit and Human Cardiac Muscle by A-myosin Motor Gene Transfer. FASEB J. 2010, 24, 415–424. [Google Scholar] [CrossRef][Green Version]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin–Proteasome System by the FoxO Transcriptional Network during Muscle Atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, X.; Gao, T.; Tian, H.; Zhou, D.; Zhang, L.; Li, G.; Wang, X. The Autophagic-Lysosomal and Ubiquitin Proteasome Systems Are Simultaneously Activated in the Skeletal Muscle of Gastric Cancer Patients with Cachexia. Am. J. Clin. Nutr. 2020, 111, 570–579. [Google Scholar] [CrossRef]
- Springer, J.; Palus, S.; Rauchhaus, M.; Anker, S.D. Experimental Cancer Cachexia Severely Impairs Heart Function. J. Card. Fail. 2008, 14, S18. [Google Scholar] [CrossRef]
- Springer, J.; Tschirner, A.; Grzesiak, A.; Kaschina, E.; von Haehling, S.; Anker, S.D. Cancer Cachexia Therapy: A Matter of Helping the Heart? J. Card. Fail. 2010, 16, S12. [Google Scholar] [CrossRef]
- Manne, N.D.P.K.; Lima, M.; Enos, R.T.; Wehner, P.; Carson, J.A.; Blough, E. Altered Cardiac Muscle MTOR Regulation during the Progression of Cancer Cachexia in the ApcMin/+ Mouse. Int. J. Oncol. 2013, 42, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Hyltander, A.; Drott, C.; Körner, U.; Sandström, R.; Lundholm, K. Elevated Energy Expenditure in Cancer Patients with Solid Tumours. Eur. J. Cancer Clin. Oncol. 1991, 27, 9–15. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific Metabolic Rates of Major Organs and Tissues across Adulthood: Evaluation by Mechanistic Model of Resting Energy Expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef]
- Weiss, R.G.; Gerstenblith, G.; Bottomley, P.A. ATP Flux through Creatine Kinase in the Normal, Stressed, and Failing Human Heart. Proc. Natl. Acad. Sci. USA 2005, 102, 808–813. [Google Scholar] [CrossRef]
- Gibbs, C.L. Cardiac Energetics. Physiol. Rev. 1978, 58, 174–254. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Redox-Optimized ROS Balance: A Unifying Hypothesis. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2010, 1797, 865–877. [Google Scholar] [CrossRef]
- Wisneski, J.A.; Gertz, E.W.; Neese, R.A.; Mayr, M. Myocardial Metabolism of Free Fatty Acids. Studies with 14C-Labeled Substrates in Humans. J. Clin. Investig. 1987, 79, 359–366. [Google Scholar] [CrossRef]
- Drott, C.; Lundholm, K. Glucose Uptake and Amino Acid Metabolism in Perfused Hearts from Tumor-Bearing Rats. J. Surg. Res. 1990, 49, 62–68. [Google Scholar] [CrossRef]
- Lee, D.E.; Brown, J.L.; Rosa-Caldwell, M.E.; Perry, R.A.; Brown, L.A.; Haynie, W.S.; Washington, T.A.; Wiggs, M.P.; Rajaram, N.; Greene, N.P. Cancer-induced Cardiac Atrophy Adversely Affects Myocardial Redox State and Mitochondrial Oxidative Characteristics. JCSM Rapid Commun. 2021, 4, 3–15. [Google Scholar] [CrossRef]
- Devine, R.D.; Bicer, S.; Reiser, P.J.; Wold, L.E. Increased Hypoxia-Inducible Factor-1α in Striated Muscle of Tumor-Bearing Mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1154–H1162. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sugimoto, K.; Fujimoto, T.; Xie, K.; Takahashi, T.; Akasaka, H.; Kurinami, H.; Yasunobe, Y.; Matsumoto, T.; Fujino, H.; et al. Preventive Effects of Low-Intensity Exercise on Cancer Cachexia-Induced Muscle Atrophy. FASEB J. 2019, 33, 7852–7862. [Google Scholar] [CrossRef] [PubMed]
- Asp, M.L.; Tian, M.; Wendel, A.A.; Belury, M.A. Evidence for the Contribution of Insulin Resistance to the Development of Cachexia in Tumor-Bearing Mice. Int. J. Cancer 2010, 126, 756–763. [Google Scholar] [CrossRef]
- Copeland, G.P.; Leinster, S.J.; Davis, J.C.; Hipkin, L.J. Insulin Resistance in Patients with Colorectal Cancer. Br. J. Surg. 2005, 74, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Raun, S.H.; Knudsen, J.R.; Han, X.; Jensen, T.E.; Sylow, L. Cancer Causes Dysfunctional Insulin Signaling and Glucose Transport in a Muscle-type-specific Manner. FASEB J. 2022, 36, e22211. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Novinger, L.J.; Huot, J.R.; Harris, R.A.; Couch, M.E.; O’Connell, T.M.; Bonetto, A. PDK4 Drives Metabolic Alterations and Muscle Atrophy in Cancer Cachexia. FASEB J. 2019, 33, 7778–7790. [Google Scholar] [CrossRef]
- Fukawa, T.; Yan-Jiang, B.C.; Min-Wen, J.C.; Jun-Hao, E.T.; Huang, D.; Qian, C.-N.; Ong, P.; Li, Z.; Chen, S.; Mak, S.Y.; et al. Excessive Fatty Acid Oxidation Induces Muscle Atrophy in Cancer Cachexia. Nat. Med. 2016, 22, 666–671. [Google Scholar] [CrossRef]
- Wyart, E.; Reano, S.; Hsu, M.Y.; Longo, D.L.; Li, M.; Hirsch, E.; Filigheddu, N.; Ghigo, A.; Riganti, C.; Porporato, P.E. Metabolic Alterations in a Slow-Paced Model of Pancreatic Cancer-Induced Wasting. Oxidative Med. Cell. Longev. 2018, 2018, 6419805. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Pietzsch, S.; Stapel, B.; Ricke-Hoch, M.; Lee, C.W.; Bankstahl, J.P.; Scherr, M.; Heineke, J.; Scharf, G.; Haghikia, A.; et al. Insulin Supplementation Attenuates Cancer-Induced Cardiomyopathy and Slows Tumor Disease Progression. JCI Insight 2017, 2, e93098. [Google Scholar] [CrossRef]
- Sassoon, D.A. Fatty Acid Metabolism-the First Trigger for Cachexia? Nat. Med. 2016, 22, 584–585. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and Function of Mitochondrial Membrane Protein Complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Anselmi, C.; Wittig, I.; Faraldo-Gómez, J.D.; Kühlbrandt, W. Structure of the Yeast F 1F O-ATP Synthase Dimer and Its Role in Shaping the Mitochondrial Cristae. Proc. Natl. Acad. Sci. USA 2012, 109, 13602–13607. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Ultrastructural Bases for Metabolically Linked Mechanical Activity in Mitochondria. I. Reversible Ultrastructural Changes with Change in Metabolic Steady State in Isolated Liver Mitochondria. J. Cell Biol 1966, 30, 269–297. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Lawrence, M.M.; Borowik, A.; Oliver, L.; Peelor, F.F.; van Remmen, H.; Miller, B.F. Tumor Burden Negatively Impacts Protein Turnover as a Proteostatic Process in Noncancerous Liver, Heart, and Muscle, but Not Brain. J. Appl. Physiol. 2021, 131, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Nukaga, S.; Mori, T.; Miyagawa, Y.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Nakashima, C.; et al. Combined Administration of Lauric Acid and Glucose Improved Cancer-Derived Cardiac Atrophy in a Mouse Cachexia Model. Cancer Sci. 2020, 111, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Murat Zergeroglu, A.; Demirel, H.A. Mitochondrial Signaling Contributes to Disuse Muscle Atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Melser, S.; Lavie, J.; Bénard, G. Mitochondrial Degradation and Energy Metabolism. Biochim. Et Biophys. Acta Mol. Cell Res. 2015, 1853, 2812–2821. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Pietzsch, S.; Ricke-Hoch, M.; Stapel, B.; Hilfiker-Kleiner, D. Modulation of Cardiac AKT and STAT3 Signalling in Preclinical Cancer Models and Their Impact on the Heart. Biochim. Et Biophys. Acta Mol. Cell Res. 2020, 1867, 118519. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Zhu, X.; Burfeind, K.G.; Krasnow, S.M.; Levasseur, P.R.; Morgan, T.K.; Marks, D.L. Establishment and Characterization of a Novel Murine Model of Pancreatic Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Clark, Y.Y.; Wold, L.E.; Szalacha, L.A.; McCarthy, D.O. Ubiquinol Reduces Muscle Wasting but Not Fatigue in Tumor-Bearing Mice. Biol. Res. Nurs. 2015, 17, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. The Respiratory Chain and Oxidative Phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 2006, 17, 65–134. [Google Scholar]
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control: An Introduction to OXPHOS. 5th ed. Bioenerg. Commun. 2020, 2020, 112. [Google Scholar]
- Schmidt, C.A.; Fisher-Wellman, K.H.; Darrell Neufer, P. From OCR and ECAR to Energy: Perspectives on the Design and Interpretation of Bioenergetics Studies. J. Biol. Chem. 2021, 297, 101140. [Google Scholar] [CrossRef]
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef]
- de Castro, G.S.; Simoes, E.; Lima, J.D.C.C.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Hinch, E.C.A.; Sullivan-Gunn, M.J.; Vaughan, V.C.; McGlynn, M.A.; Lewandowski, P.A. Disruption of Pro-Oxidant and Antioxidant Systems with Elevated Expression of the Ubiquitin Proteosome System in the Cachectic Heart Muscle of Nude Mice. J. Cachexia Sarcopenia Muscle 2013, 4, 287–293. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Nukaga, S.; Mori, T.; Fujiwara-Tani, R.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Sasaki, T.; Nakashima, C.; et al. Evaluation of Cancer-Derived Myocardial Impairments Using a Mouse Model. Oncotarget 2020, 11, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.H.; Marinello, P.C.; Cecchini, A.L.; Blegniski, F.P.; Guarnier, F.A.; Cecchini, R. Oxidative and Proteolytic Profiles of the Right and Left Heart in a Model of Cancer-Induced Cardiac Cachexia. Pathophysiology 2014, 21, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.R.; Pin, F.; Narasimhan, A.; Novinger, L.J.; Keith, A.S.; Zimmers, T.A.; Willis, M.S.; Bonetto, A. ACVR2B Antagonism as a Countermeasure to Multi-Organ Perturbations in Metastatic Colorectal Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1779–1798. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative Stress and Disuse Muscle Atrophy: Cause or Consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant Properties of Resveratrol: A Structure-Activity Insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Shadfar, S.; Couch, M.E.; McKinney, K.A.; Weinstein, L.J.; Yin, X.; Rodriguez, J.E.; Guttridge, D.C.; Willis, M. Oral Resveratrol Therapy Inhibits Cancer-Induced Skeletal Muscle and Cardiac Atrophy in Vivo. Nutr. Cancer 2011, 63, 749–762. [Google Scholar] [CrossRef]
- Springer, J.; Tschirner, A.; Hartman, K.; von Haehling, S.; Anker, S.D.; Doehner, W. The Xanthine Oxidase Inhibitor Oxypurinol Reduces Cancer Cachexia-Induced Cardiomyopathy. Int. J. Cardiol. 2013, 168, 3527–3531. [Google Scholar] [CrossRef]
- Zhang, V.X.; Sze, K.M.F.; Chan, L.K.; Ho, D.W.H.; Tsui, Y.M.; Chiu, Y.T.; Lee, E.; Husain, A.; Huang, H.; Tian, L.; et al. Antioxidant Supplements Promote Tumor Formation and Growth and Confer Drug Resistance in Hepatocellular Carcinoma by Reducing Intracellular ROS and Induction of TMBIM1. Cell Biosci. 2021, 11, 217. [Google Scholar] [CrossRef]
- Assi, M.; Derbré, F.; Lefeuvre-Orfila, L.; Rébillard, A. Antioxidant Supplementation Accelerates Cachexia Development by Promoting Tumor Growth in C26 Tumor-Bearing Mice. Free Radic. Biol. Med. 2016, 91, 204–214. [Google Scholar] [CrossRef]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Cancer: Antioxidants Accelerate Lung Cancer Progression in Mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef]
- Wang, X.; Pickrell, A.M.; Zimmers, T.A.; Moraes, C.T. Increase in Muscle Mitochondrial Biogenesis Does Not Prevent Muscle Loss but Increased Tumor Size in a Mouse Model of Acute Cancer-Induced Cachexia. PLoS ONE 2012, 7, e33426. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.R.; Beasley, G.S.; Miller, N.; Goldberg, J.F.; Jefferies, J.L. Clinical Insights Into Heritable Cardiomyopathies. Front. Genet. 2021, 12, 663450. [Google Scholar] [CrossRef] [PubMed]
- Law, M.L.; Cohen, H.; Martin, A.A.; Angulski, A.B.B.; Metzger, J.M. Dysregulation of Calcium Handling in Duchenne Muscular Dystrophy-Associated Dilated Cardiomyopathy: Mechanisms and Experimental Therapeutic Strategies. J. Clin. Med. 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Ross, J.M.; Loiselle, D.S.; Han, J.C.; Crossman, D.J. Right-Sided Heart Failure Is Also Associated with Transverse Tubule Remodeling in the Left Ventricle. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H940–H947. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Busquets, S.; Argilés, J.M. Experimental Cancer Cachexia: Evolving Strategies for Getting Closer to the Human Scenario. Semin. Cell Dev. Biol. 2016, 54, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ballarò, R.; Costelli, P.; Penna, F. Animal Models for Cancer Cachexia. Curr. Opin. Supportive Palliat. Care 2016, 10, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Herron, T.J.; Devaney, E.J.; Metzger, J.M. Modulation of Cardiac Performance by Motor Protein Gene Transfer. Ann. N. Y. Acad. Sci. 2008, 1123, 96–104. [Google Scholar] [CrossRef]
- Wessels, A.; Sedmera, D. Developmental Anatomy of the Heart: A Tale of Mice and Man. Physiol. Genom. 2004, 15, 165–176. [Google Scholar] [CrossRef]
| Patients (Sample Size) | Study Design | Major Findings | |
|---|---|---|---|
| Functional Remodeling | |||
| Anker, Ebner, Hildebrandt, et al. 2016 [45] | NSCLC, pancreatic, CRC (145) | Prospective, longitudinal | HR > 75 bpm predicted for mortality |
| Anker, von Haehling, Coats, et al. 2021 [53] | NSCLC, pancreatic, CRC (120) | Prospective, longitudinal | - ↑ NSVT vs. control - NSVT & PVC predicted for mortality |
| Cramer, Hildebrandt, Kung, et al. 2014 [46] | CRC (50) | Prospective, single timepoint | ↑ HR, ↓ HRV, ↓ LVEF vs. control |
| Kazemi-Bajestani, Becher, Butts, et al. 2019 [54] | NSCLC (50) | Prospective, longitudinal | ↓ LVEF, ↓ GLS at 4-month follow-up |
| Kazemi-Bajestani, Becher, Butts, et al. 2019 [56] | NSCLC (70) | Prospective, single timepoint | ↓ LVEF (<50%) incidence is higher in cachectic vs. non-cachectic patients |
| Lee, Park, Lim, et al. 2016 [44] | Breast cancer (4786) | Retrospective, longitudinal | ↑ HR predicted for mortality |
| Structural Remodeling | |||
| Barkhudaryan, Scherbakov, Springer, et al. 2017 [57] | Lung, pancreatic, GI cancer (177) | Retrospective, single timepoint | ↓ Heart weight in cachectic vs. non-cachectic patients |
| Cai, Mao, Yang, et al. 2020 [58] | Pancreatic cancer (98) | Retrospective, longitudinal | ↓ LVMA, LVMRA associated with mortality |
| Kazemi-Bajestani, Becher, Butts, et al. 2019 [54] | NSCLC (50) | Prospective, longitudinal | Cardiac atrophy associated with ↑ DLT, ↓ treatment response, ↓ physical functioning, ↑ mortality |
| Springer, Tschirner, Haghikia, et al. 2014 [48] | NSCLC, pancreatic, GI cancer (12 cancer, 14 cancer cachexia) | Post-mortem | Cachexia associated with ↓ heart mass, ↓ LVWT |
| Metabolic Remodeling | |||
| Hyltander, Körner, Lundholm, 1993 [59] | Weight-losing cancer patients (60) | Randomized, controlled trial | SNS activation is a main driver of increased REE |
| Hyltander, Daneryd, Sandström, et al. 2000 [47] | Weight-losing cancer patients (10) | Randomized, crossover | SNS attenuation via β-blockers caused ↓ REE, ↓ HR, ↓ O2 uptake |
| Heckmann, Totakhel, Finke, et al. 2019 [60] | Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, non-lymphatic cancer (337) | Retrospective, single timepoint | - Hodgkin’s lymphoma associated with ↑ cardiac glucose uptake - Chemotherapy associated with ↓ cardiac glucose uptake |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiggs, M.P.; Beaudry, A.G.; Law, M.L. Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors. Cells 2022, 11, 1931. https://doi.org/10.3390/cells11121931
Wiggs MP, Beaudry AG, Law ML. Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors. Cells. 2022; 11(12):1931. https://doi.org/10.3390/cells11121931
Chicago/Turabian StyleWiggs, Michael P., Anna G. Beaudry, and Michelle L. Law. 2022. "Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors" Cells 11, no. 12: 1931. https://doi.org/10.3390/cells11121931
APA StyleWiggs, M. P., Beaudry, A. G., & Law, M. L. (2022). Cardiac Remodeling in Cancer-Induced Cachexia: Functional, Structural, and Metabolic Contributors. Cells, 11(12), 1931. https://doi.org/10.3390/cells11121931






